Abstract
Summary
This study’s purpose was to clarify the cost-effectiveness of osteoporosis treatment. Denosumab treatment was cost-effective compared with alendronate treatment for elderly Japanese women at high risk of fragility fractures. Denosumab treatment might be cost-effective for patients with lower bone mineral density.
Purpose
In Japan’s super-aged society, the prevention and treatment of osteoporosis are a critical issue with implications for the medical economy. This study’s purpose was to clarify the cost-effectiveness of osteoporosis treatment with denosumab versus weekly alendronate for elderly Japanese women at high risk of fragility fractures.
Methods
A Markov model was used for simulation analysis. The modeled population was 75-year-old Japanese women with a bone mineral density (BMD) of 65% of the young adult mean (YAM) (T-score, − 2.87) and a history of previous vertebral body fracture. The simulation model was repeated until patient age reached 100 years or death. Analysis was performed from the societal perspective. Costs and epidemiological data were derived from previous studies. The incremental cost-effectiveness ratio (ICER) was calculated from the simulation. We compared the ICER with willingness-to-pay. Additional analyses were performed with different combinations of age and BMD. Sensitivity analysis verified the robustness of the analysis.
Results
For the modeled population, the ICER of denosumab versus alendronate treatment was estimated at US$40,241/quality-adjusted life year (QALY). The ICER of denosumab for 80-year-old women whose BMD was 60% of YAM was estimated at US$22,469/QALY.
Conclusions
Assuming willingness-to-pay as US$50,000/QALY, denosumab treatment for 75-year-old Japanese women with a BMD of 65% of YAM and a history of previous vertebral body fracture was cost-effective compared with alendronate treatment. Among over 75 years of age, denosumab treatment might be more cost-effective than alendronate for patients with a BMD of 65% of YAM or lower.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The number of osteoporosis patients is increasing in the super-aged society in Japan. Osteoporotic fragility fractures are also increasing [1]. Although Imai et al. reported that the age-specific incidence of hip fractures in Niigata Prefecture was lower in 2015 than in 2010 [2], the total number of hip fractures in Japan continues to rise.
Among osteoporotic fragility fractures, hip fractures and vertebral fractures have a great impact in clinical practice. These fractures significantly degrade patients’ quality of life (QOL) [3] and are related to an increased mortality rate [4]. In addition, hip fracture treatment is expensive because it usually requires surgery. Hip fracture is a major factor leading to the need for long-term care and also increases nursing care expenses [5]. The increased expense associated with treating these fragility fractures is an urgent issue for health economics in Japan.
Osteoporosis treatment aims to prevent fragility fractures by raising bone mineral density (BMD). Japanese guidelines for the prevention and treatment of osteoporosis (2015 edition) provide standard treatment guidance [6]. The evidence for various osteoporosis treatment drugs is provided in the guideline; doctors use their experience and knowledge to select the most appropriate drug according to the patient’s age and fracture risk.
The Japanese guideline recommends alendronate, risedronate, and denosumab for patients with a high risk of hip fracture [6]. Major risk factors for hip fracture include female sex, low BMD, older age, and a history of previous fragility fractures. Additional risk factors include smoking, alcohol use, family history of hip fracture, exercise, BMI, and low calcium intake [6].
In recent years, the importance of health economic analysis has been recognized; results of economic analysis are required to be used in clinical decision-making and in medical policy development in Japan. A trial of cost-effectiveness evaluation was implemented beginning in fiscal year 2016; the results will be adapted to adjust the price of drugs and medical devices in the future [7]. In 2017, an official guideline for economic evaluation was developed in response to the request of the Central Social Insurance Medical Council (Chuikyo) [7]. Because osteoporosis treatment is associated with health economic problems in Japan, it is very important to analyze treatment from the viewpoint of health economics.
Osteoporosis treatment is generally considered to be cost-effective when used in patients with a high risk of fracture [6]. The purpose of this study was to analyze the cost-effectiveness of 5 years’ treatment with denosumab or alendronate in elderly Japanese women with low BMD and existing previous vertebral fracture, who were considered to have a high risk of fragility fractures.
As mentioned above, the guideline recommends alendronate, risedronate, and denosumab for patients at high risk of hip fracture [6]. In Japan, alendronate has the largest market share among bisphosphonates, so we chose alendronate as the representative bisphosphonate [8]. The relative risk reduction for fracture occurrence reported in a network meta-analysis was nearly equal for alendronate and risedronate [9]. In addition, drug adherence, side effects, and the offset time effect of the two bisphosphonate are thought to be almost the same. Because risedronate and alendronate produce very similar results, this study only included weekly alendronate in analysis.
Materials and methods
Target population
In Japan, osteoporosis is defined as a T-score lower than − 2.5 or BMD lower than 70% of the young adult mean (YAM), measured with dual-energy X-ray absorptiometry (DXA) [6]. In addition, patients with previous hip or vertebral fracture are diagnosed with osteoporosis, regardless of T-score or BMD [6]. Risk factors for hip fracture include female sex, old age, low BMD, and history of vertebral fracture [6].
The reference value for BMD in the proximal femur of Japanese women aged 20 to 29 years is 0.790 ± 0.090 g/cm2. Thus, 70% of the YAM for BMD is 0.553 g/cm2, 65% of YAM is 0.5135 g/cm2, and 60% of YAM is 0.474 g/cm2. T-scores were calculated using NHANES III reference value of 0.858 ± 0.120 g/cm2; 70% of YAM was equivalent to a T-score of − 2.54, 65% of YAM was equivalent to a T-score of − 2.87, and 60% of YAM was equivalent to a T-score of − 3.2 [10].
The target population in this study was individuals at high risk for fragility fractures, especially hip fractures. Specifically, the population was 75-year-old Japanese women with a BMD of 65% of YAM (T-score, − 2.87) and a history of previous vertebral body fracture. We used the population of women with a BMD of 65% of YAM (T-score, − 2.87), which is 5% lower than the diagnostic criteria, as a population at higher risk of fractures. Because Japanese epidemiological data indicate that hip fractures markedly increase when patients reach their 70s [11], we regarded the high-risk population for hip fracture as Japanese women aged 75 and older.
Markov model
Markov model analysis was performed to estimate the cost-effectiveness of osteoporosis treatment, comparing 5-year treatment with subcutaneous denosumab every 6 months versus weekly oral alendronate or no treatment.
In this model, the cohort was transitioned between health states in a 1-year cycle and was followed from the time of treatment initiation until death or 100 years of age, which was defined as a lifelong time horizon.
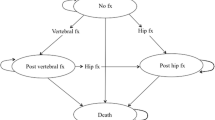
The model consisted of 12 health states: “event free,” “hip fracture,” “post-hip fracture,” “second hip fracture,” “post-second hip fracture,” “vertebral fracture,” “post-vertebral fracture,” “second vertebral fracture,” “post-second vertebral fracture,” “bed ridden,” “drop out,” and “death” (Fig. 1).
Markov model used in this study. Patients start in event-free state and move to other states with a certain transition probability in a 1-year cycle. Dropout may occur until the third year after start of drug treatment. Patients can move from all states to the state of “dead”; the arrows representing this movement are omitted
In this model, all patients began in the “event-free” state and were exposed to the possibility of an event occurring with a certain probability every year. Major events were “hip fracture” and “vertebral fracture.” In a cycle in which fracture did not occur, the patient remained in the “event-free” state. A patient who had hip fracture or vertebral fracture moved to the “post-hip fracture” or “post-vertebral fracture” state. We set the model so that hip fracture and vertebral fracture could occur up to twice. Models in previous reports [12] commonly allowed patient to have multiple vertebral fractures. Because the target population of this study was 75-year-old Japanese women, we considered that it would be rare for a patient to receive treatment for multiple vertebral fractures before death. Therefore, we set our model so that patients could have a maximum of two clinical vertebral fractures. Our intention was to set the number of vertebral fractures slightly conservatively.
Patients who had vertebral fracture could have hip fracture, but patients who had hip fracture could not have vertebral fracture, as in a previous report [12]. After hip fracture, patients had a certain probability of becoming bedridden and entering a nursing home. We assumed that patients who entered a nursing home stayed there until death without experiencing a new event. Therefore, patients in the state “bedridden” had only two possibilities: death or remaining bedridden. “Death” could occur in all states. Patients could discontinue osteoporosis treatment up to 3 years after treatment initiation; patients who continued treatment until 3 years continued treatment thereafter [13].
This model did not consider fragility fractures other than hip fracture and clinical vertebral fracture (e.g., wrist fracture or pelvic fracture) because data derived from the Japanese population are insufficient. We considered clinical vertebral fractures in this model; asymptomatic morphometric vertebral fractures were not considered. Thirty percent of all vertebral fractures are considered clinical vertebral fractures [14].
This model was constructed and analyzed with TreeAge Pro 2016 (TreeAge Software, Williamstown, MA, USA).
Outcome estimation
Transition probabilities
The annual incidence rates of hip and vertebral fracture were estimated with the equations of Moriwaki et al. [14] (Supplemental Table 1). These equations included patient age and BMD and epidemiological data from post-menopausal Japanese women with osteoporosis and osteopenia [14]. The transition probability (p) of events occurring within a certain time (t) was calculated using the incidence rate (r), according to the following formula: p = 1 − exp(−rt) [14]. In this study, the transition probability of clinical vertebral fractures was derived from the incidence rates of patients with previous vertebral fractures (Supplemental Table 1).
The relative risk of hip fracture in patients with previous vertebral fracture was derived from the report of Kanis et al. [15]. The relative risk of subsequent hip fracture in Japanese patients with previous hip fracture was derived from the report of Hagino et al. [16].
The age-specific mortality rate was derived from the 22nd Life Table published by the Ministry of Health and Welfare of Japan [17].
The odds ratio of death after hip fracture was determined with the formula reported by Moriwaki et al. [4, 14]. The odds ratio was calculated from the first to the tenth year; each odds ratio was incorporated into the model. We assumed that patients would continue to have an increased mortality rate after more than 10 years after a hip fracture.
The probability that a patient would become bedridden and enter a nursing home after hip fracture was derived from the report of Hayashi et al. [18].
Clinical efficacy
To use data close to the conditions that we established, we chose the data on fracture-prevention effect. The relative risks of hip and vertebral fracture in patients receiving alendronate were derived from data on the fracture-prevention effect of alendronate in patients with previous vertebral fracture in the sub-analysis of the FIT trial [19]. The relative risk of hip fracture in patients receiving denosumab was derived from data on patients aged over 75 years with T-scores below − 2.5 reported by Boonen et al. [20]. The relative risk of vertebral fracture in patients receiving denosumab was derived from data on patients aged over 75 years reported by McClung et al. in the sub-analysis of the FREEDOM trial [21] (Table 1).
In our model, the post-cessation drug effects continued for the same period as the treatment period; this period was called the offset time [12]. The effects of 5-year treatment with alendronate or denosumab were assumed to linearly approach zero at 5 years after stopping treatment, as in past reports [12, 23, 32].
Drug adherence consists of “persistence,” which is duration of treatment; “compliance,” which is evaluated with the medication possession ratio (MPR); and “primary non-adherence” [13]. We considered “persistence.” Strom et al. assumed that patients were at risk of dropping out during the first 3 years of treatment [13]; we used a similar setting. The persistence rate of alendronate use until the third year was derived from the prescription data of 13 Japanese university hospitals reported by Kishimoto et al. [22]. Because there is no literature on the 3-year persistence rate for denosumab use in Japan, we used the 2-year persistence rate of 2315 patients in Sweden reported by Karlsson et al. [24]. Because there were no data for the third year in that report, we estimated the 3-year persistence rate from the data of Karlsson et al., using the ratio of second-year to third-year alendronate persistence rates of Kishimoto et al. [22, 24] (Table 1).
We considered the reduction in fracture prevention effect resulting from partial drug compliance with the following equation: partial efficacy of fracture prevention effect = 1 − (relative risk of fracture by drug) × (MPR of drug).
For alendronate compliance, we used the MPR of weekly bisphosphonate in the first year from the report of Kishimoto et al. [22]. However, because there are no data on denosumab compliance in Japan, denosumab compliance information was obtained from the report by Hadji et al., which analyzed denosumab adherence in the first 12 months in routine practice in four European countries [25]. That study used the term “adherence” to described compliance.
The drug effect in patients who dropped out in the first year was assumed to be half of the original fracture-preventing effect in the first year. Patients who dropped out in the third year were assumed to have the original fracture-prevention effect in the first and second years and half the drug effect in the third year. After the third year, the drug effect decreased linearly, becoming zero in the fifth year.
The general strategy of osteoporosis treatment in this model was as follows. At the time of the initial hospital visit, the patient’s BMD was measured with DXA. Blood tests and X-ray of the lumbar spine were performed to confirm the presence or absence of secondary osteoporosis or vertebral fracture. After measuring biochemical markers of bone turnover, the doctor started osteoporosis treatment.
The patient visited the hospital once every 3 months and markers were measured within 6 months from the start of treatment. Patients treated with denosumab received daily calcium, magnesium, and natural vitamin D3 supplementation. Calcium and creatinine levels were checked every 3 months.
Hip fracture patients were assumed to receive osteoporosis treatment with denosumab or alendronate until their deaths.
The “no-treatment” group visited the hospital and continued follow-up with blood tests or DXA, but received no osteoporosis treatment. After fracture, they received fracture treatment and follow-up observation, but did not receive osteoporosis treatment. The “no-treatment” group was set for reference only for comparison with other treatments.
Costs
To estimate the opportunity cost of resource use from a societal perspective, we aggregated the direct medical costs borne by patients and third-party payers including public medical expenses and public nursing care expenses.
All costs were calculated using US$1.0 for Japanese Yen 100, for convenience. Costs are shown in Table 1.
Drugs costs were derived from a 2018 Japanese price list [26]. The cost of denosumab included the cost of daily calcium, magnesium, and natural vitamin D3 supplements.
The annual costs of hospital visits and examinations were calculated according to the Japanese tariffs in 2016 [33]. Treatment costs for hip fractures were calculated using the average values at a university hospital and two private hospitals reported by Kondo et al. [27]. Treatment costs for vertebral fractures were calculated using average values of treatment costs, including inpatient and outpatient treatment excluding surgery, from a questionnaire survey in Japan reported by Konno et al. [28]. The annual cost of level 5 nursing home care was calculated from the 2015 Japanese benefit for nursing home care [29]. We analyzed this model using a 3% discount rate for all costs.
Utilities
We used quality-adjusted life years (QALYs) as a measure of the treatment effect. Each utility value is shown in Table 1.
We used the utility values according to age for the health condition of elderly Japanese women reported by Nawata et al. [30]. We calculated the rate of change of the utility value resulting from fragility fractures in elderly Japanese women using the report of Hagino et al. [3]. The utility value after fracture was calculated by multiplying the utility value for each age in the event-free state by the rate of change due to fragility fracture. The utility value of the bedridden state was obtained from data reported by Imai et al. [31]. Outcomes were discounted at a rate of 3%.
Base-case analysis
In base-case analysis, we estimated the lifetime costs and QALYs of 5-year treatment with denosumab, alendronate, or no treatment in 75-year-old Japanese women with a BMD at 65% of YAM (T-score, − 2.87) and with a history of previous vertebral body fracture. The main purpose of this study was to compare the ICER of denosumab treatment versus alendronate treatment. The ICER was defined as follows:
ICER = incremental cost/incremental effectiveness = cost (denosumab treatment) − cost (alendronate treatment) / effectiveness (denosumab treatment) − effectiveness (alendronate treatment).
In addition, we calculated the ICERs for various combinations of age and BMD in the target population. Three levels of the YAM value (70% [T-score, − 2.54], 65% [T-score of − 2.87], 60% [T-score, − 3.2]) and three ages (75 years, 80 years, 85 years) were set; each combination of parameters was analyzed.
Sensitivity analysis
To clarify the robustness of the results of this study, deterministic analysis was performed. For the data range used in deterministic sensitivity analysis, the confidence interval shown in the literature was used. Costs were verified in the range of ± 30%. Changes in utility values were verified in the range of ± 10%.
Probabilistic sensitivity analysis was also performed with a 1000-times Monte Carlo simulation. The effects of drugs, utility values, dropout ratio, MPR of drugs, and odds ratio of death after hip fracture were randomly sampled from beta distribution and gamma distribution. Cost items were sampled from a triangular distribution.
Results
Validation
The number of fractures occurring in 10 years in this model was calculated and compared with the predicted fracture incidence according to fracture risk assessment tool: FRAX® over a 10-year periods [34]. In the no-treatment group of the base case, the incidence of hip fracture was 6.4% over 10 years and that of vertebral fracture was 28.1%. In other words, 10-year probability of major fracture was above 34.5%. In contrast, if we input BMD and age of our base case, using the average height and weight of 75-year-old Japanese women [35], FRAX predicted that the 10-year probability of hip fracture was 9.2% and that of the major fracture was 29%. Thus, the 10-year probability of vertebral fracture according to FRAX was below 20%.
Although the incidence of hip fractures in this model was slightly more conservative than that of FRAX, the incidence rates are similar. In contrast, the 10-year probability of vertebral fracture in this model was 28.1%, whereas the probability was less than 20% according to FRAX. In this model, vertebral fractures occurred somewhat more frequently than predicted by FRAX. We believe that the validity of this model was confirmed. If we set this model so that vertebral fracture could occur three or more times, the probability of vertebral fracture would further increase.
Cost-effectiveness according to base-case analysis
Table 2 shows the results of cost-effectiveness analysis of osteoporosis treatment for 75-year-old Japanese women with a BMD of 65% of YAM (T-score, − 2.87) and a history of previous vertebral fracture. Compared with alendronate treatment, the additional lifetime cost of 5-year treatment with denosumab was US$1770; the additional lifetime effect was 0.04 QALY. Thus, the ICER was US$40,241/QALY.
Table 3 shows the ICERs associated with different ages and BMDs (T-scores) in the target population. For 75-year-old women with a BMD of 60% of YAM (T-score, − 3.2), the ICER was US$22,759/QALY; for those with 70% of YAM (T-score, − 2.54), the ICER was US$63,941/QALY.
In the target population with a BMD less than 65% of YAM (T-score, − 2.87), which is an extremely low value, when willingness to pay was set at US$50,000 [36], denosumab was cost-effective in all age groups.
Deterministic sensitivity analysis
The results of deterministic sensitivity analysis are shown in Fig. 2. The parameters that had a significant effect on the outcome were the relative risks of hip fracture with denosumab and alendronate treatment, the compliance of denosumab and alendronate, the cost of denosumab, and the offset time of denosumab.
Probabilistic sensitivity analysis
The acceptability curve and the scatter plot obtained from probabilistic sensitivity analysis is shown in Fig. 3. In the base-case analysis, the probabilities that the ICER of 5-year treatment with denosumab or alendronate will be less than US$50,000 were 52.3% and 47.7%, respectively.
Discussion
When willingness-to-pay was set at US$50,000, osteoporosis treatment with denosumab was cost-effective compared with weekly alendronate treatment for 75-year-old Japanese women with a BMD of 65% of YAM (T-score, − 2.87) and a history of previous vertebral body fracture. This study clarifies how the ICERs of denosumab treatment to alendronate treatment change with different combinations of age and BMD values. In the target population with a BMD of 65% or less of YAM (T-score, − 2.87 or lower), ICERs were lower than US$50,000. Our findings also suggest that for the same BMD, treatment of 80-year-old women is more cost-effective than treatment of the other two age groups. When the target treatment population was set at 85 years, the overall mortality rate increased, the number of prevented fractures decreased, and the efficiency deteriorated. Within each age group, the cost-effectiveness of denosumab tended to be better for populations with lower BMD.
In deterministic sensitivity analysis, the relative risk of hip fracture in patients taking denosumab versus alendronate had the greatest influence on results. This finding is similar to that of a previous cost-effectiveness analysis [32, 37]. To improve the robustness of cost-effectiveness analysis for osteoporosis treatment, it is important that the values of relative risk for hip fracture in patients receiving each drug be more specific.
The model was sensitive to denosumab compliance in deterministic sensitivity analysis. Jonsson et al. did not take compliance into consideration in their study because of lack of data, but proposed that not only a cost of drug and its fracture-prevention effects but also a country’s own persistence and compliance data are important [12]. Because there is little difference in the fracture prevention effect of alendronate versus denosumab for hip fracture, the high persistence rate and better compliance rate of denosumab are greatly related to its cost-effectiveness. In this study, the model was sensitive to the persistence rates of both denosumab and alendronate.
The parameter with the next greatest influence in sensitivity analysis was the combined cost of annual denosumab and daily calcium, magnesium, and natural vitamin D3 supplements. Karnon et al. conducted a cost-effectiveness analysis of denosumab, zoledronic acid, and alendronate in Australia [38]. In Australia, the price of alendronate has declined by approximately 65%, while the prices of denosumab and zoledronic acid have not declined. That study reported that denosumab was not cost-effective because its price had not declined. We conducted this analysis using the drug price revised in 2018 [26]; however, the result of cost-effectiveness analysis is affected by increases in drug prices. In this study, if the cost of denosumab was lower than US$820, which is currently 89.0% of the price, the ICER was always below the threshold in base-case analysis. To evaluate cost-effectiveness, more discussion of the cost of denosumab may be necessary.
The model was also sensitive to the offset time of denosumab in deterministic sensitivity analysis. In this study, the offset time of denosumab was set similarly to that of alendronate, as in previous reports [12, 32, 37]. It has been suggested that BMD may decrease quickly after discontinuation of denosumab, resulting in vertebral fractures [39]. Therefore, we need to verify in future studies whether it is reasonable to determine the offset time of denosumab in the same way as for alendronate. In this study, deterministic sensitivity analysis verified the effect of the offset time of denosumab in the range of 0 to 10 years. Assuming no offset time with denosumab, the ICER was US$60,906, which exceed the threshold. Offset time of denosumab has a large influence on the results, so careful consideration is necessary.
Several cost-effectiveness analyses of denosumab and alendronate have been conducted in other countries. Many of these studies have found that the cost-effectiveness of denosumab was better than that of alendronate [12, 37, 40]. The study reported by Karnon et al. is the only study reporting that denosumab was not cost-effective, because of its price [38].
Mori et al. performed the first cost-effectiveness analysis comparing denosumab with alendronate, and their study is the only prior research on the subject in Japan [32]. In an analysis of four age groups and multiple factors, that study found that denosumab was more cost-effective than alendronate. A comparison between that study and the present study is shown in Supplemental Table 2 [41, 42].
Our model was essentially not very different from that of Mori et al. Patients who had hip fracture could not have vertebral fracture. Other fragility fractures (e.g., wrist or pelvic fracture) were not considered. Mori et al. also set their model so that patients could have a maximum of two hip fractures, as in our model. The major difference between the models is that patients could have a maximum of two clinical vertebral fractures in our model, as discussed above.
Another difference between the structure of our model and that of Mori et al. is the determination of the transition probability for hip and vertebral fracture. To calculate transition probability, we used a formula to calculate the annual incidence rate of fracture according to age and BMD in accordance with the method of Moriwaki et al. [14]. In contrast, Mori et al. calculated the annual fracture rates by multiplying the age- and sex-specific fracture risk in the general population by the relative risk based on the presence of osteoporosis [32].
The advantage of the mathematical formula used in this study to determine the fracture transition probability is that it is possible to classify conditions according to age and BMD. Using this formula, it is possible to verify how drug efficacy changes with BMD and age. Evaluating efficacy with a combination of age and BMD is useful when making decisions in actual clinical situations and may also be useful for policy-making decisions. The validity of this formula can be discussed, but it has been sufficiently verified in previous studies [14]. In this model, we confirmed the validity using FRAX.
Mori et al. used the fracture incidence rate of each age and multiplied it by the relative risk of osteoporosis. One great advantage of that method is that it is very concise and is based on epidemiological data. However, it is difficult to classify conditions based on BMD, and there is the problem that the “degree” of osteoporosis cannot be verified.
There are also differences in the data on the drug effects of denosumab and alendronate. Mori et al. used data from the network meta-analysis of 2012 [9, 32]. The data used in the present study were derived from sub-analysis of large clinical trials of denosumab and alendronate use [19,20,21]. The reason for using these data was to match the subject of the present study, which was elderly women with a high risk of fragility fracture and a history of previous vertebral fracture.
Although there were some differences in the data used in the two studies, the cost-effectiveness of osteoporosis treatment using denosumab was better than that of alendronate treatment in both studies.
A strength of this study is that the analysis was performed with conditionalization according to BMD and age group. No previous studies of denosumab have examined how the combination of age and BMD affects cost-effectiveness. In clinical practice, doctors select drugs after considering several parameters, such as patient age, BMD, and history of previous fracture. As this study indicates, it is clinically important to analyze the cost-effectiveness of osteoporosis treatment according to BMD and age.
Although the cost-effectiveness of denosumab was good for the target population in base-case analysis, the cost-effectiveness may be higher for 80-year-old patients and for those with markedly low BMD values. Our results indicate that from a health-economic viewpoint, denosumab treatment should be considered for elderly osteoporotic women with markedly low BMD. Our results can be the basis for new treatment decisions in daily practice and for determining medical policy.
Health economic analysis indicated that denosumab treatment may not be recommended for patients over 85 years of age, even those with very low BMD, because the efficiency decreased as the mortality rate increased and the total number of preventable fractures decreased for patients aged 85 years and older. It is necessary to comprehensively evaluate the indications for denosumab treatment in super-aged patients, considering their background and risks.
There are several limitations in this study. First, other fragility fractures such as wrist fractures and pelvic fractures were not considered. The main reason for this exclusion is that domestic data on other fragility fractures are insufficient.
Second, data on the persistence rate of 3 years or more of denosumab treatment in Japan are insufficient, so we used data from other countries. The data we used were 2-year persistence rates for denosumab, so we assumed a third-year persistence rate.
Third, the offset time of effect after discontinuation of denosumab is controversial. It is necessary to sufficiently evaluate how to set the offset time for denosumab in the future.
Fourth, we did not consider the side effects of denosumab or alendronate. Side effects include gastroesophageal reflux disease with alendronate and cellulitis with denosumab; common problems of bone resorption inhibitors include osteonecrosis of the jaw and atypical femoral fracture. However, because these serious side effects are very infrequent, the current consensus is that the benefits of fracture prevention with osteoporosis drugs exceed the risk of these side effects [43]. Bone fusion is difficult in atypical femoral fractures, resulting in a high rate of nonunion [44], which decreases QOL and is a clinical problem that should not be ignored. It is necessary to consider incorporating these side effects into future models.
In this study, we set the willingness to pay threshold at US$50,000. Thresholds for judging cost-effectiveness analysis remain controversial in Japan. However, in many domestic medical economic analyses to date, the threshold value has been based on the report of Shiroiwa et al. [36]. In that report, the threshold in Japan was five million JPY, which we recognize as being common in Japan [36]. The Japanese cost-effectiveness evaluation expert group of the Central Social Insurance Medical Council (Chuikyo) mentions the report of Shiroiwa et al. as a criterion, and a willingness-to-pay threshold of US$50,000 has been regarded as the present standard [36, 45]. However, careful discussion is required to establish accurate standards in Japan.
In conclusion, osteoporosis treatment with denosumab was cost-effective for 75-year-old Japanese women with a BMD of 65% of YAM (T-score, − 2.87) and a history of previous vertebral body fracture, compared with treatment with weekly alendronate, when willingness-to-pay was set at US$50,000. Denosumab treatment may be cost-effective for elderly women over 75 years of age with a BMD value of 65% or less of YAM (T-score, − 3.2 or lower).
References
Orimo H, Yaegashi Y, Hosoi T, Fukushima Y, Onoda T, Hashimoto T, Sakata K (2016) Hip fracture incidence in Japan: estimates of new patients in 2012 and 25-year trends. Osteoporos Int 27:1777–1784
Imai N, Endo N, Shobugawa Y, Ibuchi S, Suzuki H, Miyasaka D, Sakuma M (2017) A decrease in the number and incidence of osteoporotic hip fractures among elderly individuals in Niigata, Japan, from 2010 to 2015. J Bone Miner Metab. https://doi.org/10.1007/s00774-017-0863-2
Hagino H, Nakamura T, Fujiwara S, Oeki M, Okano T, Teshima R (2009) Sequential change in quality of life for patients with incident clinical fractures: a prospective study. Osteoporos Int 20:695–702
Tsuboi M, Hasegawa Y, Suzuki S, Wingstrand H, Thorngren KG (2007) Mortality and mobility after hip fracture in Japan: a ten-year follow-up. J Bone Joint Surg Br 89:461–466
Harada A, Matsui Y, Takemura M, Wakao N, Ota T (2005) Cost-utility analysis of osteoporosis. Nihon Ronen Igakkai Zasshi 42:596–608 In Japanese
Committee of Japanese Guidelines for the Prevention and Treatment of Osteoporosis (2015) The Japanese guidelines for the prevention and treatment of osteoporosis (2015 edn). Life Science Publishing, Tokyo. In Japanese
Shiroiwa T, Fukuda T, Ikeda S, Takura T, Moriwaki K (2017) Development of an official guideline for the economic evaluation of drugs/medical devices in Japan. Value Health 20:372–378
Nagase T (2016) Market of osteoporosis treatment drugs. Kokusai Iyakuhin Jouhou 1072:6–14 In Japanese
Murad MH, Drake MT, Mullan RJ, Mauck KF, Stuart LM, Lane MA, Abu Elnour NO, Erwin PJ, Hazem A, Puhan MA, Li T, Montori VM (2012) Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab 97:1871–−1880
Looler AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC Jr, Lindsay R (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489
Orimo H, Yaegashi Y, Onoda T, Fukushima Y, Hosoi T, Sakata K (2009) Hip fracture incidence in Japan: estimates of new patients in 2007 and 20-year trends. Arch Osteoporos 4:71–77
Jonsson B, Strom O, Eisman JA, Papaioannou A, Siris ES, Tosteson A, Kanis JA (2011) Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 22:967–982
Strom O, Borgstrom F, Kanis JA, Jonsson B (2009) Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int 20:23–34
Moriwaki K, Komaba H, Noto S, Yanagisawa S, Takiguchi T, Inoue H, Toujo T, Fukagawa M, Takahashi HE (2013) Cost-effectiveness of alendronate for the treatment of osteopenic postmenopausal women in Japan. J Bone Miner Res 28:395–403
Kanis JA, Johnell O, De Laet C et al (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382
Hagino H, Sawaguchi T, Endo N, Ito Y, Nakano T, Watanabe Y (2012) The risk of a second hip fracture in patients after their first hip fracture. Calcif Tissue Int 90:14–21
Japan Ministry of Health, Labour and Welfare (2018) Life table for Japanese. http://www.mhlw.go.jp/toukei/saikin/hw/life/22th/dl/22th_04.pdf. Accessed 31 Mar 2018
Hayashi Y (2007) Economical viewpoint of treatment of osteoporosis. Nihon Rinsho 65:609–614 In Japanese
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Boonen S, Adachi JD, Man Z, Cummings SR, Lippuner K, Törring O, Gallagher JC, Farrerons J, Wang A, Franchimont N, San Martin J, Grauer A, McClung M (2011) Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 96:1727–1736
McClung MR, Boonen S, Torring O et al (2012) Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 27:211–−218
Kishimoto H, Maehara M (2015) Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch Osteoporos 10:231
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26:2401–2411
Hadji P, Papaioannou N, Gielen E, Feudjo Tepie M, Zhang E, Frieling I, Geusens P, Makras P, Resch H, Möller G, Kalouche-Khalil L, Fahrleitner-Pammer A (2015) Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int 26:2479–2−89
National Health Insurance Drug List (2018) Jihou Press, Tokyo. In Japanese
Kondo A, Zierler BK, Isokawa Y, Hagino H, Ito Y (2009) Comparison of outcomes and costs after hip fracture surgery in three hospitals that have different care systems in Japan. Health Policy 91:204–210
Konno S, Togawa D, Kamae I, Inoue Y, Kikuchi S (2009) Burden of illness of conservative medical management to vertebral compression fracture in Japanese osteoporosis patients. Orthop Surg 60:1022–1039 In Japanese
Reward for Nursing Care (2015) Igaku-tsushin-sha, Tokyo, Japan. In Japanese
Nawata S, Yamada Y, Ikeda S, Ikegami N (2000) EuroQol study of the elderly general population: relationship with IADL and other attributes. Iryo to shakai 10:76–86 In Japanese
Imai H, Fujii Y, Fukuda Y, Nakao H, Yahata Y (2008) Health-related quality of life and beneficiaries of long-term care insurance in Japan. Health Policy 85:349–355
Mori T, Crandall CJ, Ganz DA (2017) Cost-effectiveness of denosumab versus oral alendronate for elderly osteoporotic women in Japan. Osteoporos Int 28:1733–1744
Medical Fee Schedule (2016) Igaku-tsushin-sha, Tokyo, Japan. In Japanese
University of Sheffield (2018) FRAX WHO Fracture Risk Assessment Tool. Japan. https://www.sheffield.ac.uk/FRAX/tool.aspx?country=3. Accessed 6 Aug 2018. In Japanese
Japan Ministry of Health, Labour and Welfare (2018) Average value of height / weight. https://www.mhlw.go.jp/toukei/youran/indexyk_2_1.html. Accessed 6 Aug 2018. In Japanese
Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K (2010) International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ 19:422–437
Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D (2013) Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy 11:485–497
Karnon J, Shafie AS, Orji N, Usman SK (2016) What are we paying for? A cost-effectiveness analysis of patented denosumab and generic alendronate for postmenopausal osteoporotic women in Australia. Cost Eff Resour Alloc 14(11)
Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guanabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC (2017) Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone 105:11–17
Hiligsmann M, Reginster JY (2011) Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium. PharmacoEconomics 29:895–911
Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ (2007) Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 82:1493–1501
Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J, Kendler DL (2012) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23:317–326
Adler RA, El-Hajj Fuleihan G, Bauer DC et al (2016) Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 31:16–−35
Teo BJ, Koh JS, Goh SK, Png MA, Chua DT, Howe TS (2014) Post-operative outcomes of atypical femoral subtrochanteric fracture in patients on bisphosphonate therapy. Bone Joint J 96-b:658–−664
Japan Ministry of Health, Labour and Welfare (2018) the Central Social Insurance Medical Council, cost effectiveness assessment expert group meeting. https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000179573.pdf Accessed 31 Mar 2018. In Japanese
Acknowledgments
We would like to express our greatest appreciation to Dr. Shu-Ling Hoshi, from the Department of Health Care Policy and Health Economics, Faculty of Medicine, University of Tsukuba, for her useful discussion and idea regarding this work and to Rebecca Tollefson, DVM, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(DOC 87 kb)
Rights and permissions
About this article
Cite this article
Yoshizawa, T., Nishino, T., Okubo, I. et al. Cost-effectiveness analysis of drugs for osteoporosis treatment in elderly Japanese women at high risk of fragility fractures: comparison of denosumab and weekly alendronate. Arch Osteoporos 13, 94 (2018). https://doi.org/10.1007/s11657-018-0509-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-018-0509-6