Abstract
Background
In the US, 26 % of women aged ≥65 years, and over 50 % of women aged ≥85 years are affected with postmenopausal osteoporosis (PMO). Each year, the total direct health care costs are estimated to be $US12–18 billion.
Objective
The cost effectiveness of denosumab versus oral bisphosphonates in postmenopausal osteoporotic women from a US third-party payer perspective was evaluated.
Methods
A lifetime cohort Markov model was developed with seven health states: ‘well’, hip fracture, vertebral fracture, ‘other’ osteoporotic fracture, post-hip fracture, post-vertebral fracture, and dead. During each cycle, patients could have a fracture, remain healthy, remain in a post-fracture state or die. Relative fracture risk reductions, background fracture risks, mortality rates, treatment-specific persistence rate, utilities, and medical and drug costs were derived using published sources. Expected costs and quality-adjusted life years (QALYs) were estimated for generic alendronate, denosumab, branded risedronate, and branded ibandronate in the overall PMO population and high-risk subgroups: (a) ≥2 of the following risks: >70 years of age, bone mineral density (BMD) T score less than or equal to −3.0, and prevalent vertebral fracture; and (b) ≥75 years of age. Costs and QALYs were discounted at 3 % annually, and all costs were inflated to 2012 US dollars. Sensitivity analyses were conducted by varying parameters e.g., efficacies of interventions, costs, utilities, and the medication persistence ratio.
Results
In the overall PMO population, total lifetime costs for alendronate, denosumab, risedronate, and ibandronate were $US64,400, $US67,400, $US67,600 and $US69,200, respectively. Total QALYs were 8.2804, 8.3155, 8.2735 and 8.2691, respectively. The incremental cost-effectiveness ratio (ICER) for denosumab versus generic alendronate was $US85,100/QALY. Risedronate and ibandronate were dominated by denosumab. In the high-risk subgroup (a), total costs for alendronate, denosumab, risedronate and ibandronate were $US70,400, $US70,800, $US74,000 and $US76,900, respectively. Total QALYs were 7.2006, 7.2497, 7.1969 and 7.1841, respectively. Denosumab had an ICER of $US7,900/QALY versus generic alendronate and dominated all other strategies. Denosumab dominated all strategies in women aged ≥75 years. Base-case results between denosumab and generic alendronate were most sensitive to the relative risk of hip fracture for both drugs and the cost of denosumab.
Conclusion
In each PMO population examined, denosumab represented good value for money compared with branded bisphosphonates. Furthermore, denosumab was either cost effective or dominant compared with generic alendronate in the high-risk subgroups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
• Although denosumab is slightly more expensive than other branded oral osteoporotic treatments, it offers more benefits and results in lower lifetime costs.
• In elderly patients (>75 years of age) and those who are at higher risk of osteoporotic fracture, who have higher costs and mortality associated with fracture, denosumab offers good value for money.
• Denosumab is cost effective compared with the oral bisphosphonates because of the differences in fracture risk reduction and improved persistence due to the twice-yearly injection instead of daily oral medication with other treatments.
1 Introduction
Osteoporosis is a systemic skeletal disorder characterized by low bone mass and compromised bone strength, predisposing individuals to an increased risk of fracture [1]. Osteoporosis affects 200 million women worldwide: approximately 10 % of women aged 60 years, and two-thirds of women aged 90 years [2]. In the US, 26 % of women aged ≥65 years and over 50 % of women aged ≥85 years are affected with postmenopausal osteoporosis (PMO) [3]. It is estimated that the lifetime risk of osteoporotic fracture for Caucasian women over 50 years of age is 50 % [4].
In the US, over 1.5 million fractures per year are attributable to osteoporosis; these fractures result in 500,000 hospitalizations, 800,000 emergency room visits, 2.6 million physician visits, 180,000 nursing-home placements. The total direct health care costs are estimated to be $US12–18 billion each year [5]. In addition to cost, fractures also result in loss of function and have a negative impact on a patient’s psychological status [5]. There are many pharmacological treatments of osteoporosis, ranging from bisphosphonates, hormone replacement therapy, calcitonin, calcitriol, strontium ranelate, selective estrogen receptor modulator (SERM), and teriparatide. These treatments have been shown to be effective in reducing the risk of vertebral fracture or in reducing the risk of both vertebral and non-vertebral fractures [6]. However, the effectiveness of some of these treatments in clinical practice is limited because of poor compliance. Poor compliance could be due to the asymptomatic nature of the disease, adverse effects, frequent dosing schedules, and/or complex administration instructions for several agents [7]. About 70 % of the women who initiate osteoporotic treatment are non-adherent within 12 months and 47 % have discontinued the therapy within 1 year [8]. The compliance with weekly bisphosphonate was no better than that with osteoporosis medications requiring daily dosing [8].
Denosumab is a fully human monoclonal antibody that binds with affinity and specificity to RANK ligand, a key mediator of the formation, function, and survival of osteoclasts, the cells that are responsible for bone resorption. In the US, denosumab is indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy. Denosumab is administered by a healthcare professional once every 6 months as a subcutaneous injection. In an international, multicenter, randomized, double-blind, placebo-controlled study (Fracture REduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial [9]) of postmenopausal women with osteoporosis, denosumab significantly reduced the risks of new vertebral, non-vertebral, and hip fractures by 68, 20, and 40 %, respectively, versus placebo in 3 years. In a 2-year, randomized, crossover study comparing denosumab with a weekly bisphosphonate, it was shown that the 6-month dosing regimen of denosumab improved adherence, potentially leading to improved effectiveness [10].
With the introduction of generic bisphosphonates, there is a substantial decrease in price which could potentially impact prescribing patterns. Although there are no published studies reporting the cost effectiveness of denosumab versus other treatments in the US from a third-party payer perspective, several studies have shown denosumab to be cost effective compared with other osteoporosis treatments across Europe [11–14]. In the current study, a previously published economic model [11] for denosumab in postmenopausal osteoporotic women was used to assess the cost effectiveness of denosumab in the US compared with oral bisphosphonates, including generic alendronate, the most commonly used bisphosphonate. Additional analyses evaluated the cost effectiveness of denosumab in higher risk populations including the elderly, i.e. aged 75 years and older, as fracture is more common in this age group and these individuals are most vulnerable to the debilitating effects of fracture [15].
2 Methodology
A previously published Markov cohort model examined the cost effectiveness of denosumab compared with generic alendronate, risedronate and strontium ranelate in a Swedish population from a societal perspective [11]. The target population of the original model included postmenopausal osteoporotic women in Sweden, mean age 71 years, with a T score less than or equal to −2.5 and prevalent vertebral fracture in 34 % of patients.
Based on the previous model, the current study compares the cost effectiveness of denosumab with generic alendronate, branded risedronate, and branded ibandronate in the US from a third-party payer perspective. The target population of the current study in the base case was similar to patients in the FREEDOM trial, i.e. women with a mean age of 72 years and mean femoral neck T score of −2.16; 23 % of patients had a baseline vertebral fracture [16].
The Markov cycle length in the model was 6 months, and all patients were followed from treatment initiation to death or age 100 years. The model estimated total costs and quality-adjusted life years (QALYs) for each treatment strategy over a lifetime horizon. The cost effectiveness of denosumab versus the comparators was estimated as incremental cost-per-QALY gained. The model was built in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
2.1 Model Structure
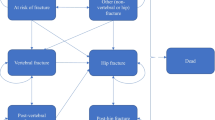
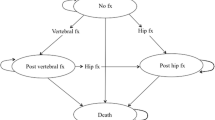
The structure of the cohort Markov state-transition model is depicted in Fig. 1. The model consists of seven health states: ‘well’, hip fracture, vertebral fracture, ‘other’ osteoporotic fracture, post-hip fracture, post-vertebral fracture, and dead. All patients in the cohort enter the model in the ‘well’ health state. During each Markov cycle (i.e. every 6 months) patients in the cohort have a probability of sustaining a fracture, remaining healthy or dying. Patients in the cohort who experience a fracture, depending on fracture type, may transition to the hip fracture, vertebral fracture or ‘other’ osteoporotic fracture health state. After 1 year in a given fracture state, the patients can (i) sustain a new fracture’ (ii) move to the post-fracture state (either post-hip or post-vertebral fracture, depending on the previous health state); (iii) move back to the ‘well’ state (‘other’ fracture patients only); or (iv) die.
Patients in the post-vertebral fracture state can either stay in this state, experience a new vertebral fracture, experience a new hip fracture or die. From the post-hip fracture state, it is only possible to remain in the post-hip fracture state, sustain another hip fracture or die.
2.2 Model Estimation
Once patients experienced a fracture, a fracture-specific cost and reduction in utility were allocated. Hip and vertebral fractures were assumed to have a direct impact on costs and quality of life during the first year after the fracture, and an additional cost and utility reduction every subsequent year following the event. Patients with ‘other’ osteoporotic fractures were assumed to only have an impact on costs and quality of life during the first year after the event. The model also takes into account the relative risk (RR) of mortality due to fracture and the risk of discontinuing treatment [11]. The model parameters and the base-case default values are described in more detail below.
2.2.1 Treatment Efficacy
The efficacy data for denosumab was derived from the FREEDOM trial [9]. The anti-fracture efficacies for alendronate, ibandronate and risedronate were obtained from the meta-analyses conducted by the National Institute for Health and Care Excellence (NICE) (see Table 1) [17]. In the meta-analyses, the risk reported for non-vertebral fractures, which may include hip and other fractures, was used in the model for ‘other’ fractures. In the absence of evidence for fracture reduction for a particular treatment at a particular skeletal site, 0 % fracture risk reduction was assumed.
2.2.2 Persistence
The risk of dropping out within the first 3 years for the comparators was estimated for the default case using persistence data obtained from Weycker et al. [8]. The 3-year cumulative incidence of patients dropping out, i.e. with a prescription-fill gap of ≥90 days, was used to calculate the 6-month discontinuation rate using the following equation:
where cumulative incidence is the discontinuation rate at 3 years and t is the total time, i.e. 36 months. A weighted average of the 6-month discontinuation rate was calculated using daily and weekly dosage of bisphosphonates; the weights were the numbers of patients taking weekly versus daily bisphosphonates. The 6-month discontinuation rate for bisphosphonates was 23.6 %. In deterministic sensitivity analyses, this rate was varied by ±25 % of its base-case value (17.7–29.5 %), as a standard error or confidence interval was not available.
The persistence rate for denosumab was based on the Denosumab Adherence Preference Satisfaction (DAPS) study [10], where patients on denosumab were found to be 50 % less likely to discontinue treatment at month 12 (p-value 0.029) compared with patients on alendronate. The expected time-specific non-persistence with denosumab was estimated by multiplying the discontinuation rate for bisphosphonates by 0.50 (95 % CI 0.34–0.74, normal distribution) the ratio of non-persistence rate for denosumab and alendronate, referred to as DAPS ratio. In the model, the 6-month discontinuation rate for denosumab was 11.8 %.
2.2.3 Treatment Duration and Offset Time
Treatment duration for all comparators was assumed to be 5 years. Anti-fracture efficacy is likely to persist for a period of time (offset time) after treatment is stopped. This could affect the number of sustained fractures and mortality, and, consequently, costs and quality of life. In the absence of more conclusive evidence for differential offset time, the model assumed that all treatments have an equal offset time of 2 years [18]. The treatment effect declined linearly over the specified offset time. It was assumed that patients on denosumab were persistent for at least 6 months while patients on oral bisphosphonates discontinue treatment earlier. In the model, patients on oral bisphosphonates who drop out lost half of the treatment effect in the last cycle, whereas patients on denosumab received the full effect. This adjustment was done to reflect that patients who discontinue treatment on average will do so in the middle of the cycle (i.e. after 3 months). Patients who dropped out in the first cycle did not receive any offset time.
2.2.4 Incidence of Fractures
The incidence of hip and ‘other’ osteoporotic fractures were derived from the Rochester Epidemiology Project [19]. The incidences of fracture by age group reported by Melton et al. are shown in Table 2. Melton et al. [20] also estimated the prevalence of vertebral fractured identified morphometrically. The clinically diagnosed vertebral fracture was derived from another population-based study using residents from Rochester County in Minnesota (see Table 2). [21] For age-specific incidence, the data were linearly extrapolated and interpolated.
In this model, the risk of sustaining a fracture in the model depends on three elements: (i) risk of fracture in the general population; (ii) increased fracture risk associated with osteoporosis (the RR); and (iii) risk reduction, if any, resulting from a treatment. Thus, the risk of experiencing a fracture in the model was calculated as:
2.2.5 Mortality
The age-specific baseline mortality in the US normal population for women was derived from http://www.mortality.org [22]. Because there was a lack of US-specific data relating to mortality due to hip, clinical vertebral and ‘other’ fractures, the default values were derived from Swedish data used in a previously published cost-effectiveness model for denosumab (Table 3) [11].
Some studies suggest that patients with osteoporosis have a greater morbidity than the general population, and that excess mortality after fracture is not solely due to the fracture event [23–27]. Given this evidence, the model assumed that 30 % of the excess mortality (compared with normal mortality) after hip, vertebral and other fractures was associated with the fracture event.
Although the literature is varied on the duration of this increased mortality after fracture, it was assumed to be 8 years based on two studies by Kanis et al. [23, 27]. This is likely a conservative assumption because, according to a recent meta-analysis, excess mortality after fracture did not return to normal age and sex-matched mortality rates even 10 years after the fracture [28].
2.2.6 Utility
Peasgood et al. [29] conducted a literature search for health-state utility values in men and women with established osteoporosis, vertebral fracture, hip fracture, wrist fracture, or shoulder fracture, across all countries. The EQ-5D data from multiple studies were pooled to estimate the impact of hip fracture on quality of life, in the first and subsequent years, as well as the impact of vertebral fracture in the first year [29]. The utility multiplier during the second and following years for a clinical vertebral fracture was derived from Borgstrom et al. [30]. Borgstorm et al. assumed a utility multiplier of 0.93 for clinical vertebral fracture in the second and following years based on a case-control study of patients enrolled in the Multiple Outcomes of Raloxifene trial [31], as well as other studies indicating a reduction in utility after the first year of vertebral fracture [32–34]. The disutility associated with ‘other’ fractures in the first year was derived from another study by Borgstrom et al. [35]. Due to lack of data in ‘other’ osteoporotic fractures, the assumptions used by Borgstrom et al. (a previously published cost-effectiveness model in strontium ranelate for osteoporosis) to estimate the disutility from other fractures was used in the model. The model assumed that the ‘other’ fractures did not have an impact on patients’ quality of life in the second and subsequent years. The fracture-specific utility multipliers, as shown in Table 4, were used, together with the baseline utility values for healthy US women, using EQ-5D [36].
2.2.7 Resource Use and Costs
The model included costs associated with the drug intervention, costs of treating fractures, drug administration and monitoring costs and long-term care costs (see Table 5). Treatment costs, including administration and monitoring, were applied while patients received the medication, and were zero if treatment was discontinued. Age-specific fracture costs by fracture site were derived from an analyses of commercial and Medicare members using Marketscan database [37]. When patients suffered specific recurrent events (e.g., second hip or vertebral fracture), the impact on costs were equal to that of the first event.
Costs associated with long-term care were considered in the model because many people with a hip fracture are discharged to a long-term care facility. Age-specific discharge rates to long-term care institutions such as nursing homes following hip fracture were obtained from the 2006 National Hospital Discharge Survey data based on the proportion of women aged 50 years and older with a first-listed diagnosis of hip fracture (International Classification of Diseases, Ninth Revision (ICD-9)-808 and 820.0) who were discharged to a long-term care institution [38]. A study by Bentler et al. [39] showed that only 51 % of these patients discharged to a nursing home remained there after 90 days. Therefore, long-term nursing-home costs were applied only to 51 % of those who were discharged to a nursing home. The model did not apply the additional long-term nursing-home costs to the remainder of women who had a hip fracture (49 %); it was assumed that nursing-home costs were accounted for in the direct medical costs of fracture. It was assumed that women who went to long-term care institutions remained there for the rest of their lives. Age-specific discharge rates were included in the probabilistic sensitivity analyses, assuming a standard error equal to 5 % of the base-case value since a standard error was not available. Patients with vertebral and ‘other’ fractures were assumed not to be associated with any long-term costs. All costs were inflated to 2012 $US using standard consumer price indices.
2.2.8 Side Effects
In the model, the risk of gastrointestinal effects with all three bisphosphonates and cellulitis with denosumab was considered. The assumptions relating to gastrointestinal effects were chosen to be similar to those used by NICE [40]. It was estimated that patients treated with alendronate or risedronate required 0.041 extra general practitioner (GP) consultations during the first cycle (6 months) and 0.021 GP consultations during the following cycles on treatment, as well as a proton-pump inhibitor (PPI) for each visit. The rate of skin infections, including cellulitis, was reported more frequently with denosumab in the FREEDOM trial, i.e. 0.0031 annually [9], therefore it was included in the analysis. The cost of treating cellulitis was estimated using the length of inpatient stay reported in the FREEDOM trial [9] (7.8 days) and the per day inpatient cost of treating cellulitis from the Healthcare Costs and Utilization Project (HCUP) Database [16, 41, 42].
In the absence of utility multipliers for cellulitis, the mean utility value for an active foot ulcer reported by Redekop et al. [43] was used in the model. Redekop et al. interviewed the general public (17–70 years of age) from The Netherlands using the time-trade-off method. For patients with gastrointestinal events, Stevenson and Davis [40] assumed that women with bisphosphonate-related adverse events had 91 % of the utility of women who did not have such side effects. Therefore, in the model, utility multiplier of 0.910 was applied to patients with gastrointestinal side effects.
2.3 Analyses
2.3.1 Base-Case Analysis
The model was used to estimate the number of QALYs (calculated by summing the product of utility weights associated with each health state with the time spent in the health state) and life years (LYs) [calculated by summing time spent in the non-death health states] over a lifetime horizon. The model also estimated the 10-year incidence of all fracture types. Total costs were estimated as the sum of the costs for the treatment intervention (both drug costs and osteoporosis management costs), direct costs of fracture and the long-term care costs of a nursing home over the lifetime horizon for each treatment strategy. Incremental cost-effectiveness ratios (ICERs) were estimated as the cost per QALY gained and cost per LY saved. Costs and health outcomes were discounted at 3 % annually.
As is customary in cost-effectiveness analysis with three or more comparators, strategies with higher cost and lower effectiveness (QALYs or LYs) than another strategy were excluded before calculating ICERs, as these strategies are considered to be dominated. Remaining strategies were then ranked in increasing order of cost and effectiveness, and ICERs were calculated sequentially, comparing each pair of successively more effective and expensive strategies. Strategies with higher ICERs than a more effective strategy were excluded, and ICERs for the remaining (undominated) strategies were calculated.
2.3.2 Sensitivity Analyses
Deterministic sensitivity analyses were performed to assess the robustness of the results to changes in key parameters, including all costs (fracture costs in the first and subsequent years, drug costs, and unit costs for a nursing-home day, bone mineral density (BMD) measurement, physician visit, hospital day for cellulitis, PPI for gastrointestinal event and nurse visit), all utility multipliers, RRs of fracture for denosumab and the comparator drug, the DAPS ratio, and the dropout rate for the comparator drug. Parameters were varied using published confidence intervals or standard errors, where available, and by 25 % above and below their base-case values where not available. Published confidence intervals or standard errors were not available for subsequent-year fracture costs for hip and vertebral fractures, drug costs, nursing-home day costs, PPI for gastrointestinal event costs, utility multiplier for subsequent-year vertebral fractures, utility multiplier for other fractures, and the dropout rate for the comparator drug. While holding the other parameters fixed, the model was re-run.
To assess uncertainty in the cost-effectiveness analysis, a probabilistic sensitivity analysis (PSA) was performed. The PSA was performed by simultaneously drawing from appropriate distribution functions for each model parameter according to their means and standard errors. This process of drawing parameters and running the model was repeated 1,000 times and the results are presented graphically. In the PSA, efficacy of denosumab and the comparators, costs of fractures (in the first and subsequent years), utilities (for fractures only), the DAPS ratio, and proportion of patients going to long-term care after hip fracture were included. Parameters were varied using published confidence intervals or standard errors, where available. Published confidence intervals or standard errors were not available for subsequent-year fracture costs for hip and vertebral fractures, so a range of ±10 % of the base case was used. Published confidence intervals or standard errors were also unavailable for the utility multipliers for subsequent-year vertebral fractures and other fractures (±25 % was used), as well as the proportion of patients going to long-term care (5 % of the mean was used as a standard error).
In addition, because patients at high risk for fracture are of particular clinical interest, two subgroup analyses were conducted. The first subgroup was comprised of postmenopausal women with at least two of the following risk factors: (i) >70 years of age; (ii) BMD T score less than or equal to −3.0; and (iii) prevalent vertebral fracture. The second subgroup was comprised of postmenopausal women over 75 years of age. The patient characteristics for these two subgroups are listed in Table 6.
3 Results
3.1 Base-Case Results
Results of the multiway cost-effectiveness analysis suggest that generic alendronate has the lowest costs, followed by denosumab (Table 7). Compared with alendronate, the lifetime costs associated with denosumab are approximately $US3,000 higher per patient. However, patients on denosumab have 0.04 additional QALYs per patient. The ICER for denosumab relative to alendronate is $US85,100 per QALY gained. Denosumab dominates both branded risedronate and branded ibandronate by having lower costs and better outcomes (i.e. higher QALYs).
Compared with oral bisphosphonates, patients on denosumab have lower 10-year risks of vertebral and hip fractures (Table 8). Figure 2 displays the disaggregated costs. Across all four treatment strategies, nursing-home care constitutes the majority of disease costs.
3.2 Sensitivity Analyses
3.2.1 Deterministic Sensitivity Analyses
Figure 3 illustrates only the top ten parameters that are most sensitive to model results. The ICER for denosumab versus generic alendronate is most sensitive to changes in the RR of hip fracture with denosumab. When this risk is varied from 0.37 to 0.97, the ICER for denosumab relative to alendronate ranges from $US10,000 per QALY gained to as high as $US487,000 per QALY gained. Other influential parameters include the RR of hip fracture on generic alendronate and drug cost of denosumab (Fig. 3). Parameters such as the drug cost of generic alendronate, the RRs of other osteoporotic fractures, and the DAPS ratio are not illustrated in Fig. 3 because the cost-effectiveness results did not vary substantially from the base case.
In a scenario analysis, instead of constant persistence rate, the dropout rate reported by Weycker et al. [8] at every 6 months over a 36-month follow-up period was used for the bisphosphonates. Results were consistent with the base case; denosumab had an ICER of $US84,200 per QALY gained compared with alendronate.
3.2.2 Probabilistic Sensitivity Analyses
The probability of denosumab being cost effective compared with the bisphosphonates, including generic alendronate, at a threshold of $US100,000 per QALY is 49.4 % (Fig. 4).
3.2.3 Subgroup Analyses
In the subgroup analysis, higher risk patients (as defined by at least two out of three risk factors) on generic alendronate once again have the lowest cost compared with others, followed by denosumab. The ICER for denosumab versus alendronate is $US7,900. Denosumab dominates both branded risedronate and branded ibandronate (see Table 9). In ≥75-year-old patients, denosumab has the lowest cost of all comparators and dominates all other treatment strategies—generic alendronate, branded risedronate and branded ibandronate (see Table 10).
4 Discussion
In this study, the cost effectiveness of denosumab compared with oral bisphosphonates (generic alendronate, branded ibandronate, and branded risedronate) was evaluated. In women with a mean age of 72 years and a mean T score of −2.16, the ICER for denosumab versus generic alendronate was $US85,100 per QALY gained. Denosumab dominated both risedronate and ibandronate. Compared with all other treatments, the probability of denosumab being cost effective at a threshold of $US100,000 per QALY is 49.4 %. The results are most sensitive to the RR of hip fracture with denosumab, the RR of hip fracture with generic alendronate, and the cost of denosumab. In women with a higher risk of fracture, the ICER for denosumab versus generic alendronate is $US7,900, and denosumab dominates branded risedronate and branded ibandronate. In women over 75 years of age, denosumab dominates all oral bisphosphonates.
Jonsson et al. [11], using a similar Markov model in their study, evaluated the cost effectiveness of denosumab versus oral bisphosphonates in Sweden. Compared with generic alendronate, patients on denosumab had 0.04 more QALYs, similar to the current study (0.04); denosumab was cost effective compared with alendronate.
Although several other European studies have reviewed the cost effectiveness of denosumab, the results from these studies cannot be compared with the present study because of differences in treatment comparators, patient population, and study perspective. Hiligsmann and Reginster [12] compared denosumab with no treatment using the base-case population from the FREEDOM trial [9] from the Belgian health care perspective. The authors found that denosumab was cost effective and produced an additional 0.03 QALYs per patient compared with no treatment. In another study, Hiligsmann and Reginster [13], using data from the FREEDOM trial [9], compared alendronate (both branded and generic) and risedronate with denosumab from the Belgian health care perspective. Although, in the study, the authors conducted scenario analyses with different age groups, the study population was not comparable with the present study—women from FREEDOM with a BMD T score less than or equal to −2.5, and women from FREEDOM with a prevalent vertebral fracture. Compared with alendronate, patients on denosumab were found to gain 0.02 and 0.03 QALYs per patient in these two subgroups, respectively. Scotland et al. [14] evaluated the cost effectiveness of denosumab from the UK health and social care perspective. The patient population in the study included patients from FREEDOM [9] who were over 70 years of age and with a BMD T score less than or equal to −2.5, both with and without a prior fracture. Denosumab dominated ibandronate in both of these subgroups. Denosumab was also compared with no treatment, strontium ranelate, raloxifene, zoledronic acid, and teriparatide, which were not included in our study of oral bisphosphonates.
The results from the present study should be interpreted in light of the numerous assumptions that were made in terms of the model structure and model inputs. First, the cohort Markov model assumes a hierarchical structure; patients from the post-hip fracture state can either remain in the post-hip fracture state or sustain another hip fracture or die. They cannot experience a vertebral fracture or an ‘other’ osteoporotic fracture. Patients with vertebral fractures can only incur new vertebral fractures, hip fractures, or die. Therefore, the number of milder fractures in the cohort is likely to be slightly underestimated. Also, increased risk of subsequent fractures, after the initial model fracture, was not included in the model. This may underestimate the number of fractures, since it is likely that patients with a history of fracture have a higher risk of subsequent fracture.
Second, in the base case, the target population was similar to women in the FREEDOM trial and might not be applicable to all osteoporotic patients; However, this was addressed by examining the cost effectiveness of denosumab in other risk groups.
In the base case, all patients were assumed to be at risk of dropping out during the first 3 years. After that, patients remain on treatment until treatment is terminated at 5 years. This assumption was based on long-term studies indicating that dropout rates are highest shortly after the initiation of treatment, after which dropout rates plateau and remain stable for 5 or more years [44, 45].
In the absence of conclusive evidence on differential offset of bone remodelling, the model assumed that all treatments have an equal offset time of 2 years, i.e. antifracture effect continues for 2 years after treatment discontinuation. Therefore, in the base case, it was assumed that persistent population have some treatment effect for 7 years (5 years of full effect while on the drug and 2 years of a linearly declining effect after treatment discontinuation). Patients who dropout in the first cycle did not receive any offset time.
In the model, only persistence to treatment was considered, compliance was not evaluated. While compliance to bisphosphonates is known to be lower, excluding it from the analyses probably did not impact the results because denosumab is known to have better compliance compared with alendronate; therefore, results from this study are more conservative [10].
The model analyzes fracture RR reductions at different skeletal sites separately. In the NICE meta-analysis, hip fracture RR reductions may be reported separately and may be included in a composite non-vertebral fracture RR reduction value (used as ‘other’ osteoporotic fractures in our model). For products that have separate fracture RR reduction at the hip site, there may be added benefit when calculating costs offsets from reductions in fractures.
The current study assumed that the generic formulation of alendronate would have comparable efficacy and safety data as branded alendronate. However, in a recent study, Kanis et al. [46] suggest that there is some evidence that generic alendronate might be less well tolerated than the branded alendronate and that this might lead to poorer adherence resulting in poorer fracture outcomes which could potentially impact the cost-effectiveness results.
Lastly, in the absence of US-specific data, the base-case estimates are based on data from a Swedish study [11]. In order to account for excess mortality in women, the model assumes 30 % excess mortality after hip, vertebral and ‘other’ fractures, and the duration is assumed to be 8 years.
5 Conclusions
The results from this study suggest that denosumab is a cost-effective option compared with the existing oral bisphosphonates, for the treatment of postmenopausal osteoporotic women in the US with characteristics similar to those in the FREEDOM trial. With the generic bisphosphonates now available, patients might have a cheaper alternative such as alendronate. Using a threshold of $US100,000 per QALY, denosumab is a cost-effective option compared with generic alendronate. Reasons for denosumab being cost effective compared with the oral bisphosphonates include the differences in fracture risk reduction and improved persistence with denosumab. Hip and vertebral fractures are more common in elderly patients and are associated with higher economic costs, morbidity, and mortality [15]; therefore, the economic benefits of denosumab were even more pronounced in the high-risk groups, including those who were aged 75 years and older. In the high-risk populations, denosumab either dominates (lower costs and higher QALYs) or is cost effective compared with oral bisphosphonates, including generic alendronate. These data highlight the importance of selecting the most appropriate treatment for postmenopausal women at high risk for fracture.
References
National Institutes of Health (NIH) Consensus Development Panel. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95.
Kanis JA. WHO Technical Report 2007. University of Sheffield, UK.
Melton LJ III, Chrischilles EA, Cooper C, et al. Perspective: how many women have osteoporosis? J Bone Miner Res. 1992;7(9):1005–10.
US Department of Health and Human Services. Bone health and osteoporosis: a report of the Surgeon General. Rockville: US Department of Health and Human Services, Office of the Surgeon General; 2004.
Gass M, Dawson-Hughes B. Preventing osteoporosis-related fractures: an overview. Am J Med. 2006;119(4 Suppl 1):S3–11.
Delmas PD, Rizzoli R, Cooper C, Reginster JY. Treatment of patients with postmenopausal osteoporosis is worthwhile. The position of the International Osteoporosis Foundation. Osteoporos Int. 2005;16:1–5.
Osterberg L, Blaschke T. Adherence to medication. NEJM. 2005;353:487–97.
Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int. 2006;17:1645–52.
Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. NEJM. 2009;361(8):756–65.
Freemantle N, Satram-Hoang S, Tang E-T, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23(1):317–26.
Jonsson B, Strom O, Eisman JA, et al. Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2011;22:967–82.
Hiligsmann M, Reginster JY. Potential cost-effectiveness of denosumab for the treatment of postmenopausal osteoporotic women. Bone. 2010;47(1):34–40.
Hiligsmann M, Reginster JY. Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium. Pharmacoeconomics. 2011;29(10):895–911.
Scotland G, Waugh N, Royle P, McNamee P, Henderson R, Hollick R. Denosumab for the prevention of osteoporotic fractures in post-menopausal women: a NICE single technology appraisal. Pharmacoeconomics. 2011;29(11):951–61.
Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab. 2011;96(6):1727–36.
Denosumab (Prolia) Prescribing Information, June 2010I.
National Institute for Health and Clinical Excellence (UK). Systematic reviews of clinical effectiveness prepared for the guideline ‘Osteoporosis: assessment of fracture risk and the prevention of osteoporotic fractures in individuals at high risk’. National Collaborating Centre for Nursing and Supportive Care, September 2008. http://www.nice.org.uk/guidance/index.jsp?action=download&o=42362.
Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972–80.
Melton LJ III, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9(1):29–37.
Melton LJ III, Lane AW, Cooper C, et al. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3:113–9.
Cooper C, Atkinson EJ, O’Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7(2):221–7.
http://www.Mortality.org. US Life Tables 2007.
Kanis JA, Oden A, Johnell O, et al. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int. 2004;15(2):108–12.
Poor G, Atkinson EJ, O’Fallon WM, et al. Determinants of reduced survival following hip fractures in men. Clin Orthop. 1995;319:260–5.
Kanis J, et al. Excess mortality after vertebral fracture. Sheffield: WHO Collaborating Centre for Metabolic Bone Diseases; 2002.
Parker MJ, Anand JK. What is the true mortality of hip fractures? Public Health. 1991;105(6):443–6.
Kanis JA, Oden A, Johnell O, et al. The components of excess mortality after hip fracture. Bone. 2003;32(5):468–73.
Haentjens P, Magaziner J, Colon-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Int Med. 2010;152:380–90.
Peasgood T, Herrmann K, Kanis JA, et al. An updated systematic review of health state utility values for osteoporosis related conditions. Osteoporos Int. 2009;20(6):853–68.
Borgstrom F, Johnell O, Kanis JA, et al. Cost effectiveness of raloxifene in the treatment of osteoporosis in Sweden: an economic evaluation based on the MORE study. Pharmocoeconomics. 2004;22(17):1153–65.
Oleksik A, Lips P, Dawson A, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000;15(7):1384–92.
Tosteson AN, Gabriel SE, Grove MR, Moncur MM, Kneeland TS, Melton LJ 3rd. Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int. 2001;12(12):1042–9.
Silverman SL, Minshall ME, Shen W, Harper KD, Xie S. The relationship of health-related quality of life to prevalent and incident vertebral fractures in postmenopausal women with osteoporosis: results from the Multiple Outcomes of Raloxifene Evaluation Study. Arthritis Rheum. 2001;44(11):2611–9.
Hall SE, Criddle RA, Comito TL, Prince RL. A case-control study of quality of life and functional impairment in women with long-standing vertebral osteoporotic fracture. Osteoporos Int. 1999;9(6):508–15.
Borgstrom F, Jonsson B, Strom O, et al. An economic evaluation of strontium ranelate in the treatment of osteoporosis in a Swedish setting: based on the results of the SOTI and TROPOS trials. Osteoporos Int. 2006;17(12):781–93.
Hanmer J, Lawrence WF, Anderson JP, et al. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Mak. 2006;26(4):391–400.
Marketscan Database Analysis. Data on file-Amgen 2010.
National Hospital Discharge Survey (NHDS) Data 2006—National Center for Health Statistics: Centers for Disease Control and Prevention.
Bentler SE, Liu L, ObrizanM, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epi. 2009;170(10):1290–9.
Stevenson M, Davis S. Analyses of the cost-effectiveness of pooled alendronate and risedronate, compared with strontium ranelate, raloxifene, edifronate and teriparatide. 2006. http://www.nice.org.uk/page.aspx?0=370643. Accessed 12 May 2008.
Healthcare Costs & Utilization Project Database based on US Nationwide Inpatient Sample. http://hcupnet.ahrq.gov/.
Denosumab Clinical Study Report, Nov 2008.
Redekop WK, Stolk EA, Kok E, et al. Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes Metab. 2004;30(6):549–56.
Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed in a managed care population. Bone. 2006;38(6):922–8.
Solomon D, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch inter Med. 2005;165(20):2414–9.
Kanis JA, Reginster JY, Kaufman JM, et al. A reappraisal of generic bisphosphonates in osteoporosis. Osteoporos Int. 2012;23(1):213–21.
Boonen S, McClung M, Minisola S, et al. 2009. Effect of denosumab on the incidence of hip, new vertebral and nonvertebral fractures over 3 years among postmenopausal women with higher fracture risk: a subgroup analysis from the FREEDOM study. J Bone Miner Res 24(Suppl 1). http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=da35831c-de93-4607-8b12-b7fcaf42f45d. Accessed March 23 2012.
Inderjeeth CA, Foo ACH, Lai MMY, et al. Efficacy and safety of pharmacological agents in managing osteoporosis in the old old: review of the evidence. Bone. 2009;44(5):744–51.
McClung MR, Boonen S, Torring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal ostoeoporosis. J Bone Miner Res. 2011. doi:10.1002/jbmr.536.
McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344(5):333–40.
Meadows ES, Klein R, Rousculp MD, et al. Cost-effectiveness of preventative therapies for postmenopausal women with osteopenia. BMC Womens Health. 2007;7(6):1–9.
National Osteoporosis Foundation. Osteoporos Int. 1998; (Suppl 4)S7–S80.
Liu H, Michaud K, Nayak S, et al. The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Arch Intern Med. 2006;166:1209–17.
Physician’s Fee and Coding Guide 2012. MAG Mutual Healthcare Solutions, 2011.
Drug Topics Red Book. Montvale: Thomson Healthcare; 2010.
AnalySource 2011. WAC Pricing, November 1, 2011.
Acknowledgments
Funding for this study was provided by Amgen, who provided input and review in the design of the model and writing of this manuscript.
Conflicts of interest
Anju Parthan and Morgan Kruse are employees of OptumInsight, which received funding from Amgen to conduct the study. Douglas Taylor was an employee of OptumInsight at the time this work was conducted. Nicole Yurgin and Hema Viswanathan are employees of Amgen, Inc., and own stocks in Amgen, Inc. Nicole Yurgin also owns stocks in Eli Lilly and Company. At the time this study was conducted, Joice Huang was employed by Amgen, Inc. and owns Amgen stocks.
Author contributions
All authors contributed to the study design. Anju Parthan and Morgan Kruse conducted the study analyses; Anju Parthan, Morgan Kruse, Nicole Yurgin, Joice Huang and Hema Viswanathan contributed to writing of the manuscript. Anju Parthan is the guarantor of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parthan, A., Kruse, M., Yurgin, N. et al. Cost Effectiveness of Denosumab versus Oral Bisphosphonates for Postmenopausal Osteoporosis in the US. Appl Health Econ Health Policy 11, 485–497 (2013). https://doi.org/10.1007/s40258-013-0047-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-013-0047-8