Abstract
Summary
This study analyzed data on 87,224 osteoporotic patients with up to 18 years of computerized medical history. Patients with osteoporosis and type 2 diabetes had higher bone density yet more fractures than non-diabetic osteoporotic patients. Fracture incidence among the diabetic patients was associated with retinopathy and cardiovascular disease, but not with diabetes duration.
Purpose
Little is known about the association between type 2 diabetes mellitus (T2DM) and fragility fractures or the mechanism(s) involved. We examined fracture correlates among T2DM patients with osteoporosis.
Methods
We used electronic health records of an osteoporosis (OP) registry cross-linked with a diabetes registry of a large payer provider healthcare organization in Israel. A cross-sectional analysis compared osteoporosis patients with and without T2DM, and a longitudinal Cox proportional hazard regression was used to identify incident fracture correlates.
Results
As of December 2015 a total of 87,224 current OP patients were identified, of whom 15,700 (18%) had T2DM. The T2DM OP patients were characterized by older age (mean 74.6 vs. 69.5), more males (20.3 vs. 14.0%), and a higher rate of chronic comorbidities compared to OP without diabetes. All major OP fractures (hip, spine, humerus, and forearm) were significantly more prevalent among T2DM OP patients (44 vs. 32%), with an overall age-standardized ratio of 1.22 (95% CI 1.19 to 1.25) and 1.15 (95% CI 1.10 to 1.21) for females and males respectively. The average T-scores were higher (femur neck − 1.8 vs. − 1.9, total hip − 1.2 vs. − 1.6, and vertebrae − 1.3 vs. − 1.7) for the T2DM OP patients compared to the non-T2DM OP patients. Among women with coexisting T2DM and osteoporosis (n = 10,812), fracture incidence was significantly associated with retinopathy (HR = 1.24, 95% CI 1.05 to 1.47) and cardiovascular disease (HR = 1.22, 95% CI 1.10 to 1.36) after controlling for age, bone mineral density T-score, rheumatoid arthritis, glucocorticoids, alcohol, and smoking).
Conclusion
This large population-based study confirms the higher fracture risk of osteoporotic patients with T2DM, as compared to osteoporotic patients without T2DM, despite higher bone mineral density levels. The presence of micro- and macrovascular disease appears to increase this risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both diabetes (DM) and osteoporosis are increasing health problems worldwide. The reports in the literature suggest an association between diabetes and bone disease [1,2,3], but the mechanism(s) involved remains to be clarified. Osteoporosis, which is characterized by decreased bone strength, is diagnosed mainly by measuring low bone mineral density (BMD) detected by dual-energy X-ray absorptiometry (DXA). Some parameters related to bone strength, such as bone microarchitecture, cannot be detected in BMD. Patients with type 2 diabetes (T2DM) are usually characterized by low turnover osteoporosis, decreased osteoblastic activity, and decreased damage repair, features which cannot be detected by BMD, and thus BMD has limitations in predicting fracture risk in diabetics [4,5,6,7,8]. Increased microvascular damage in DM patients may affect bone quality. [9]. Another possible explanation may lie in a lower frequency of weight-bearing activity [10] which interferes with bone health [11]. The extra-skeletal risk of altered gait and falls may also be elevated in this population due to the complications of diabetes, such as vision disorders, nerve damage, or hypoglycemia [12]. In addition, several anti-diabetic therapeutic strategies have shown a deleterious effect on bone metabolism [13].
A systematic review of 15 T2DM studies worldwide reported an increased risk of hip fractures (relative risk [RR] = 1.7, 95% confidence interval [CI] 1.3 to 2.2), which persisted after controlling for age, physical activity and body mass index (BMI: RR = 2.6, 95% CI 1.5 to 4.5), and weaker non-significant risks for fractures at other sites [14]. An especially increased risk was reported for males, albeit based on a small population [14]. A recent review on prevalence and determinants of osteoporosis in T2DM patients [15] concluded that clinical studies on this population suffer from small sample sizes and heterogeneous methods, whereas epidemiological studies were mostly limited to self-reports without precise information on diabetes classification or control [16,17,18,19].
Little is known about the epidemiology of osteoporosis among diabetic patients in terms of incidence, prevalence, and distribution of fracture types. Describing the special characteristics and progress of osteoporosis in T2DM patients and assessing their risk heterogeneity could further improve the understanding of this disease and identify patients who would benefit most from therapy. The aims of the current investigation were to characterize osteoporosis patients with T2DM in comparison with osteoporosis patients without DM, and to explore possible diabetes-related determinants of fractures among osteoporotic patients in a large population with a longitudinal follow-up.
Methods
Study design and participants
All the data were obtained from the computerized database of Maccabi Healthcare Services (MHS), a large health maintenance organization (HMO) in Israel. According to the 1995 national health insurance law, HMOs may not deny coverage to applicants on any grounds, including age or state of health. MHS insures and provides health services to two million members. Its central database contains patients’ demographics, physician diagnoses, medical procedures, hospitalizations, and full capture of all prescription medication dispensations and laboratory tests since 1999. There is a 99% retention rate with loss to follow-up due mainly to mortality (with such data being available for research purposes). MHS has developed several computerized registries of major chronic diseases, such as ischemic heart disease, oncologic diseases, diabetes, and osteoporosis, in order to improve the quality of chronic care delivery to its members. The registries are continuously updated, and they identify patients via automatic search formulas, as opposed to being dependent upon active reporting by physicians.
The current study utilized the diabetes and osteoporosis registries. Their construction has been previously described elsewhere, and a comprehensive approach was used to cross-validate them and ensure high specificity [20,21,22]. Briefly, the osteoporosis registry identifies patients by diagnosis, by at least two dispensations of medications for osteoporosis, by BMD in the osteoporotic range (T-score ≤ − 2.5), or by a major osteoporotic fracture (MOF) which occurred at a typical age (50+ years for females and 60+ years for males) after excluding fractures due to motor vehicle accidents. MOF sites included the femur neck, clinical spine, forearm, and proximal humerus fractures, in accordance with fracture risk assessment definitions [19]. Registry entry date was defined as the earliest of all the above criteria. Patients with multiple myeloma or Paget’s disease were excluded. The DM registry identifies patients according to HbA1c values and glucose test results, DM therapy dispensations, and an active diagnosis of DM from relevant physicians, with an overall specificity of 99.99%.
Considering the different pathogenetic mechanisms of type 1 and type 2 diabetes, the current study was focused on type 2 diabetes as compared with no diabetes.
Primary objective: cross-sectional comparison
Our primary objective was to compare the profile of osteoporosis patients with and without T2DM in terms of osteoporosis-related risk factors and DM-related complications. The population included all active (alive and enrolled) MHS osteoporosis registry members on December 31, 2015. Patients who were identified as having T1DM [23] were excluded due to their different fracture risk and smaller population.
We utilized the MHS central database of medical records to extract data on demographics, smoking status, body mass index (BMI), comorbidities, and laboratory tests on December 31, 2015. The following osteoporosis-related and diabetes-related conditions were extracted from documented diagnoses according to ICD-9 codes (Appendix Table 4): rheumatoid arthritis, history of falls, cardiovascular disease, peripheral vascular disease, neuropathy, nephropathy, retinopathy, pancreatitis, and hypoglycemia. A history of hypoglycemia was assessed by either a coded diagnosis or any dispensations of glucagon hypo kits. In addition to recorded diagnoses, data on falls were also extracted from the MHS physiotherapy database: as of 2007, all MHS members who visited a physiotherapy clinic at the age of 65 years or more were queried about the occurrence and number of falls during the preceding year. These data were divided between “any falls” and “no falls” and compared among the osteoporosis patients with and without T2DM for whom the data were available (13,956, 16% of the study population).
Glycemic control was assessed by HbA1c. Missing HbA1c values were completed by glucose levels where available using an accepted conversion formula [24]. The most recent HbA1c/glucose test was used for the cross-sectional analysis, while the last one before the index date or within 90 days post-index date was used for the longitudinal analysis.
BMD T-scores were extracted from over 230,000 historical densitometry reports of tests performed in Assuta hospitals (a chain of seven country-wide medical centers, where approximately 86% of BMD scans of MHS members are performed). All scans were executed using the same standardized model of GE-lunar prodigy scanner. Scan reports are available as of June, 2006 to the present, excluding 2010. Numeric test results were extracted from historical PDF files using optical character recognition technology.
In addition, we extracted patients’ exposures to selected anti-diabetic medications with potential influence on bone metabolism (thiazolidinediones [25, 26], insulin, inhibitors of dipeptidyl peptidase 4, and glucagon-like peptide-1). The dispensation history for each patient at the index date was categorized into at least 12-month cumulative dispensations vs. less than 12 months.
Secondary objective: fracture correlates
Fracture correlates were obtained by means of a population-based retrospective cohort study. Follow-up began at the time of osteoporosis diagnosis (the index date) and continued until the first MOF incidence, disenrollment from MHS, death, or on December 31, 2015, whichever occurred first. The osteoporosis status was defined by the earliest recorded diagnosis, medication dispensation (bisphosphonates, raloxifene, strontium ranelate, denosumab, teriparatide, or calcitonin), or BMD in the osteoporotic range.
The population in the longitudinal analysis included all MHS females in the osteoporosis registry and in the DM registry, after excluding those who were younger than 50 years of age on the index date, those with cancer on the index date (according to the Israel National Cancer Registry), and those with a prior MOF on the index date. Males were not included in the longitudinal analysis due to gender disparities in fracture epidemiology and the substantially smaller population size of men in the MHS osteoporosis registry (15% males). Also excluded were patients who had been members of MHS less than 1 year before the index date in order to ensure the detection of a primary MOF.
The MHS DM registry entrance date served as a proxy for DM onset, to calculate duration of diabetes at index.
We retrieved the patients’ demographics and comorbidities at the index date. Comorbidities were extracted in accordance with the data sources and definitions used for the primary objective analysis. BMI and smoking became obligatory fields (requiring yearly updating) in 2013 and were therefore mostly available for the cross-sectional analysis but largely missing for the longitudinal analysis. A missing baseline BMI was completed with the first available BMI, based on the high stability of BMI during adulthood [27]. Missing smoking status was completed with the first documented smoking status, in accordance with previous investigations that reported low levels of smoking initiation among adults aged 35 years or more [28, 29]. Baseline BMD T-score levels (measured before or up to 1 year after the index date) were missing for 76% of the population. Consequently, T-score values were completed with the first available T-score post-index date (45% still missing at study closure).
Accurate daily alcohol units were not available; however, excess alcohol intake was detected by diagnoses of alcoholism and alcohol disorders as well as anamnesis of drinking habits.
Oral glucocorticoids exposure was computed according to dispenses of more than 3 months at a dose equivalent or higher than prednisolone 5 mg daily. Lastly, vitamin D level at index was added as a covariate in the longitudinal analysis, using the last level measured before or up to 60 days following the index date (missing for 76.1%, deficient for 43.3% of those with available data).
Statistical analysis
Primary objective
Descriptive statistics were generated with mean and standard deviation or percentage. Differences between osteoporosis patients with and without T2DM were analyzed using ANOVA or the Kruskal Wallis and chi-squared tests for continuous and categorical variables, respectively. Considering the large population size, p values were accompanied with standard mean difference (SMD), and a SMD > 0.1 was considered meaningful [30].
Age- and sex-standardized fracture rates were calculated with standardized morbidity ratio, which is a ratio of observed and expected cases. The observed cases are the total number of fracture cases among T2DM patients. The expected cases were derived by multiplying the fracture prevalence rates among the DM-free patients per each age category (in 5-year groups) by the number of T2DM patients in the corresponding age category and summing the expected cases of all age categories.
Secondary objective
The Cox proportional hazard model was used with age as a time scale. The multivariate model also included BMD T-score, BMI, smoking status, and the following baseline variables: rheumatoid arthritis, DM duration, HbA1c, and Vitamin D level, history of hypoglycemia events, pancreatitis, micro- and macrovascular DM complications, as well as prior use of thiazolidinediones (for at least 1 year). Exposure to inhibitors of dipeptidyl peptidase-4 and glucagon-like peptide-1was not included in the model due to the small number of users at index date (< 2%). The proportional hazard assumption was tested based on Schoenfeld residuals regressed on follow-up time, and it was met by all covariates (p > 0.05).
All statistical analyses were performed with R statistical programming language [31].
Role of the funding source
The funders (Merck & Co. Inc.) reviewed the study design and participated in the final interpretation of the results and in drafting the report. The funders had no role in the collection or analysis or initial interpretation of the data. The corresponding author had full access to all the data and the final responsibility to submit for publication.
Results
Primary objective: cross-sectional comparison
A total of 91,422 current osteoporosis patients were identified (Fig. 1a) of whom 71,846 (79%) were diabetes-free, 15,378 were identified as having T2DM, 821 as having T1DM, and 3377 whose DM status was undetermined. Among those having both T2DM and OP, DM preceded OP for 68.2% of the patients. A total of 63.8% of the patients were entered into osteoporosis registry based on T-score ≤ − 2.5 or MOF.
Diabetic osteoporosis patients were older than non-DM osteoporosis patients (mean current age 74.6 vs. 69.5 years, respectively) and more likely to be males (20.3 vs. 14.0%, respectively, p < 0.001) (Table 1). Despite their older age, the average T-scores for the T2DM patients were higher in all sites compared to the non-DM patients (femur neck − 1.8 vs. − 1.9, total hip − 1.2 vs. − 1.6, vertebrae −1.3 vs. − 1.7), with similar screening rates (80.4 vs. 81.7% underwent BMD evaluations). A total of 37% T2DM patients were within the osteoporotic range (T-score ≤ − 2.5) compared to 45% non-DM patients. Moreover, despite their older age, the T2DM patients were slightly less likely to be treated for osteoporosis compared with the non-DM patients (65.0 vs. 70.8%, respectively). In subjects who had vitamin D levels available (92%), vitamin D deficiency was significantly more prevalent among the T2DM patients (29.2 vs. 21.6%). History of falls, hypoglycemia, cardiovascular disease, retinopathy, neuropathy, nephropathy, and peripheral vascular disease were also more prevalent among the TD2M subgroup of OP patients. The prevalence of major osteoporotic fractures (hip, spine, humerus, and forearm) was significantly higher among the T2DM patients compared to the non-DM patients across all fracture subtypes, with an overall age-standardized ratio of 1.22 (95% CI 1.19 to 1.25) and 1.15 (95% CI 1.10 to 1.21) for females and males, respectively (Table 2).
Secondary objective: fracture correlates (Table 3)
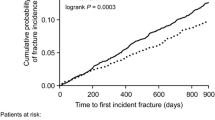
A total of 10,812 females met the inclusion criteria for this objective. They were identified as having both osteoporosis and T2DM, they were 50+ years of age at osteoporosis diagnosis, they had no prior fractures or cancer, and they were MHS members for at least 1 year (Fig. 1b). There was a total of 2300 MOFs (22% of the population) over a mean follow-up of 9 years. BMD was strongly associated with fracture risk in a gradual manner as was the presence of rheumatoid arthritis.
Fracture incidence among this population was associated with traditional risk factors such as bone density, glucocorticoid exposure (HR = 1.29, 95% CI 1.06 to 1.56), and rheumatoid arthritis (HR = 1.28, 95% CI 1.02 to 1.59). In addition, a significant association was observed with retinopathy (HR = 1.24, 95% CI 1.05 to 1.47) and cardiovascular disease (HR = 1.22, 95% CI 1.10 to 1.36) (Table 3). A non-significant increased risk was also observed in patients with baseline neuropathy (HR = 1.13, 95% CI 0.94 to 1.36) or pancreatitis (HR = 1.38, 95% CI 0.82 to 2.34). The diabetes duration was not a significant indicator of increased fracture incidence, nor was the baseline glycemic control, although some elevated risk was observed for patients with HbA1c > 7.5% compared with HbA1c < 6.5% (HR = 1.12, 95% CI 0.94 to 1.33), and history of hypoglycemia (HR = 1.10, 95% CI 0.95 to 1.27).
Long-term users of thiazolidinediones on the index date exhibited a non-significantly increased fracture risk (HR = 1.16, 95% CI 0.85 to 1.59). The use of insulin was not associated with fracture hazard in this subcohort. Similar results were obtained when the T-score was not adjusted for in the multivariable model (HR = 1.24, 95% CI 1.05 to 1.47 for retinopathy, HR = 1.24 95% CI 1.11 to 1.38 for cardiovascular disease, HR = 1.25 95% CI 1.00 to 1.56 for rheumatoid arthritis, and HR = 1.14 95% CI 0.96 to 1.36 for patients with HbA1c > 7.5% vs. HbA1c < 6.5%).
Discussion
In this large population-based study of patients with osteoporosis, T2DM patients comprised 18% of the osteoporosis population. Osteoporotic patients with diabetes exhibited substantially more comorbidities, with a 16% higher fracture prevalence compared with osteoporotic patients without diabetes, after adjusting for age and sex distribution. Despite their older age, the T2DM patients were characterized by higher BMD levels compared with the non-DM osteoporosis patients. The observed risk heterogeneity between osteoporosis patients with and without diabetes raises some concern regarding the applicability of pivotal osteoporosis trials for diabetic patients, where they were excluded or substantially under-represented [32, 33]. The impact of anti-osteoporosis treatment on DM patients with fractures and normal BMD levels has not been studied because such treatment was not indicated for these patients. Considering their different clinical profile in terms of older age and increased comorbidity, their different disease presentation (higher BMD), and the considerable proportion of osteoporosis patients who are also diabetic, the efficacy of current therapeutic intervention strategies among this special subgroup of patients warrants further elucidation [34].
The increased fracture prevalence observed among the T2DM patients in our cross-sectional analysis is similar to that reported by an earlier meta-analysis [35] and supports the findings of a large prospective study of osteoporosis fractures (SOF) [36]. An even higher excessive fracture risk for diabetics was observed in SOF, a disparity which may be explained by its prospective design or by its having been conducted in the 1980s. The findings of both the SOF [36] and the current study suggest a slightly higher risk for humerus and Colles’ fractures compared with other types of MOFs.
Our exploratory analysis aimed to identify diabetes-specific factors potentially associated with fracture risk. The findings of that analysis suggest that ophthalmic and cardiovascular complications are associated with increased fracture incidence among T2DM patients, consistent with previous findings on Swedish T1DM patients [37] as well as those of a meta-analysis on both T1DM and T2DM [35]. While BMD is largely criticized for its limited capture of bone quality, it is important to take into account these extra-skeletal risk factors for falls and fractures. Additional diabetes-related factors include glycemic control, diabetes duration, and long-term use of anti-diabetic medications. In the current study, after adjusting for diabetes complications, neither diabetes duration nor glycemic control played a key role in fracture occurrence, confirming the conclusions of a meta-analysis [35] as well as those of a small recent study on the lack of an association of these factors with BMD [38]. Missing data on baseline glycemic control (23%) usually signifies lower healthcare service utilization, typical for neglected patients with less frequent disease management, which may explain their elevated fracture risk. BMI was not associated with fracture risk, in accordance with a recent large population-based study [39].
Nationally representative risk estimates based on large-scale cohorts with extensive clinical data are usually difficult to obtain. The current study leveraged two validated, automated registries of diabetes and osteoporosis patients with a high retention rate, thus providing a good source for evaluating disease prevalence as well as disease correlates. However, several study limitations bear mention. The primary analysis utilized cross-sectional data of current osteoporosis patients. The surprisingly younger age at MOF occurrence may be explained by survival bias, or, alternatively, it may be due to the registry’s definition of MOFs as fractures occurring only after the age 50 years for females or 60 years for males. The study’s exploratory cohort analysis was adjusted to baseline covariates at osteoporosis onset and did not account for post-index date changes in clinical characteristics or exposures, which may vary during the course of follow-up and influence the risk of fracture (e.g., osteoporosis therapy, diabetes therapy, glycemic control, and kidney function). The average age at osteoporosis diagnosis was 63 years, whereas the average age at the first MOF occurrence was 70 years. In light of this long follow-up and the slow progression of osteoporosis, future research may benefit from more complex designs which incorporate time-dependent risk predictors. Non-severe hypoglycemia events may not be well documented and captured retrospectively. Lastly, data on physical activity habits were not available since they were not routinely coded in the electronic medical records.
In summary, this study describes the unique clinical characteristics of osteoporotic patients with coexisting T2DM and underscores their elevated risk for fractures, particularly for patients with macro- or microvascular complications. The substantially different risk profile of osteoporosis patients with coexisting diabetes calls for further clarification regarding the efficacy of current osteoporosis therapeutic intervention strategies among these patients.
References
Ishii S, Cauley JA, Crandall CJ, Srikanthan P, Greendale GA, Huang MH, Danielson ME, Karlamangla AS (2012) Diabetes and femoral neck strength: findings from the hip strength across the menopausal transition study. J Clin Endocrinol Metab 97:190–197
Kurra S, Siris E (2011) Diabetes and bone health: the relationship between diabetes and osteoporosis-associated fractures. Diabetes Metab Res Rev 27:430–435
Hamann C, Kirschner S, Gunther KP, Hofbauer LC (2012) Bone, sweet bone—osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol 8:297–305
Giangregorio LM, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA (2012) FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res 27:301–308
Jackuliak P, Payer J (2014) Osteoporosis, fractures, and diabetes. Int J Endocrinol 2014:820615
Tuominen JT, Impivaara O, Puukka P, Rönnemaa T (1999) Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care 22:1196–1200
Botella Martinez S, Varo Cenarruzabeitia N, Escalada San Martin J, Calleja Canelas A (2016) The diabetic paradox: bone mineral density and fracture in type 2 diabetes. Endocrinol Nutr 63:495–501
Goldshtein I, Gerber Y, Ish-Shalom S, Leshno M (2018) Fracture risk assessment with FRAX using real-world data in a population-based cohort from Israel. Am J Epidemiol 187:94–102
Vogt MT, Cauley JA, Kuller LH, Nevitt MC (1997) Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res 12:283–289
Sibai AM, Costanian C, Tohme R, Assaad S, Hwalla N (2013) Physical activity in adults with and without diabetes: from the ‘high-risk’ approach to the ‘population-based’ approach of prevention. BMC Public Health 13:1002
Chastin SF, Mandrichenko O, Helbostadt JL, Skelton DA (2014) Associations between objectively-measured sedentary behaviour and physical activity with bone mineral density in adults and older adults, the NHANES study. Bone 64:254–262
Lord SR, Dayhew J (2001) Visual risk factors for falls in older people. J Am Geriatr Soc 49:508–515
Palermo A, D’Onofrio L, Eastell R, Schwartz AV, Pozzilli P, Napoli N (2015) Oral anti-diabetic drugs and fracture risk, cut to the bone: safe or dangerous? A narrative review. Osteoporos Int 26:2073–2089
Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166:495–505
Leidig-Bruckner G, Grobholz S, Bruckner T, Scheidt-Nave C, Nawroth P, Schneider JG (2014) Prevalence and determinants of osteoporosis in patients with type 1 and type 2 diabetes mellitus. BMC Endocr Disord 14:33
Hofbauer LC, Brueck CC, Singh SK, Dobnig H (2007) Osteoporosis in patients with diabetes mellitus. J Bone Miner Res 22:1317–1328
Leidig-Bruckner G, Ziegler R (2001) Diabetes mellitus a risk for osteoporosis? Exp Clin Endocrinol Diabetes 109(Suppl 2):S493–S514
de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA (2005) Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam study. Osteoporos Int 16:1713–1720
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
Chodick G, Heymann AD, Shalev V, Kookia E (2003) The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol 18:1143–1146
Shalev V, Chodick G, Goren I, Silber H, Kokia E, Heymann AD (2011) The use of an automated patient registry to manage and monitor cardiovascular conditions and related outcomes in a large health organization. Int J Cardiol 152:345–349
Goldshtein I, Chandler J, Shalev V et al (2015) Osteoporosis in the community: findings from a novel computerized registry in a large health organization in Israel. J Aging Res Clin Practice 4:59–65
Sella T, Shoshan A, Goren I, Shalev V, Blumenfeld O, Laron Z, Chodick G (2011) A retrospective study of the incidence of diagnosed type 1 diabetes among children and adolescents in a large health organization in Israel, 2000–2008. Diabet Med 28:48–53
Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ (2008) Translating the A1C assay into estimated average glucose values. Diabetes Care 31:1473–1478
Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G, for the A Diabetes Outcome Progression Trial (ADOPT) Study Group (2008) Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care 31:845–851
Aubert RE, Herrera V, Chen W, Haffner SM, Pendergrass M (2010) Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes Obes Metab 12:716–721
Heo M, Faith MS, Pietrobelli A (2002) Resistance to change of adulthood body mass index. Int J Obes Relat Metab Disord 26:1404–1405
Health UDo, Services H (2012) Preventing tobacco use among youth and young adults: a report of the surgeon general. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA, p 3
Edwards R, Carter K, Peace J, Blakely T (2013) An examination of smoking initiation rates by age: results from a large longitudinal study in New Zealand. Aust N Z J Public Health 37:516–519
Cohen J (1977) Statistical power analysis for the behavioral sciences, chapter 2: the t test for means. Academic Press, Inc., Toronto
Team RC (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. 2013
Keegan TH, Schwartz AV, Bauer DC, Sellmeyer DE, Kelsey JL (2004) Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care 27:1547–1553
Johnell O, Kanis JA, Black DM, Balogh A, Poor G, Sarkar S, Zhou C, Pavo I (2004) Associations between baseline risk factors and vertebral fracture risk in the Multiple Outcomes of Raloxifene Evaluation (MORE) study. J Bone Miner Res 19:764–772
Dede AD, Tournis S, Dontas I, Trovas G (2014) Type 2 diabetes mellitus and fracture risk. Metabolism 63:1480–1490
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int 18:427–444
Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR, Study of Osteoporotic Features Research Group (2001) Older women with diabetes have an increased risk of fracture: a prospective study. The Journal of Clinical Endocrinology & Metabolism 86:32–38
Miao J, Brismar K, Nyren O, Ugarph-Morawski A, Ye W (2005) Elevated hip fracture risk in type 1 diabetic patients: a population-based cohort study in Sweden. Diabetes Care 28:2850–2855
Shanbhogue VV, Hansen S, Frost M, Jørgensen NR, Hermann AP, Henriksen JE, Brixen K (2016) Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol 174:115–124
Leslie WD, Morin SN, Majumdar SR, Lix LM (2018) Effects of obesity and diabetes on rate of bone density loss. Osteoporos Int 29:61–67
Acknowledgements
We are grateful for the assistance of Mrs. Racheli Katz and Cheli Meltzer Cohen in data extraction and Mrs. Esther Eshkol in language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Sofia Ish-Shalom has received research grants and consulting, advisory board, lecture fees, and any combination of the three from Merck Sharp & Dohme, Eli Lilly, Enterabio, GlaxoSmithKline, and Novartis. Anne E. dePapp and Allison Martin Nguyen are employees of Merck & Co., Inc. Julie Chandler was an employee of Merck & Co. at the time of this analysis and manuscript preparation. Inbal Goldshtein, Varda Shalev, and Gabriel Chodick declare that they have no conflict of interest.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Goldshtein, I., Nguyen, A.M., dePapp, A.E. et al. Epidemiology and correlates of osteoporotic fractures among type 2 diabetic patients. Arch Osteoporos 13, 15 (2018). https://doi.org/10.1007/s11657-018-0432-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-018-0432-x