Abstract
This study describes a plantlet regeneration protocol of somatic embryos in Rosa ‘John F. Kennedy’ (hybrid tea rose). Different somatic embryo sizes exhibited significant differences in the single bud (SB type) regeneration rate and multiple bud (MB type) regeneration rate. The highest single bud (SB type) regeneration rate (27.10%) was obtained from the large size (4 mm × 5 mm). The multiple bud regeneration rate was highest at 39.60% for the medium size (3 mm × 4 mm). Changes in the endogenous hormone content and ratios of various types of embryogenic cultures were clearly diverse: higher contents of abscisic acid (ABA) and indole-3-acetic acid (IAA) occurred in the SPC explant (single-piece cotyledonary somatic embryo) with a regenerated single bud (SB type). In a MW-type somatic embryo (milky-white single-piece-cotyledon explant), the gibberellic acid (GA3)/ABA ratio was the highest (1.807), and the IAA/GA3 ratio was the lowest (0.902). However, the highest ratios of IAA/GA3 (6.159) and the lowest ratios of GA3/ABA (0.383) appeared in SB-type cultures. Additionally, the highest IAA/ABA ratios (6.535) and higher ratios of GA3/ABA (1.729) were found in MB-type cultures. This indicated that ways to regulate plant cell totipotency in Rosa ‘John F. Kennedy’ somatic embryos differed between single bud (SB type) regeneration and multiple bud (MB type) regeneration. Finally, this study classified and summarized common intermediate materials in in vitro culture based on morphological characteristics and plantlet regeneration pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rose (Rosa hybrida L.) is a plant in the Rosaceae family with a long history of cultivation. Modern rose hybridization and selection began in Europe in the early sixteenth century, and there are now nearly 20,000 commercial varieties worldwide, making it one of the most popular ornamental plants (Vergne et al. 2010; Qi et al. 2018; Khatami et al. 2020). Because of their rich flower color, repeat flowering ability, diverse varieties, and strong adaptability, the modern rose is widely used as cut flowers, potted plants, and landscaping, and some of them are extremely important in the edible, medicinal, essential oil extraction, and cosmetic industries (Nguyen and Van 2020). As a result, the modern rose is one of the world’s most important commercial flower crops.
Despite the fact that modern roses are diverse and have a wide range of flower colors, there are no blue-purple varieties due to a lack of delphinidin-based anthocyanins, which are typically the main component of violet and blue flowers (Katsumoto et al. 2007). Improving floral color traits is one of the main goals of modern rose breeding programs (Bao et al. 2012), but conventional breeding to generate new varieties is a long and arduous task, as the genetic improvement of modern roses can be limited by a high degree of genetic heterozygosity, lack of blue flower allelic variation, and interspecific discordance or different ploidy (Liu et al. 2021). Molecular breeding technology has the ability to cross species boundaries and modify plant genetic material, providing a technical approach for targeted plant breeding. Using molecular breeding techniques, recipient plants can produce a wider range of floral colors by utilizing exogenous control pathways of floral color genes (Tanaka et al. 2010).
In recent years, molecular breeding techniques have been widely used to improve rose varieties, which have enabled significant progress in flower color. Katsumoto et al. (2007) used rose embryogenic calluses as explants and downregulated the endogenous dihydroflavonol 4-reductase (DFR) gene in rose while overexpressing the viola F3′5'H gene and the Iris × hollandica DFR gene in transgenic rose petals to accumulate delphinidin and produce blue-purple flowers. Nakamura et al. (2015) used an Agrobacterium-mediated method to express the pansy F3′5'H gene and the torenia A3′5′OMT gene in embryogenic callus, and the regenerated transgenic plants accumulated methylated anthocyanins and turned purple-red in the petals. Therefore, using molecular breeding techniques to improve rose flower color is feasible; however, an important prerequisite for successful molecular breeding is the establishment of plant regeneration systems through tissue culture techniques.
Tissue culture technology has been used significantly in the large-scale production and genetic improvement of plants (Pati et al. 2004; Azadi et al. 2018). It has been reported that by selecting tissues and organs, such as leaves, petioles, stem segments, isolated roots, and immature embryos as explants, modern roses can be regenerated in vitro into plantlets by organogenesis (Tian et al. 2008; Pourhosseini et al. 2013; Samiei et al. 2021) and somatic embryogenesis (Kim et al. 2003; Bao et al. 2012; Chen et al. 2014). Samiei et al. (2021) used Rosacanina bud-bearing stem segments as explants, induced in vitro axillary bud germination, and regenerated plants via organogenesis. Kim et al. (2003) used Rose ‘Sumpath’ immature zygotic embryo cultured explants and directly generated somatic embryos. Lee et al. (2013) obtained regenerated plants by somatic embryogenesis in vitro using rose roots as explants. It is well known that somatic embryos are bipolar and produce fewer chimaeras in transgenic plants than organogenesis; therefore, they have been more widely used in genetic engineering (Rout et al. 1991; Liu et al. 2021).
Plant endogenous hormones play an important role in plant development. Therefore, the relationship between changes in endogenous hormone content and somatic embryogenesis in vitro has also attracted the attention of researchers (Farias-Soares et al. 2014). High-performance liquid chromatography (HPLC) is currently the mainstream method for the accurate determination of endogenous hormones, which can effectively separate different endogenous hormones with a high sensitivity (Forcat et al. 2008). The study by Nic-Can et al. (2016) has shown that auxin (IAA) is involved in the establishment and maintenance of cell polarity and promotes the early development of somatic embryos. For example, the IAA content of carrot embryogenic cells is 13 times that of non-embryogenic cells (Sasakin 1994). Like auxins, cytokinins (CKs) are also critical for somatic embryo induction, and the balance between cytokinins and auxins determines the dedifferentiation and redifferentiation states of cells (Wu et al. 2021). Abscisic acid (ABA) is associated with the synthesis of storage material at the maturation stage and has been found to play an important role in regulating the development of plant somatic embryos and to inhibit the early germination of somatic embryos and the development of malformed embryos (Su et al. 2013). Kępczyńska (2021) found that GAs were involved in the acquisition of embryonic competence in Medicago truncatula leaf somatic cells. Similarly, Mikuła (2021) found in their study of Cyathea delgadii that large amounts of GAs were characteristic of the formation of unicellular somatic embryos. Liang et al. (2022) reported the endogenous phytohormone changes with embryogenic cultures in Korean pine, where endogenous hormone contents and endogenous hormone ratios had some effect on the high embryogenic potential maintenance or the morphological differentiation for somatic embryos. This showed that plant regeneration via somatic embryogenesis may be affected by the relationship between the changes in the contents of the abovementioned endogenous hormones and the development process of somatic embryos, which could provide a clear strategy and pathway for subsequent artificial regulation of plant regeneration for somatic embryos.
Rosa ‘John F. Kennedy’ (hybrid tea rose) was chosen as the subject in this study, and different explant types were used for the development of in vitro cultures capable of plantlet regeneration by somatic embryogenesis. Furthermore, to provide a research basis for artificially controlling the plant regeneration pathway, the relationship between changes in endogenous hormone contents and various types of embryogenic cultures during somatic embryo regeneration was investigated. Finally, this study aimed to offer a reference for tissue culture research by classifying and summarizing common intermediate materials in in vitro culture based on morphological characteristics and plant regeneration pathways.
Material and methods
Plant material
Annual green and healthy branches from Rosa ‘John F. Kennedy’ (hybrid tea rose) were taken from the rose germplasm resources nursery of the Nanyang Academy of Agricultural Sciences in May 2020. The annual branches were cut into 1.0- to 1.5-cm stem segments with an axillary bud and sterilized with disinfectant mercuric chloride (0.1% (v/v) HgCl2; Tongxin, Guizhou, China). After that, these sterilized materials were transferred to Murashige and Skoog (MS; Murashige and Skoog 1962; Solarbio; Beijing, China) medium containing 30 g L−1 sucrose (Kemiou; Tianjin, China), 8.0 g L−1 agar (Biosharp; Hefei, China), 1.0 mg L−1 6-benzyladenine (BA; Solarbio), and 0.1 mg L−1 indole-3-butyric acid (IBA; Solarbio) (P medium) for propagation, and the sterile shoots with higher multiplication coefficients were selected to establish the clonal line from a single bud through subculture every 30 d.
Embryogenic callus (EC) induction from leaf explants, somatic embryo induction from EC, and somatic embryo propagation were carried out by following the procedure described previously by Zhu (2022). Briefly, the leaves of sterile seedlings of Rose ‘KND-3’ were chosen for dark cultivation in MS medium with 7 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D; Solarbio); and, after a period of time, EC was induced. On MS medium containing 1.0 mg L−1 BA, 0.1 mg L−1 α-naphthalene acetic acid (NAA; Solarbio), and 0.1 mg L−1 gibberellic acid (GA3; Solarbio), embryogenic callus (EC) formed somatic embryos. After successfully obtaining somatic embryos from the EC, the somatic embryos were inoculated into MS medium containing 0.5 mg L−1 BA and 0.1 mg L−1 IBA (SER medium), and proliferation of the embryos was observed after repeated passages. These proliferative materials were at the same genetic background from the clonal line of Rose ‘KND-3.’
Medium and culture conditions
Murashige and Skoog (MS) basal medium was used for in vitro culture of the modern rose, supplemented with 30 g L−1 sucrose, 8.0 g L−1 agar, and plant growth regulators at different concentrations, which were used for several purposes in this research, followed by adjustment of the pH to 6.0 prior to autoclaving (121℃, 20 min). Somatic embryo regeneration medium (SER medium) was used for two purposes in this research, including somatic embryo propagation and regeneration induction. Sterile material proliferation medium (P medium) was used for sterile seedlings propagation. The medium composition was described above. Hormone-free MS medium was used for rooting induction of regenerated seedlings. The culture temperature was 24 ± 2℃; embryogenic callus and somatic embryo induction were induced in the dark while other treatments were conducted in light under a 16-hr light and 8-hr dark photoperiod with a light intensity of 40 micromoles m−2 s−1.
Plantlet regeneration Via Somatic Embryogenesis
Somatic embryos were cultured on SER medium in the light; and when new somatic embryos were observed, they were immediately subcultured every 30 d to SER medium in clusters for propagation. Single-piece cotyledon explants (SPC explants) with different sizes (2 mm × 3 mm; 3 mm × 4 mm; 4 mm × 5 mm) were chosen as plant material and cultured in SER medium for plantlet regeneration induction. After 25 d of regeneration induction, the regeneration induction frequency (%) was recorded. Each experiment was repeated three times with 16 explants per treatment. SPC explants forming at least 2.0 buds were considered responding explants and scored for SPC explants with multiple bud induction frequencies.
The regenerated bud was separated from cotyledon explants and transferred to the same SER medium. Two wk of light culture later, multiple shoots could be observed. One stem segment with one node explants was approximately 1.5 cm long from the 30-d-culture regenerated seedlings that were selected and subcultured every 30 d in SER medium for regenerated plantlet propagation. Strong rootless seedlings with terminal buds (ST explants) were screened and cultured in hormone-free MS medium for rooting.
Endogenous hormone determination from somatic embryos during various types of embryogenic cultures
Single-piece cotyledonary somatic embryos (SPC explants) were cultured on SER medium in the light to promote the differentiation of the embryogenic structures, and then embryogenic cultures containing various developmental stages were obtained. Endogenous ABA, GA3, IAA, and ZT (zeatin) were determined simultaneously in initial explants and tissues during the induction phase and plantlet regeneration on days 0, 5, and 25. After 25 d of SPC culture, the embryogenic cultures were classified into two types according to the number of regenerated buds: SPC explant with a single bud and SPC explant with multiple buds (> 2). Each type of embryogenic culture was 0.5 g of fresh weight (FW), which was quickly frozen in liquid nitrogen and stored at − 80℃ for endogenous hormone content determination. After sample collection was completed, the samples were packaged on dry ice and transported to Nanjing Cavensis Testing Technology Co., Ltd. (Nanjing China) for endogenous hormone determination by ultra-performance liquid chromatography tandem mass spectrometry. Each sample assay was repeated three times. After excluding outlier data, the data of the three replicates were averaged for analysis.
Statistical analysis
The data collected from the experiments were processed and analyzed in SPSS Statistics 22.0 and Origin 2019 software with Duncan’s multiple range tests at a level of significance of P = 0.05. Percentage data were transformed via arcsine before analysis.
Results and discussion
Plantlet regeneration via Somatic Embryogenesis of Rosa ‘John F. Kennedy’
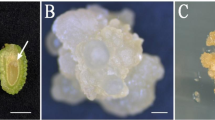
After leaf explants approximately 1.0 cm long were isolated from the seedlings of the ‘KND-3’ line and cultured according to the procedure described earlier by Zhu (2022), milky cotyledonary somatic embryos (CSE explants) could be derived from a few induced calluses (Fig. 1a). After somatic embryos were transferred to SER medium in the light, some explants turned green and expanded, and new somatic embryos could still be obtained on the cultured material. These somatic embryos were subcultured to SER medium in clusters (cluster culture) in the light every 30 d, which accelerated CSE propagation (Fig. 1b). In this research, plant materials were derived from the same clonal line; and, therefore, all of the obtained CSE explants had the same genetic background.
Plantlet regeneration via somatic embryogenesis of Rosa ‘John F. Kennedy.’ (a) Cotyledonary somatic embryo induced from callus of rose P explant; bar, 5.0 mm. (b) Somatic embryo propagation; bar, 5.0 mm. (c) SPC (single-piece cotyledonary somatic embryo) culture of somatic embryo (MW type); bar, 5.0 mm. (d) SPC explant turning green under light (TG type); bar, 5.0 mm. (e) Single bud sprouting from single somatic embryo (SB type); bar, 5.0 mm. (f) Growth of single regenerated seedling; bar, 5.0 mm. (g) Dicotyledon seed–like germination of somatic embryo; bar, 5.0 mm. (h) Growth of seed-like germinated seedling; bar, 5.0 mm. (i) Multiple buds induced from SPC explant (MB type); bar, 5.0 mm. (j) Growth of multiple regenerated seedlings; bar, 5.0 mm. (k) Regenerated shoots propagation; bar, 10.0 mm. (l) ST explant; bar, 10.0 mm. (m) Complete regenerated plantlet; bar, 10.0 mm.
Single-piece cotyledonary somatic embryos (SPC explants) were isolated from somatic embryo clusters, which were strong and tight in structure at different sizes; SPC explants (Fig. 1c) were used as the plant material for somatic embryo regeneration induction. After 5 d in light culture, the milky white SPC explant turned green (Fig. 1d). As shown in Table 1, after 25 d of single-piece cotyledon culture (SPC culture) on SER medium, the explants could regenerate, some single-piece explants could sprout a single bud (Fig. 1e), few explants could germinate like dicotyledon seeds (Fig. 1g) with low frequency (< 5%), and multiple buds could be derived from anywhere in the explants (Fig. 1i).
The regeneration capability of the SPC explants was compared for different explant sizes. Different explant sizes exhibited a statistically significant difference in the single bud regeneration rate and multiple bud regeneration rate (P < 0.05). The highest single bud regeneration rate (27.10%) was obtained for the SPC size of 4 mm × 5 mm. The multiple bud regeneration rate was highest at 39.60% for the SPC size of 3 mm × 4 mm.
In this study of SPC culture for Rosa ‘John F. Kennedy,’ the plantlet regenerated via single bud formation (SBF of plantlet regeneration type) with only one bud deriving from one explant. Normally, a single bud could be formed from the base of SPC explant where there was the point for somatic embryos propagating aggregation (Fig. 1e), and the regenerated bud grew along the longitudinal axis on which its developmental characteristics were similar with monocotyledon seed germination (Fig. 1f). In quite low frequency (< 5%), the SPC explants could be observed to germinate like dicotyledon seeds (Fig. 1g, h). Furthermore, the plantlet regenerated via multiple bud formation (MBF of plantlet regeneration type) with multiple buds deriving from one explant. Normally, clusters of multiple buds could be formed at any position from the explants (Fig. 1i), and the regenerated buds propagated along the horizontal axis in which its developmental characteristics were similar with organogenesis in vitro culture (Fig. 1j). These data showed that regeneration approaches were affected by the sizes of somatic embryos even if they were on the same induction medium. And, the ways to regenerate a single bud and multiple buds from SPC explants might be truly different plantlet regeneration patterns in which SBF of plantlet regeneration type and MBF of plantlet regeneration type could be considered as the simulation of seed propagation and vegetative propagation in nature.
The regenerated shoots were transferred to SER medium; and after 30 d of light culture, the shoots were observed for development and propagation (Fig. 1k). The regenerated shoots would gain the development of the shoots after the extension of subculture time (for example 45 d). A regenerated shoot with a 2.0-cm-long terminal bud (ST explant) (Fig. 1l) should be selected and transferred to hormone-free MS medium for adventitious roots induction. It should ensure no meristems found in the base of the shoot were inserted into MS medium for easy rooting. After 15 d of root induction treatment with the rooting rate of over 80%, the complete regenerated plantlet was obtained (Fig. 1m).
Endogenous hormones and endogenous hormone ratios from various types of embryogenic cultures
During the process of tissue culture for SPC explants of Rosa ‘John F. Kennedy,’ the embryogenic cultures showed different results: at the initial culture (day 0), all the SPC explants were milky-white (MW type, Fig. 1c) whereas after 5 d of induction culture, they turned green (TG type, Fig. 1d). After 25 d, the regenerated materials could be classified into two types according to their external morphological characteristics (such as color, shape, and the number of regenerated buds per explant), in which the materials were single bud formed from SPC explants (SB type, Fig. 1e), and multiple buds formed from SPC explants (MB type, Fig. 1i). These representative samples from different SPC culture period were harvested and pooled together or maintained separately for endogenous hormone determination.
Changes in the contents of single endogenous hormones
In Fig. 2, the contents of IAA and GA3 among the different types of embryogenic cultures were higher than that of ABA, and those of ZT were not detected at all except for in the MW type. The content of IAA decreased first and then increased during the regeneration process (the process from TG type to SB type and that from TG type to MB type). The analysis of endogenous hormones revealed that the content of IAA was the highest, ranging from 4.637 to 6.585 ng g−1FW; and the highest IAA content was in the MW type, but the concentration difference between the SB type and MB type was not statistically significant. The above results indicated that high levels of IAA in Rosa ‘John F. Kennedy’ might be beneficial for maintaining a high embryogenic potential but less effective for regenerating plantlets; it was consistent with the previous study of Korean pine (Liang et al. 2022).
Changes in the content of endogenous hormones in different types of embryogenic cultures of Rosa ‘John F. Kennedy.’ A. Changes in IAA content in different types of embryonic cultures; B. Changes in ZT content in different types of embryonic cultures; C. Changes in GA3 content in different types of embryonic cultures; D. Changes in ABA content in different types of embryonic cultures. Note: The data are expressed as the means ± SD, and means followed by the same letters are not significantly different by Duncan’s multiple tests (P < 0.05). MW type, SPC (single-piece cotyledonary somatic embryo) culture of somatic embryo; TG type, SPC explant turning green under light; SB type, single bud sprouting from single somatic embryo; MB type, multiple buds induced from SPC explant. IAA, indole-3-acetic acid; ZT, zeatin; GA3, gibberellic acid; ABA, abscisic acid.
Similar to IAA, the contents of GA3 and ABA in the different 4 types were high, and the maximum concentrations of GA3 and ABA in these materials were 7.301 and 4.045 ng g−1FW, respectively. However, unlike IAA, the content of GA3 or ABA decreased during the regeneration process. The interesting detail about the endogenous hormone determination in this study was that there was a significant difference between the regenerated materials of the SB type and MB type in the content of GA3 and ABA in which the SB type had the lowest GA3 content and a higher level of ABA content while the MB type showed the opposite results. These results suggested that the single-piece somatic embryo-derived single bud (SB type) and multiple bud (MB type) might be obviously different culture materials according to the different contents of endogenous hormones, and somatic embryo regeneration from Rosa ‘John F. Kennedy’ might involve the development of different patterns.
These findings indicated that a relatively high IAA, GA3, and ABA content and a low ZT content have a positive effect onsomatic embryo formation and development. A high IAA level was the premise for somatic embryo development and polarity establishment, and high GA3 and ABA contents promoted the maturation and germination of somatic embryos in the latter stage whereas regenerating plantlets required low levels of GA3 and ABA.
The effect of the ratio of endogenous hormone content on the differentiation of somatic Embryos in Rosa ‘John F. Kennedy’
In this study, the proportions of endogenous hormones in different types of embryonic cultures showed different changing trends and ranges of change (Fig. 3). Specifically, the ratios of IAA/ABA, GA3/ABA, and IAA/GA3 were clearly higher (0.383 to 6.535) than the ratios of ZT/ABA, ZT/IAA, and ZT/GA3 (all < 0.01). The ratio of IAA/ABA (1.633) and IAA/GA3 (0.902) in MW-type somatic embryos were the lowest while the IAA/ABA (6.535) value of the MB-type cultures was the highest. However, the highest ratios of IAA/GA3 (6.159) and the lowest ratios of GA3/ABA (0.383) appeared in SB-type cultures. It was worth noting that high ratios of IAA/GA3 and low ratios of GA3/ABA could cause somatic embryo germination similar to seeds. There were no obvious changes in the ratios of ZT/ABA, ZT/IAA, and ZT/GA3 among different types of embryogenic cultures, indicating that these ratios had little relevance with somatic embryo differentiation. These results showed that changes in the endogenous hormone content and ratios of various types of embryogenic cultures were clearly diverse during the plantlet regeneration process for somatic embryos of Rosa ‘John F. Kennedy’.
Changes in the ratio of endogenous hormones in different types of embryogenic cultures of Rosa ‘John F. Kennedy.’ Note: The data are expressed as the means ± SD. According to Duncan’s multiple tests, the mean values marked with the same letters do not differ significantly at the 0.05 confidence level. MW type, SPC (single-piece cotyledonary somatic embryo) culture of somatic embryo; TG type, SPC explant turning green under light; SB type, single bud sprouting from single somatic embryo; MB type, multiple buds induced from SPC explant. IAA, indole-3-acetic acid; ZT, zeatin; GA3, gibberellic acid; ABA, abscisic acid.
Classification and regeneration characteristics of intermediate material in In Vitro culture
For this study, the diverse regeneration results described above could be observed in the different sizes of SPC explants on the same induction medium (Fig. 1e, Fig. 1g, and Fig. 1i) in which single-piece somatic embryos could sprout similarly to monocotyledon germination (single bud derived from SPC explant) (Fig. 1e), induce multiple buds at any position from the explant (Fig. 1i), or germinate similarly to seeds with roots and buds with quite low induction rates (< 5%) (Fig. 1g).
In this study, SPC explants of Rosa ‘John F. Kennedy’ could be observed to have multiple bud formation via organogenesis but also germination-like seeds (classical somatic embryogenesis). Cotyledonary somatic embryos induced adventitious buds and regenerated plantlets via organogenesis, which was also observed in similar cases in Rosa hybrida ‘Tineke’ (Zhu et al. 2022) and the woody plant camphor tree (Du 2005). According to these regeneration studies on somatic embryos, this structure should be seen as a special category of intermediate material in in vitro culture. In other words, there might be no significant difference between somatic embryogenesis and organogenesis in view of somatic embryo structure as an intermediate material that can develop in various ways, except whether plantlets are regenerated via somatic embryo germination (Zhu. 2022).
On the basis of previous studies, there are several types of intermediate materials in in vitro culture, including calluses, protocorm-like bodies (PLBs) (Fig. 4b), and embryoid bodies (somatic embryos) (Fig. 4h). Based on their structural features, the calluses derived from various explants are divided into two major types, namely, non-embryogenic calluses (Fig. 4c) and embryogenic calluses (Fig. 4e); generally, non-embryogenic calluses can regenerate plantlets via organogenesis (Gao 2004; Srinivasan et al. 2021), and embryogenic calluses can easily induce somatic embryos (Du 2005; Du et al. 2007). Cotyledonary somatic embryos can be observed to both regenerate similarly to seeds (Fig. 4k) and induce multiple buds (Fig. 4i) by use of different cytokinin treatments (Du et al. 2007; Ebrahimi et al. 2018; Zhu et al.2022). For Orchidaceae in vitro culture, a special material, a protocorm-like body, can be obtained, which has the characteristics of both somatic embryogenesis and organogenesis (Lin et al. 2011; Bustam et al. 2013). In view of the cytological observation and regeneration approach of this structure that could easily gain adventitious buds from PLB, this material should be considered the metamorphosis of stems (the developmental process is shown in Fig. 4 from b, f, to a). In summary, the different explants regenerated plantlets mainly through organogenesis. The other regeneration approaches were mostly the metamorphosis of organogenesis, except that somatic embryo explants germinated similarly to seeds at low frequency. The classification and regeneration characteristics of the intermediate material as described above are represented in Fig. 4, and the details are shown in Table 2.
Schematic representation of the plantlet regeneration approaches from different intermediate material in in vitro culture. (a) Adventitious buds derived from leaflet. (b) Protocorm-like bodies (PLBs). (c) Non-embryogenic callus. (d) Cotyledonary somatic embryo. (e) Embryogenic callus. (f) Adventitious buds derived from PLBs. (g) Adventitious buds derived non-embryogenic callus. (h) Single-piece cotyledonary somatic embryo. (i) Multiple buds regenerated from cotyledonary somatic embryo. (j) Somatic embryos induced from embryogenic callus. (k) Somatic embryo germinating bud- and root-like seed.
Conclusion
In cell totipotency, cells of plants with the whole genetic material for a species have the potential to develop into embryos and plants. Under certain conditions (for example, in vitro culture, use of exogenous hormones), it can regenerate plantlet via simulating of seed propagation in nature (somatic embryo germinating like a seed) or via simulating of vegetative propagation in nature (explant deriving vegetative organs). In in vitro culture of some species (for example, Rosa ‘John F. Kennedy’), it can also be observed that the plantlet regenerates by like seed propagation and like vegetative propagation appearing simultaneously.
Data availability
All the key data are presented in the article.
References
Azadi P, Kermani MJ, Samiei L (2018) Somatic embryogenesis in Rosa hybrida. In: Jain, S., Gupta, P. (eds) Step wise protocols for somatic embryogenesis of important woody plants. Forestry Sciences 85: 161–170.Springer, Cham. https://doi.org/10.1007/978-3-319-79087-9_13
Bao Y, Liu GF, Shi XP, Ning GG, Liu J, Bao MZ (2012) Primary and repetitive secondary somatic embryogenesis in Rosa hybrida ‘Samantha.’ Plant Cell Tiss Org Cult 109:411–418. https://doi.org/10.1007/s11240-011-0105-6
Bustam S, Sinniah UR, Kadir MA, Zaman QZ, Subrananiam S (2013) Selection of optimal stage for protocorm-like bodies and production of artificial seeds for direct regeneration on different media and short term storage of Dendrobium Shavin White. Plant Growth Regul 69:215–224. https://doi.org/10.1007/s10725-012-9763-6
Chen JR, Wu L, Hu BW, Yi X, Liu R, Deng ZN, Xiong XM (2014) The influence of plant growth regulators and light quality on somatic embryogenesis in China Rose (Rosa chinensis Jacq.). J Plant Growth Regul 33:295–304. https://doi.org/10.1007/s00344-013-9371-3
Du L (2005) Preliminary studies on plant regeneration via somatic embryogenesis and Agrobacterium-mediated transformation of Camphor Tree (Cinnamomum camphora L.). PhD Dissertation. Huazhong Agricultural University, Wuhan, Hubei, China. (in Chinese)
Du L, Zhou S, Bao MZ (2007) Effect of plant growth regulators on direct somatic embryogenesis in camphor tree (Cinnamomumcamphora L.) from immature zygotic embryos and embryogenic calli induction. For Stud China 9:267–271 (in Chinese)
Ebrahimi M, Mokhtari A, Amirian R (2018) A highly efficient method for somatic embryogenesis of Kelussiaodorotissima Mozaff., an endangered medicinal plant. Plant Cell Tiss Org Cult 132:99–110. https://doi.org/10.1007/s11240-017-1314-4
Farias-Soares FL, Steiner N, Schmidt EC, Pereira MLT, Rogge-Renner GD, Bouzon ZL, Floh ESI, Guerra MP (2014) The transition of proembryogenic masses to somatic embryos in Araucaria angustifolia (Bertol.) Kuntze is related to the endogenous contents of IAA, ABA and polyamines. Acta Physiol Plant 36:1853–1865
Forcat S, Bennett MH, Mansfield JW, Grant MR (2008) A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods 4:1–8. https://doi.org/10.1186/1746-4811-4-16
Gao LP (2004) Establishment of plant regeneration system and studies on Agrobacterium-mediated transformation of Rosa hybrida cv. Samantha. PhD Dissertation. Huazhong Agricultural University, Wuhan, Hubei, China. (in Chinese)
Katsumoto Y, Fukuchi-Mizutani M, Fukui Y, Brugliera F, Holton TA, Karan M, Nakamura N, YOnekura-Sakakibara K, Togami J, Pigeaire A, Tao GQ, Nehra NS, Lu CY, Dyson BK, Tsuda S, Ashikari T, Kusumi T, Mason JG, Tanaka Y (2007) Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol 48:1589–600. https://doi.org/10.1093/PCP/PCM131
Kępczyńska E, Orłowska A (2021) Profiles of endogenous ABA, bioactive GAs, IAA and their metabolites in Medicago truncatula Gaertn. non-embryogenic and embryogenic tissues during induction phase in relation to somatic embryo formation. Planta 253:67. https://doi.org/10.1007/s00425-021-03582-8
Khatami F, Najafi F, Yari F, Khavari-Nejad RA (2020) Expression of etr1–1 gene in transgenic Rosa hybrida L. increased postharvest longevity through reduced ethylene biosynthesis and perception. SciHort 263:109103. https://doi.org/10.1016/j.scienta.2019.109103
Kim SW, Oh SC, Liu JR (2003) Control of direct and indirect somatic embryogenesis by exogenous growth regulators in immature zygotic embryo cultures of rose. Plant Cell Tiss Org Cult 74:61–66. https://doi.org/10.1023/A:1023355729046
Lee SY, Lee JL, Kim JH, Ko JY, Kim ST, Lee EK, Kim WH, Kwon Hyeno O (2013) Production of somatic embryo and transgenic plants derived from breeding lines of Rosa hybrida L. Hort Environ Biotechnol 54:172–176. https://doi.org/10.1007/s13580-013-0085-z
Liang Y, Xu X, Shen HL, Gao ML, Zhao Y, Bai X (2022) Morphological and endogenous phytohormone changes during long-term embryogenic cultures in Korean pine. Plant Cell Tiss Org Cult 151:253–264. https://doi.org/10.1007/s11240-022-02348-8
Lin Y, Li J, Li B, He T, Chun Z (2011) Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tiss Org Cult 105:329–335. https://doi.org/10.1007/s11240-010-9871-9
Liu GQ, Yuan Y, Jiang H, Bao Y, Ning GG, Zhao LJ, Zhou XF, Zhou HG, Gao JP, Ma N (2021) Agrobacterium tumefaciens-mediated transformation of modern rose (Rosa hybrida) using leaf-derived embryogenic callus. Hort Plant J 7:359–366. https://doi.org/10.1016/j.hpj.2021.02.001
Mikuła A, Tomaszewicz W, Dziurka M, Kazmierczak A, Grzyb M, Sobczak M, Zdankowski P, Rybczynski J (2021) The origin of the Cyathea delgadii Sternb. somatic embryos is determined by the developmental state of donor issue and mutual balance of selected metabolites. Cells 10:1388. https://doi.org/10.3390/cells10061388
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nakamura N, Katsumoto Y, Brugliera F, Demelis L, Nakajima D, Suzuki H, Tanaka Y (2015) Flower color modification in Rosa hybrida by expressing the S-adenosylmethionine: anthocyanin 3′,5′-O-methyltransferase gene from Toreniahybrida. Plant Biotechnol 32:109–117. https://doi.org/10.5511/plantbiotechnology.15.0205a
Nic-Can GI, Loyola-Vargas VM (2016) The role of the auxins during somatic embryogenesis. In: Loyola-Vargas V, Ochoa-Alejo N (eds) Somatic embryogenesis: fundamental aspects and applications. 171–182. Springer, Cham. https://doi.org/10.1007/978-3-319-33705-0_10
Nguyen NH, Van LB (2020) A simple, economical, and high efficient protocol to produce in vitro miniature rose. In Vitro Cell Dev Biol - Plant 56:362–365. https://doi.org/10.1007/s11627-019-10043-1
Pati PK, Sharma M, Sood A, Ahuja PS (2004) Direct shoot regeneration from leaf explants of Rosa damascena Mill. In Vitro Cell Dev Biol - Plant 40:192–195. https://doi.org/10.1079/IVP2003503
Pourhosseini L, Kermani MJ, Habashi AA, Khalighi A (2013) Efficiency of direct and indirect shoot organogenesis in different genotypes of Rosa hybrida. Plant Cell Tiss Org Cult 112:101–108. https://doi.org/10.1007/s11240-012-0210-1
Qi WC, Chen X, Fang PH, Shi SC, Li JJ, Liu XT, Cao XQ, Zhao N, Hao HY, Li YJ, Han YJ, Zhang Z (2018) Genomic and transcriptomic sequencing of Rosa hybrida provides microsatellite markers for breeding, flower trait improvement and taxonomy studies. BMC Plant Biol 18:119. https://doi.org/10.1186/s12870-018-1322-5
Rout GR, Debata BK, Das P (1991) Somatic embryogenesis in callus cultures of Rosa hybrida L. cv. Landora Plant Cell Tiss Org Cult 27:65–69. https://doi.org/10.1007/BF00048208
Samiei L, Pahnehkolayi MD, Tehranifar A, Karimian Z (2021) Organic and inorganic elicitors enhance in vitro regeneration of Rosa canina. J Genet Eng Biotechnol 19:60. https://doi.org/10.1186/s43141-021-00166-7
Sasakin K, Shimomura K, Kamada H, Harada H (1994) IAA metabolism in embryogenic and non-embryogenic carrot cells. Plant Cell Physiol 35:1159–1164. https://doi.org/10.1093/oxfordjournals.pcp.a078709
Srinivasan P, Raja HD, Tamilvanan R (2021) Efficient in vitro plant regeneration from leaf-derived callus and genetic fidelity assessment of an endemic medicinal plant Ranunculus wallichianus Wight & Arnn by using RAPD and ISSR markers. Plant Cell Tiss Org Cult 147:413–420. https://doi.org/10.1007/s11240-021-02134-y
Su YH, Su YX, Liu YG, Zhang XS (2013) Abscisic acid is required for somatic embryo initiation through mediating spatial auxin response in Arabidopsis. Plant Growth Regul 69:167–176. https://doi.org/10.1007/s10725-012-9759-2
Tanaka Y, Aida R (2010) Genetic engineering in floriculture. In: Jain S, Brar D (eds) Molecular techniques in crop improvement. 695–717. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-2967-6_30
Tian CW, Chen Y, Zhao XL, Zhao LJ (2008) Plant regeneration through protocorm-like bodies induced from rhizoids using leaf explants of Rosa spp. Plant Cell Rep 27:823–831. https://doi.org/10.1007/s00299-007-0504-7
Vergne P, Maene M, Gabant G, Chauvet A, Debener T, Bendahmane M (2010) Somatic embryogenesis and transformation of the diploid Rosa chinensis cv Old Blush. Plant Cell Tiss Org Cult 100:73. https://doi.org/10.1007/s11240-009-9621-z
Wu GY, Wei XL, Wang X, Wei Y (2021) Changes in biochemistry and histochemical characteristics during somatic embryogenesis in Ormosiahenryi Prain. Plant Cell Tiss Org Cult 144:505–517. https://doi.org/10.1007/s11240-020-01973-5
Zhou S, Du L, Chu XY, Bao MZ (2010) Establishment of plantlet regeneration system from leaves explants for callistephus chinensis ‘bouquet scarlet’. Acta Horticulturae Sinica 37(10):1667–1672. (in Chinese)
Zhu ZF (2022) Studies on plant regeneration via somatic embryogenesis and its characters in physiology and biochemistry of Rosa hybrida L.M. D. Dissertation. Nanyang Normal University, Nanyang, Henan, China. (in Chinese)
Zhu ZF, Zheng MY, Ma Y, Li JM, K XL, Du L (2022) Somatic embryo induction and plantlet regeneration of Rosa hybrida ‘Tineke’, Mol Plant Breed 1–9. (in Chinese)
Acknowledgements
The authors thank Ms. MYZ (Nanyang Academy of Agricultural Science) who provided plant material for this research.
Funding
This work was supported by the Postgraduate Education Reform and Quality Improvement Project of Henan Province, Grant No. YJS2021JD17; the Natural Science Foundation of Henan Province of China, Grant No. 232300420450; and the Key Research Project of Colleges and Universities of Henan Province Education Department, Grant No. 24B210008.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. LD guided the experiment, data analysis, writing the manuscript, and final editing. CYD participated in the investigation, methodology, and validation. XLK and ZFZ carried out all the experiment work and collected the data. YM, HRG, and JML participated in the writing of the introduction, data analysis, software operation, and survey validation. All authors commented on and contributed to the writing and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, L., Kang, X., Zhu, Z. et al. Plantlet regeneration via somatic embryogenesis and changes in endogenous hormone content of Rosa ‘John F. Kennedy’. In Vitro Cell.Dev.Biol.-Plant 60, 344–354 (2024). https://doi.org/10.1007/s11627-024-10426-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-024-10426-z