Abstract
We investigated the effect of red light and plant growth regulators on somatic embryogenesis in China Rose (Rosa chinensis Jacq.). Embryogenic calli that had been induced by combinations of 2,4-dichlorophenoxyacetic acid and thidiazuron in darkness were exposed to dark, red, and white light treatments. Cultures subjected to red light treatment generated the greatest number of embryos, with one (SE1 embryos) or two (SE2 embryos) expanded cotyledons. The largest numbers of shoot-like embryos without cotyledons (SE0 embryos) were produced in cultures subjected to dark treatment. The effects of different concentrations of abscisic acid (ABA) on the proliferation and germination of different types of somatic embryos were also evaluated. A concentration of 9.45 μM was found to be the most effective in promoting the proliferation and germination of SE2 embryos. The higher the concentration of ABA (from 0 to 18.90 μM), the higher the percentage of abnormal polycotyledonary embryos produced. The highest percentage of regenerated plants was obtained from SE2 embryos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Roses are attractive ornamental plants cultivated worldwide, and successful regeneration systems have been developed for a number of species. Regeneration via somatic embryogenesis has been reported for hybrid teas, floribundas, and other garden-type roses (Castillon and Kamo 2002; De Wit and others 1990; Firoozabady and others 1994; Hsia and Korban 1996; Kim and others 2003; Kintzios and others 1999; Kunitake and others 1993; Li and others 2002; Marchant and others 1996; Noriega and Söndahl 1991; Sarasan and others 2001; van der Salm and others 1996). Regeneration by means of organogenesis has been described in hybrid teas (Hill 1967), Rosa hybrida (Burger and others 1990; Dubois and de Vries 1995; Ibrahim and Debergh 2001; Rout and others 1992; van der Salm and others 1996), and R. multiflora ‘Thornless’ (Rosu and others 1995). In addition, regeneration from both organogenesis and somatic embryogenesis has been reported for R. hybrida (Hsia and Korban 1996; Li and others 2002) and R. rugosa-type roses (Kim and others 2009). To the best of our knowledge, few studies have examined the differences between organogenesis and somatic embryogenesis in roses, however, and no reports have appeared regarding the critical factors influencing callus conversion during organogenesis and somatic embryogenesis.
China Rose (R. chinensis Jacq.) is an important Chinese ornamental species from which many modern rose cultivars have been derived. In previous studies, organogenesis and transformation systems were established in this species (Chen and others 2006, 2010). Regeneration frequency from organogenesis was very low, however, with only a few shoots regenerating and becoming normal plantlets. One cultivar of R. chinensis, ‘Yueyuehong’, is an ancient ornamental with large, red flowers that has played an important role in rose breeding (Huang 2000). Although somatic embryogenesis has been observed in ‘Old Blush’, a R. chinensis cultivar with smaller, pink flowers, no studies to date have documented somatic embryogenesis in ‘Yueyuehong’. To optimize the regeneration system of China Rose and to investigate its capacity for somatic embryogenesis, the effects of thidiazuron (TDZ) in combination with 2,4-dichlorophenoxyacetic acid (2,4-D), red light, and abscisic acid (ABA) on somatic embryogenesis were investigated in this study.
ABA is an important plant growth regulator (PGR) that is involved in somatic embryogenesis in roses. In some rose species, short ABA treatments have been found to increase somatic embryo maturation (EM) and conversion and increase somatic embryogenesis (Hill 1967; Marchant and others 1996). Addition of ABA during the culturing of carrot somatic embryos has been shown to normalize the morphological development of embryoids to that of their zygotic counterparts (Rai and others 2011; Yang and Zhang 2010), whereas its addition to caraway somatic embryos has helped eliminate white-light-induced abnormalities (Ammirato 1974). Several studies have investigated ABA-level changes in developing seed embryos. In many plant species, ABA fluctuates during seed development, reaching its highest concentration when the dry weight of heart-stage embryos is increasing rapidly (Umbeck 1981). However, the critical effect of ABA on the morphological development of primary somatic embryos in rose species has not been widely investigated.
Red light treatment is another factor influencing somatic embryo development. Somatic embryogenesis has been observed to increase significantly in cultures of quince (D’Onofrio and others 1998), carrot (Michler and Lineberger 1987), and various pine species (Merkle and others 2005) subjected to red light treatment. Culture conditions (for example, subculture duration and dark vs. light exposure) greatly influence the type of calli formed and subsequent somatic embryo differentiation. In roses, however, the critical influence of red light on somatic embryogenesis has not yet been reported.
Since our previous study (Chen and others 2006) on somatic embryogenesis in China Rose, more details have been reported (Vergne and others 2010) regarding this process in R. chinensis ‘Old Blush’, including the important role of TDZ in rose somatic embryogenesis, the effects of ABA and light on cotyledon formation from somatic embryos, and the creation of new morphological types of calli and somatic embryos. The research reported in the current study was undertaken to elucidate the influence of red light on somatic embryogenesis in China Rose, to ascertain whether somatic embryogenesis is affected by ABA level, and to investigate control of morphology in cultures to increase embryogenic potential.
Materials and Methods
Plant Materials
Nodal stem segments of China Rose (R. chinensis Jacq.) ‘Yueyuehong’, 1–2 cm in size, were obtained from the nursery at Beijing Forestry University. To sprout shoots, surface-sterilized explants were placed on shoot proliferation (SP) medium (Marchant and others 1996). The SP medium consisted of MS basal medium (Murashige and Skoog 1962) combined with 4.44 μM benzyladenine, 0.27 μM 1-naphthalene acetic acid (NAA), and 8.67 μM gibberellic acid (GA3), and solidified using 6.0 g L−1 agar (Qualigens, Mumbai, India). PGRs were added to the medium and the pH was adjusted to 6.0 before autoclaving at 121 °C and 125 kPa for 15 min. The aseptic shoots were subcultured monthly onto fresh SP medium. The cultures were incubated at 25 ± 2 °C under a 16/8-h light/dark regimen (Chen and others 2006; Marchant and others 1996).
Callus Induction
Leaves with 1-mm petioles were taken from aseptic shoots and used as explants. The explants were placed abaxial side down in 9-cm Petri dishes containing one of four different solidified embryo proliferation (EP) media. The four different EP media were composed of SH (Schenk and Hildebrandt 1972) basal salts and vitamins, together with 30.0 g L−1 sucrose, 300.0 mg L−1 l proline, and 4.0 g L−1 agarose (Biowest, Shanghai YITO, China) (Marchant and others 1996) supplemented with different PGRs, that is, 13.56 μM 2,4-D (Marchant and others 1996), 2.25 μM TDZ, 11.25 μM TDZ, or 13.56 μM 2,4-D + 2.25 μM TDZ, and adjusted to pH 5.4 (Chen and others 2006). EP medium was supplemented with TDZ to investigate its effect on China Rose callus induction and to optimize regeneration. The dishes were sealed with Parafilm (Sigma-Aldrich, St. Louis, MO, USA) and maintained in the dark at 25 ± 2 °C. A minimum of 30 explants were cultured for each treatment, and each treatment was replicated three times. Subculturing was performed in 9-cm Petri dishes every 4 weeks using the same medium under the same culture conditions.
Somatic Embryogenesis on EM Medium Under Different Light Conditions
To optimize somatic embryogenesis culture conditions, we investigated the effects of red and white light exposure. In previous studies on China Rose, reddish-brown, friable callus was able to regenerate on EM medium (Chen and others 2006, 2010). This type of callus, termed embryogenic callus, quickly proliferated on EP medium and was crumbly and easily divided. The semitransparent, watery calli generated in this study were also easily divided. Twelve-week-old embryogenic calli were divided into three groups (~1,000 mg each) for dark, red light, and white light treatments and transferred from EP media to the surface of a modified EM medium (Marchant and others 1996) consisting of SH basal salts and vitamins supplemented with 2,4-D (4.52 μM), TDZ (0.45 μM), l-proline (300.0 mg L−1), ABA (3.78 μM), GA3 (8.67 μM), sucrose (30 g L−1) (Biowest, Shanghai YITO, China), and agarose at pH 5.4 (Chen and others 2006). For each treatment, embryogenic calli weighing ~1,000 mg were cultured on the surface of the medium in a Petri dish sealed with Parafilm. Each treatment was replicated three times. All cultures were maintained at 25 °C in either dark or light/dark conditions under a 16/8-h light/dark regime. Red light was supplied by 13-W Mini Twister Energy Saver Red bulbs (Philips, Guangzhou, China) at an intensity of 7.2 μmol m−2 s−1; the white light source consisted of cool white fluorescent tubes (TLP 36 W/840; Philips) with an intensity of 27.0 μmol m−2 s−1. To retain their somatic embryogenic capacity, unused embryogenic calli induced by 2,4-D (2,4-D alone or combined with TDZ) were cultured on modified EP medium containing 13.56 μM 2,4-D and 2.25 μM TDZ, whereas unused embryogenic calli induced by TDZ alone (2.25 or 11.25 μM) were cultured on EP medium supplemented with either 2.25 or 11.25 μM TDZ. Subculturing was performed every 4 weeks, and the new cultures were maintained under the same culture conditions.
In each of the three 1,000-mg callus groups, the percentage of calli that had produced somatic embryos after 12 weeks was recorded. A stereomicroscope was used to examine calli and somatic embryos.
Somatic Embryogenesis on EM Medium Containing ABA at Different Levels
Embryogenic calli generated on modified EP medium were divided into four groups (~1,000 mg each) and subcultured on EM medium containing four different concentrations of ABA (0, 3.78, 9.45, and 18.9 μM). Each group was cultured on the medium surface in a Petri dish sealed with Parafilm. The percentage of different types of somatic embryos generated was recorded after 12 weeks (three subcultures). Each treatment was replicated three times.
Germination and Plant Regeneration from Different Types of Somatic Embryos
To regenerate whole plants, the different types of somatic embryos that had formed on the embryogenic calli were excised and cultured on SP medium to induce germination and shoot regeneration. Cultures were incubated at 25 ± 2 °C under a 16/8-h light/dark regime (Marchant and others 1996). Somatic embryos generated under each treatment were counted separately for each replicate. The percentage of each type of somatic embryo producing shoots was recorded after 12 weeks, with embryos having 0, 1, or 2 cotyledons designated as SE0, SE1, and SE2, respectively, and abnormal polycotyledonary embryos were recorded as SEp.
The 3–5-cm-long shoots that had developed from somatic embryos were subcultured for root induction on MS medium containing 0.1 mg L−1 NAA. The rooted plantlets were acclimatized, transplanted into potting soil, and grown to maturity in a greenhouse (Kim and others 2004).
Statistical Analysis
Percentage data were arcsine transformed prior to ANOVA to stabilize the variance. All data were analyzed using the SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) and compared using least significant difference tests at the 5 % probability level.
Results
Induction of Embryogenic Calli by 2,4-D and TDZ
In previous experiments (Chen and others 2006), the effect of 2,4-D on callus induction in China Rose was examined using basal SH medium. That preliminary study found that preculturing for 2 weeks on medium containing 5 mg L−1 (22.60 μM) 2,4-D was harmful to callus growth. In contrast, calli from explants incubated on EP medium containing 3 mg L−1 (13.56 μM) 2,4-D grew well and differentiated into two types of calli: one hard-textured and white, the other reddish-brown and friable. Only the latter calli produced adventitious shoots.
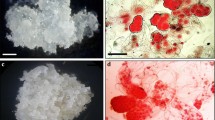
In further experiments, the effects of 2,4-D (13.56 μM), TDZ (2.25 or 11.25 μM), or a mixture of 2,4-D (13.56 μM) and TDZ (2.25 μM) on embryogenic callus formation were examined using modified EP medium. After a 12-week culture period on modified EP medium, three types of calli were produced: one hard-textured and white, another reddish-brown and friable, and the third semitransparent and watery (Fig. 1). The latter two types were able to produce regenerated plantlets upon subsequent subculturing. The explants on the medium containing only 2,4-D generated primarily reddish-brown, friable embryogenic calli (21.1 %), whereas explants on media with only TDZ (2.25 or 11.25 μM) produced mainly semitransparent, watery embryogenic calli (23.3 and 30.0 %, respectively). On medium containing a mixture of 2,4-D (13.56 μM) and TDZ (2.25 μM), ~8.3 % of the explants generated reddish-brown, friable calli and 16.7 % developed semitransparent, watery calli. These results demonstrate that 25.0 % (8.3 + 16.7 %) of the calli generated from the original leaf explants were embryogenic and able to produce embryos, similar to previous results (25.9 %) (Chen and others 2006). In addition, globular primary embryos were observed on calli generated on the medium containing a mixture of 2,4-D and TDZ (Fig. 1e).
Callus styles induced by 2,4-D and TDZ in China Rose. a, b Hard, white callus (a) and reddish-brown, friable callus (b) induced by 2,4-D on EP medium containing 13.56 μM 2,4-D. c, d Hard, white callus (c) and semitransparent, watery callus (d) induced by TDZ alone on modified EP medium with 2.25 or 11.25 μM TDZ instead of 2,4-D. e, f Semitransparent, watery calli with globular primary embryos on modified EP medium containing 13.56 μM 2,4-D and 2.25 TDZ after a 12-week culture period (e) and after an additional 8-week subculturing period (f). Scale bar 2 mm (Color figure online)
To retain their somatic embryogenic capacities, unused embryogenic calli induced by 2,4-D were cultured on modified EP medium containing both 2,4-D and TDZ, whereas embryogenic calli induced by TDZ alone (2.25 or 11.25 μM) were cultured on their same EP medium (that is, with 2.25 or 11.25 μM TDZ alone). Surprisingly, embryogenic calli subcultured on medium containing both 2,4-D and TDZ generated more globular embryos (Fig. 1f), whereas embryogenic calli cultured on medium with TDZ alone produced “wood ear”-like plantlets after an additional 8-week culture period (Fig. 2). Interestingly, these “wood ear”-like plantlets had simple leaves (Fig. 1b, c) rather than the compound leaves typical of China Rose.
Embryogenic calli generating abnormal embryos/shoots on medium containing TDZ alone. The abnormal embryos/shoots were produced after an additional 8-week culturing period on modified EP medium containing 2.25 μM (a, b) or 11.25 μM (c) TDZ instead of 2,4-D. The leaves of these embryos/shoots appeared “wood ear”-like. The embryos/shoots on medium containing a higher concentration of TDZ (11.25 μM) (c) were semitransparent, and then browned and died during subsequent subculturing. Scale bar 2 mm
Induction of Somatic Embryogenesis Under Different Light Treatments
After 12 weeks on callus induction medium, the embryogenic calli (reddish-brown and friable or semitransparent and watery) were subcultured onto modified EM medium. The calli were divided into three groups and cultured either in the dark or under a 16/8-h photoperiod treatment using red (7.2 μmol m−2 s−1) or white (27.0 μmol m−2 s−1) fluorescent light.
Induction of somatic embryogenesis took place and continued for three subculture cycles (12 weeks). As shown in Fig. 3, many somatic embryos were formed from dark-cultured calli (Fig. 3a). These somatic embryos, which were shoot-like with long, slim stems but without expanded cotyledons (SE0 embryos), grew rapidly. However, calli cultured under red- or white-light 16/8-h photoperiods produced more embryos, which possessed expanded cotyledons (SE1 or SE2 embryos) (Fig. 3b, c). The calli cultured under red light (7.2 μmol m−2 s−1) were reddish-brown and friable or watery and maintained a continuous capacity for embryogenesis (Fig. 3b). Those cultured under white light (27.0 μmol m−2 s−1) also developed SE1 or SE2 embryos, but at the same time, the calli gradually turned smooth, hard, and green, and lost their capacity for continuous embryogenesis in subsequent subcultures (Fig. 3c).
The effect of ABA on cotyledonary stage embryo development is shown in Table 1. Calli cultured in the dark displayed the highest frequencies of somatic embryo induction, but more than 86 % (19/22) of induced embryos were SE0 embryos. Those cultured under the 16/8-h photoperiod treatment using red light (7.2 μmol m−2 s−1) exhibited the highest frequencies of SE1 and SE2 embryo induction.
Maturation and Conversion of Embryos on Media with Different Concentrations of ABA
Embryogenic calli were subcultured under red light treatment on modified EM medium containing different concentrations of ABA (0, 3.78, 9.45, and 18.90 μM). Somatic embryogenesis began and continued for three subculture cycles, which lasted 12 weeks in total. As shown in Fig. 4, calli cultured on EM medium without ABA continuously generated sponge-like calli (Fig. 4a), most of which were abnormal SE0 embryos, with a few SE1 or SE2 embryos (Fig. 4b) present in subsequent subcultures. Calli cultured on EM medium containing ABA gave rise to somatic embryos with several different morphologies, including SE1 (Fig. 4c, d), SE2 (Fig. 4e, f), and SEp types such as three-cotyledon embryos (Fig. 4g, h), dactyloid clusters (Fig. 4i, j), “shooty” embryos (Fig. 4k), and “rooty” embryos (Fig. 4l). The calli cultured on EM medium with ABA were semitransparent and watery, however, and retained their capacity for continuous embryogenesis in subsequent subcultures.
Somatic embryogenesis in China Rose on EM medium with different concentrations of ABA under red light treatment. a, b Somatic embryogenesis induced on EM medium without ABA: primary embryo stage cultured for 1 week (a) and SE0 or SE2 stage after 3 weeks (b). c, d Somatic embryogenesis on EM medium containing 3.78 μM ABA: primary SE1 stage after culturing for 1 week (c) and SE1 stage after 3 weeks of culturing (d). e, f Somatic embryogenesis on EM medium containing 9.45 μM ABA: primary heart-shaped stage after 1 week (e) and SE2 stage after 3 weeks of culturing (f). g, h Somatic embryogenesis on EM medium containing 18.90 μM ABA: primary three-cotyledon stage after 1 week of culturing (g) and three-cotyledon stage after 3 weeks of culturing (h). i–l Other SEp styles such as dactyloid clusters (i, j), “shooty” embryos (k), and “rooty” embryos (l), generated on medium containing 9.45 μM ABA (i, j) or 18.90 μM ABA (k, l). Scale bar 2 mm
Induction numbers for somatic embryogenesis and cotyledon styles are given in Table 2. The lowest concentration of ABA (3.78 μM) gave rise to primarily SE1 embryos, whereas higher concentrations (9.45 or 18.90 μM) tended to induce SE2 embryos and abnormal structures such as dactyloid clusters, “shooty” embryos, and “rooty” embryos. Calli cultured on EM medium containing 9.45 μM ABA produced mostly SE2 embryos and regenerated the highest number of normal shoots in subsequent steps.
Plant Regeneration from Different Types of Embryos
We also investigated the plant regeneration frequency of the different types of somatic embryos. Plantlet regeneration was found to be heavily dependent on the type of somatic embryo. Frequencies of normal shoot regeneration are shown in Fig. 5. SE2 embryos with two cotyledons generated the highest percentages of normal shoots, whereas embryos showing abnormalities such as polycotyledonary embryos, dactyloid clusters, and “shooty” embryos generated very few normal shoots. SE2 embryos were characterized by continuously swelling cotyledons during the first 4 weeks after transfer to SP medium and displayed continuous shoot elongation during the next 3 weeks (Fig. 6, left panel). In contrast, most of the SE1 embryos and abnormalities, such as polycotyledonary embryos and dactyloid clusters, continued to produce merely calli (Fig. 6, left panel). Normal shoots regenerated from SE1 or SE2 embryos featured one or two cotyledons at the base (Fig. 6, right panel, SE1 or SE2), whereas shoots regenerated from SE0 embryos lacked cotyledons at the base (Fig. 6, right panel, SE0). After potting, the regenerated plants flowered normally in a greenhouse (Fig. 6, right panel, R).
Frequencies of normal shoots regenerated from different types of somatic embryos in China Rose. Means followed by the same letter within a column were not significantly different at P < 0.05. SE0, embryo without cotyledons; SE1, embryo with a single cotyledon; SE2, embryo with two cotyledons; SEp, abnormal embryos, such as polycotyledonary embryos, dactyloid clusters, and “shooty” embryos
Regeneration of somatic embryos in China Rose. Left Different somatic embryo germination stages on SP medium. Right Regenerated shoots with two cotyledons from SE2 embryos (SE2, arrows indicate cotyledons), with a single cotyledon from SE1 embryos (SE1, arrow indicates the cotyledon), and without cotyledons from SE0 embryos (SE0), and a regenerated rooted flowering China Rose plant (R). Scale bar 2 mm
Discussion
The application of biotechniques such as gene transfer and artificial seed production depends largely on the availability of tissue culture techniques for regenerating shoots. Increased plant regeneration efficiency through gene transformation is critical for successful rose breeding (Aldwinckle and Malnoy 2009). Since the establishment of a protocol for in vitro shoot organogenesis, several methods for regenerating roses based on shoot organogenesis and/or somatic embryogenesis have been developed (Hill 1967). Previous studies have suggested that 2,4-D, alone or in combination with other PGRs in the culture medium, is essential for inducing somatic embryogenesis in roses, and ABA has been found to be the most effective PGR for promoting the proliferation and germination of somatic embryos (Hsia and Korban 1996; Kunitake and others 1993; Li and others 2002; Marchant and others 1996; Noriega and Söndahl 1991; Rout and others 1991; van der Salm and others 1996; Visessuwan and others 1997). The types of somatic embryos or shoots produced have been quite variable, however. Single-cotyledon embryos or clusters displaying thickened cotyledons—the “classical style” (Vergne and others 2010)—seem to occur most frequently (Hsia and Korban 1996; Kunitake and others 1993; Marchant and others 1996; Rout and others 1991; van der Salm and others 1996; Visessuwan and others 1997). In addition, simultaneous somatic embryogenesis and organogenesis have been observed in a few studies (Hsia and Korban 1996; Li and others 2002). However, the critical factors controlling the morphology of somatic embryos in roses have not been studied intensively.
In previous studies on China Rose, it was observed that shoots lacking cotyledons were regenerated (Chen and others 2006, 2010). In the current study, the addition of low concentrations of TDZ increased the frequency of embryogenic callus induction and embryo production. TDZ not only induced embryogenic calli, it also produced “wood ear”-like plantlets with simple leaves. These results indicate that TDZ may be a very important regulator of somatic embryogenesis in China Rose, and may retard the formation of compound leaves. Under red light treatment, increasing the ABA concentration from 3.78 to 18.90 μM increased the number of expanded cotyledons and the frequency of polycotyledonary. ABA is required for further development of early somatic embryos into mature forms (Yang and Zhang 2010); it may also inhibit precocious germination of somatic embryos and regulate synthesis of their storage proteins (Rai and others 2011). After synthesis, storage proteins may be transferred to cotyledons, promoting their expansion. This effect seems to be synergistic with light, however, as early somatic embryos cultured in darkness seldom produced expanded cotyledons in our study. The application of reduced light intensities or ABA achieves in vitro slow-growth conservation, which is very important for further somatic embryo development (Rai and others 2011). Embryogenic competence is also correlated with photoequilibrium, with phytochrome appearing to be an important factor under low photoequilibrium conditions (D’Onofrio and others 1998). In our study, the use of red light may have created culture conditions with a low photoequilibrium and reduced light intensity, which, in turn, promoted storage protein synthesis and increased somatic embryogenesis. SE2 embryos, which had the highest normal plant regeneration frequency, were produced primarily under red light on medium containing 9.45 μM ABA, whereas SE0 embryos, which had the highest frequency of abnormal somatic embryo production, were observed mostly on modified EM medium under dark conditions or on modified EM medium without ABA. These results suggest that ABA and red light may be critical factors for normalizing morphological development of primary somatic embryos in China Rose.
Two somatic embryo types have been reported for a cultivar of R. chinensis, ‘Old Blush’ (Vergne and others 2010). An RcOBType1 embryo with expanded cotyledons, induced by 2,4-D and NAA under light treatment, was generated directly from green hard tissues and was able to develop directly into plantlets. An RcOBType2 embryo with thickened cotyledons, induced mainly by 2,4-D, NAA, and mannitol under dark treatment, did not develop RcOBType1 characteristics after exposure to light. The thicker, more numerous cotyledons associated with RcOBType2 may have been due to the dark conditions and the increased osmotic pressure from mannitol (Vergne and others 2010). In our study, induction of expanded cotyledons may have been caused by light exposure, and the number of cotyledons was largely related to both light quality and ABA addition. The use of ABA under red light was found to be critical for inducing appropriate embryo types during light regeneration. The mechanism behind cotyledon formation from rose somatic embryos requires further study.
The effect of light quality on somatic embryogenesis has been investigated in other crop plants, including quince (D’Onofrio and others 1998), carrot (Michler and Lineberger 1987), and several pine species (Merkle and others 2005). In some of those studies, somatic embryogenesis was highest in cultures subjected to red light treatment, with overall embryogenic capability correlated with photoequilibrium (D’Onofrio and others 1998). In our study, exposure to red light resulted in a higher percentage of embryos with expanded cotyledon(s) compared with culturing under darkness. Furthermore, red light cultivation was associated with a reduced frequency of abnormal shoot production. These results suggest that red light promotes somatic embryo production. Exposure to red light would not only allow maximum phytochrome activation, it would also avoid the involvement, and consequent negative effects, of blue-absorbing photoreceptors (D’Onofrio and others 1998). Treatment under white light should also result in an increased percentage of embryos with expanded cotyledons compared with dark-treatment results. In our study, somatic embryos induced under white light transformed less successfully into plantlets compared with those induced under red light or darkness.
The low conversion frequency of somatic embryos into plantlets might be attributable to their extended maintenance on a medium containing 2,4-D. Marchant and others (1996) showed that calli induced from R. hybrida ‘Trumpeter’ and ‘Glad Tidings’ retained their somatic embryogenic capacity for over 18 months without any increase in the percentage of morphologically abnormal embryos that failed to germinate. These results suggested that embryogenic callus could be transferred to 2,4-D-containing EM medium for maturation and proliferation. When somatic embryos generated during this maintenance period exhibited upward-curling cotyledons, they could be transferred to SP medium lacking 2,4-D to promote regeneration. If the embryo cotyledons were outward-spreading, the embryos would instead revert to callus on SP medium lacking 2,4-D. These results indicate that transfer of regenerated embryos to a medium lacking 2,4-D at the appropriate period is critical to plant regeneration.
In conclusion, embryogenic regeneration in China Rose plants is greatly improved by the application of red light and optimized levels of PGRs. The use of red light on modified EM medium during maturation and proliferation periods plays an important role in programming the cells toward effective formation of somatic embryos with expanded cotyledons. Our study has clearly demonstrated that 2,4-D, TDZ, and ABA are critical growth regulators for somatic embryogenesis in China Rose.
References
Aldwinckle HS, Malnoy M (2009) Plant regeneration and transformation in the Rosaceae. Transgenic Plant J 3(Special Issue 1):1–39
Ammirato PV (1974) The effects of abscisic acid on the development of somatic embryos from cells of caraway (Carum caryi L.). Bot Gaz 135:328–337
Burger DW, Liu L, Zary KW, Lee CI (1990) Organogenesis and plant regeneration from immature embryos of Rosa hybrida L. Plant Cell Tiss Org Cult 21:147–152
Castillon J, Kamo K (2002) Maturation and conversion of somatic embryos of three genetically diverse rose cultivars. HortScience 37:973–977
Chen JR, Liu R, Wang HF (2006) Plant regeneration of transgenic China Rose (Rosa chinesis Jacq.) from organogenic callus. For Stud China 8:92–97
Chen JR, Lü JJ, Liu R, Xiong XY, Guo LB, Wang HF (2010) DREB1C from Medicago truncatula enhances freezing tolerance in transgenic M. truncatula and China Rose (Rosa chinensis Jacq.). Plant Growth Regul 60:199–211
D’Onofrio C, Morini S, Bellocchi G (1998) Effect of light quality on somatic embryogenesis of quince leaves. Plant Cell Tiss Org Cult 53:91–98
De Wit JC, Esendam HF, Honkanen JJ, Tuominen U (1990) Somatic embryogenesis and regeneration of flowering plants in rose. Plant Cell Rep 9:456–458
Dubois LAM, de Vries DP (1995) Preliminary report on the direct regeneration of adventitious buds on leaf explants of in vitro grown glass house rose cultivars. Gartenbauwissenschaft 60:249–253
Firoozabady E, Moy Y, Courtney-Gutterson N, Robinson K (1994) Regeneration of transgenic rose (Rosa hybrida) plants from embryogenic tissue. Biotechnology 12:609–613
Hill GP (1967) Morphogenesis of shoot primordia in cultured stem tissue of a garden rose. Nature 216:596–597
Hsia CN, Korban SS (1996) Organogenesis and somatic embryogenesis in callus cultures of Rosa hybrida and Rosa chinensis Minima. Plant Cell Tiss Org Cult 44:1–6
Huang SW (2000) Rose Breeding. In: Cheng JS (ed) The Genetics and Breeding of Garden Plants. China Forestry Publishing House, Beijing, pp 385–402
Ibrahim R, Debergh PC (2001) Factors controlling high efficiency adventitious bud formation and plant regeneration from in vitro leaf explant of roses (Rosa hybrid L.). Sci Hortic 88:41–57
Kim SW, Oh SC, Liu JR (2003) Control of direct and indirect somatic embryogenesis by exogenous growth regulators in immature zygotic embryo cultures of rose. Plant Cell Tiss Org Cult 74:61–66
Kim CK, Oh JY, Chung JD, Burrell AM, Byrne DH (2004) Somatic embryogenesis and plant regeneration from in vitro-grown leaf explants of rose. HortScience 39(6):1378–1380
Kim SW, Oh MJ, Liu JR (2009) Somatic embryogenesis and plant regeneration in zygotic embryo explant cultures of rugosa rose. Plant Biotechnol Rep 3:199–203
Kintzios S, Manos C, Makri O (1999) Somatic embryogenesis from mature leaves of rose (Rosa sp.). Plant Cell Rep 18:467–472
Kunitake H, Imamizo H, Mii H (1993) Somatic embryogenesis and plant regeneration from immature seed-derived calli of rugosa rose (Rosa rugosa Thurb.). Plant Sci 90:187–194
Li XQ, Krasnyanski SF, Korban SS (2002) Somatic embryogenesis, secondary somatic embryogenesis, and shoot organogenesis in Rosa. J Plant Physiol 159:313–319
Marchant R, Davey MR, Lucas JA, Power JB (1996) Somatic embryogenesis and plant regeneration in Floribunda rose (Rosa hybrida L.) cvs. Trumpeter and glad tidings. Plant Sci 120:95–105
Merkle SA, Montello PM, Xia X, Upchurch BL, Smith DR (2005) Light quality treatments enhance somatic seedling production in three southern pine species. Tree Physiol 26:187–194
Michler CH, Lineberger RD (1987) Effects of light on somatic embryo development and abscisic levels in carrot suspension cultures. Plant Cell Tiss Org Cult 11:189–207
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Noriega C, Söndahl MR (1991) Somatic embryogenesis in hybrid tea roses. Biotechnology 9:991–993
Rai MK, Shekhawat NS, Harish, Gupta AK, Phulwaria M, Ram K, Jaiswal U (2011) The role of abscisic acid in plant tissue culture—a review on the recent progress. Plant Cell Tissue Organ Cult 106:179–190
Rosu A, Skirvin RM, Bein A, Norton MA, Kushad M, Otterbacher AG (1995) The development of putative adventitious shoots from a chimeral thornless rose (Rosa multiflora Thurb. ex J Murr.) in vitro. J Hort Sci 70:901–907
Rout GR, Debata BK, Das P (1991) Somatic embryogenesis in callus cultures of Rosa hybrida L. cv Landora. Plant Cell Tiss Org Cult 27:65–69
Rout GR, Samantaray S, Das P (1992) Chloropromazine induced in vitro bud break in Rosa hybrida cv Landora. Orissa J Horticult 20:8–16
Sarasan V, Roberts AV, Rout GR (2001) Methyl laurate and 6-benzyl-adenine promote the germination of somatic embryos of a hybrid rose. Plant Cell Rep 20:183–186
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50:199–204
Umbeck PF (1981) Does ABA play a role in seed germination? Israel J Bot 29:168–180
van der Salm TPM, van der Toorn CJG, Hanischten CH, Dons HJM (1996) Somatic embryogenesis and shoot regeneration from excised adventitious roots of the root stock Rosa hybrida cv Money Way. Plant Cell Rep 15:522–526
Vergne P, Maene M, Gabant G, Chauvet A, Debener T, Bendahmane M (2010) Somatic embryogenesis and transformation of the diploid Rosa chinensis cv Old Blush. Plant Cell Tiss Organ Cult 100:73–81
Visessuwan R, Kawai T, Mii M (1997) Plant regeneration systems from leaf segment culture through embryogenic callus formation of Rosa hybrida and R. canina. Breed Sci 47:217–222
Yang XY, Zhang XL (2010) Regulation of somatic embryogenesis in higher plants. Crit Rev Plant Sci 29:36–57
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31071826 and 31272208), the Special Research Fund for Doctor’s Degree Dissertation in Chinese Universities (Grant No. 20104320120008), the China Postdoctoral Science Foundation (Grant Nos. 20100471215 and 201104473), and Hunan graduates’ innovation fund (CX2012B298). English-language editing was supplied by International Science Editing and Edanz Editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ji-Ren Chen, Lian Wu and Bo-Wen Hu have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Chen, JR., Wu, L., Hu, BW. et al. The Influence of Plant Growth Regulators and Light Quality on Somatic Embryogenesis in China Rose (Rosa chinensis Jacq.). J Plant Growth Regul 33, 295–304 (2014). https://doi.org/10.1007/s00344-013-9371-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-013-9371-3