Abstract
Kelussia odoratissima Mozaff. (or Kelus) is a medicinal plant native to the Zagros Mountains in Iran. This plant is widely used as a food flavoring and for its health-promoting properties. It has been considered an endangered species by the United Nations Development Programme. In this study, a somatic embryogenesis (SE) method was developed for mass propagation of Kelus. The green globular embryogenic callus was induced on cotyledonary leaves using the Murashige and Skoog (MS) medium supplemented with 1 mg/l 2,4-dichlorophenoxyaceticacid (2,4-D) and 0.25 mg/l Kinetin. Different treatments were assayed for proliferation of the embryogenic callus. The calli remained embryogenic in an MS medium containing 2,4-D (1 mg/l). The light treatments and carbon source showed significant effects (P ≤ 0.05) on the proliferation and development of somatic embryos. These treatments improved the conversion rate of the cotyledonary-stage embryos by 100%. The average numbers of embryos in the globular, heart, torpedo, and cotyledonary stages decreased by the addition of 3 g/l case in hydrolisate. The genetic stability among tissue culture-derived plants and the mother plant were assessed using the amplification fragment length polymorphism. No polymorphic band was observed among all the plants, exhibiting the genetic stability during in vitro multiplication. This research provides a promising approach for true-to-type plant multiplication of K. odoratissima through SE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A quarter of new medicines in the market are based on plant-originated molecules (Raskin et al. 2002; Terryn et al. 2006). The plant-derived medicine industry is estimated to grow to around 35.4 billion USD between 2013 and 2020 (Giri and Zaheer 2016). However, catastrophic environmental conditions following climate change and anthropogenic activities such as overgrazing, landslides, and indiscriminate harvesting for food and medicinal purposes are the main reasons for the depletion of medicinal plant resources. Up to 80–90% of medicinal plants are harvested from their natural habitats (Nosov 2012). Around 4000–10,000 medicinal plant species are categorized as endangered (Canter et al. 2005). For example, Dioscorea balcanica, Podophyllum hexandrum, and Pilocarpus jaborandis are now regarded as extinct species and Panax ginseng has nearly disappeared in its natural growing areas (Nosov 2012).
Kelussia odoratissima Mozaff., belonging to the Apiaceae family, is found only in Iran and is locally known as “Karafse-koohi” or “Kelus” (Sajjadi et al. 2012).The natural habitats of this perennial medicinal plant is limited to heights of 2500 m above the sea in the Zagros Mountains, with at least 4–5 months of freezing temperature (Askari-Khorasgani et al. 2013; Razeghi et al. 2016).The aerial parts of K. odoratissima contain important secondary metabolites such as Z-Lingustilide and 3-e-butyl phthalide. Positive influences of Z-ligustilide on the nervous system, blood pressure, and cholesterol have been reported by Shojaei et al. (2011). These compounds are considered as anti-inflammatory, anti-cancer, neuroprotective, anti-hepatotoxic and anti-cardiovascular agents (Wu and Hsieh 2011; Chao and Lin 2012). Kelus is also used in salads, souse and as a seasoning powder in soups and dairy (Raiesi et al. 2013). It has been considered an endangered species by the United Nations Development Programme (UNDP) (Ahmadi et al. 2007). A long-term seed dormancy, slow growth rate, insufficient plant production in limited local habitats (Askari-Khorasgani et al. 2013) and over-harvesting in early growth stages (Raiesi et al. 2013) have decreased the population of Kelus in recent years. Hence, the adoption decisions such as domestication and cultivation can prevent its extinction and support a sustainable production of K. odoratissima.
Somatic embryogenesis (SE) is a tissue-culture technique with a strong potential to speed up the propagation rate in plants. By far, there is no scientific report on micropropagation of Kelususing SE. In vitro studies related to the breaking of the seed dormancy and organogenesis (Askari-Khorasgani et al. 2013; Abdollahi Sahlabadi et al. 2012), callus induction, and the production of secondary metabolites through cell-suspension culture (Razeghi et al. 2015, 2016) of K. odoratissima had been previously reported. SE is the development of embryo-like structures from somatic cells without any gametic fusion (Haque and Ghosh 2016), having three main advantages concerning medicinal plants: mass propagation of selected materials (Kumar and Sheela 2014), generation of diversity and genetic transformation (Von Arlond 2008; Vázquez-Flota et al. 2016). It has been shown that, 2,4-D at 0.5 or 1 mg/l are among the most convenient PGRs and concentrations for the induction and proliferation of embryogenic callus (Kato 1996; Pedroso and Pais 1999; Yantcheva et al. 1998; Li et al. 2006; Naing et al. 2013). Early studies, indicating the presence or absence of 2,4-D, is necessary to induce an altered genetic program in somatic cells (Fujimura and Komamine 1979; Choi and Sung 1984). The transcriptome analysis of the early stages of SE and leaf tissues in Arabidopsis indicated that the expression of more than 2500 genes changed in immature somatic embryos induced by 2,4-D (Wickramasuriya and Dunwell 2015). Besides, 2,4-D, other factors, too, have been shown to determine induction, frequency, and the development of somatic embryos in plants. For example, a combination of the cytokinins with auxin can improve the frequency of SE. Berthouly and Michaux-Ferrière (1996) produced a highly embryogenic callus in leaf explants of Coffea canephora using a combination of 2,4-D, IBA, and 2iP. Neuenschwander and Baumann (1992) showed that 4.5 μM 2,4-D with 18.4 μM kinetin improved SE in C. Arabica. Nitrogen and carbon sources are also among a set of factors determining the fate of embryogenic response of tissues, although there are controversies over the type and concentrations of these compounds (Loyola-Vargas 2016).
Plant regeneration from relatively undifferentiated callus cells induces different types of genetic changes including DNA methylations, chromosome rearrangements, and single-gene mutations, generally termed ‘somaclonal variation’ (Phillips et al. 1994). The somaclonal variation (Larkin and Scowcroft 1981) can result in useful traits (Hwang and Ko 2004; Khan et al. 2014; Slazak et al. 2015) or unwanted characteristics that limit the mass micropropagation of similar plants (Basavaraj et al. 2016). Several molecular markers such as Methylation Sensitive Amplified Polymorphism (MSAP) (Peraza-Echeverria et al. 2001), Random Amplification of Polymorphic DNA (RAPD), Simple Sequence Repeats (SSR), Inter Simple Sequence Repeats (ISSR), Amplified Fragment Length Polymorphism (AFLP) and Start Codon Targeted (SCoT) have been used for the detection of tissue-culture-induced mutations (Martins et al. 2004; Rathore et al. 2014; Sebastiani and Ficcadenti 2016; Slazak et al. 2015; Vroh-Bi et al. 2011).
Here, we developed, for the first time, an efficient protocol for the production of true-to-type plantlets in K. odoratissima Mozaff via SE. This study followed the most usual procedures for the induction of SE using 2,4-D. Moreover, the effects of the carbon source and casein hydrolysate were investigated in the proliferation of embryogenic callus. To analyze the genetic stability of in vitro regenerated plantlets, the AFLP marker was used, which produced a large number of reproducible bands (Powell et al. 1996).
Materials and methods
Plant materials
The seeds of K. odoratissima Mozaff were collected from its natural habitat of “Chaharmahal and Bakhtiari” province in Iran. The seeds were treated with 70% ethanol for 1 min, and washed immediately in sterile distilled water, followed by dipping in freshly prepared 1% (w/v) hypochlorite sodium and a few droplets of tween-20 for 15 min. Then, the seeds were rinsed three times with sterile distilled water and cultured in a hormone-free (HF) MS basal medium (Murashige and Skoog 1962) containing 3.0% (w/v) sucrose (pH 5.8) and incubated in a dark condition for 2 months at 4 °C for germination.
Embryogenic callus induction and proliferation
The hypocotyls and cotyledonary leaves were removed from in vitro germinated seeds for use as explants for callus induction. The cotyledonary leaves were cut in half and cultured with the adaxial side in the medium. For the induction of embryogenic callus, explants were cultured in an MS medium supplemented with four combinations of growth regulators 2,4-D(0.5 and1 mg/l) + Kin (0 and 0.25 mg/l). A few replicates were used as a control in a HF-MS medium. At least 4 replications with 4 petri dishes/replicate were used for each treatment. Each petri dish separately contained 25 ml of culture medium with 10 samples from each explant type. The cultures were incubated at 25 ± 1 °C in a dark condition. The percentage of embryogenic calli was recorded after 4 weeks. The proliferation of embryogenic calli was assayed by applying two different concentrations of 2,4-D (0.5 and 1 mg/l) supplemented with Kin (0.25 mg/l) with 0 and 3 g/l casein hydrolysate (pancreatic hydrolysate of casein-Duchefa/C1301) in MS basal salts. Three types of carbon source including sucrose (0 g/l) + maltose (30 g/l), sucrose (15 g/l) + maltose (15 g/l) and sucrose (30 g/l) + maltose (0 g/l) and two different light conditions(0 and 20 µM/m2/s photosynthetic photon flux density (PPFD)emitted from coolf fluorescent tubes;16/8 light/dark regime)were also tested for all treatments. The cultures were kept at 25 ± 1 °C and, after 4 weeks, the percentage of calli that remained embryogenic and the average number of embryos at different developmental stages were recorded.
Somatic embryo development
At least 50 embryos in the globular, heart, and torpedo stages from the last step were subcultured in petri dishes containing 25 ml of the HF-MS medium supplemented with sucrose (15 g/l) + maltose (15 g/l)‘according to the best medium composition from the proliferation stage’, separately. Each petri dish was considered as a replicate and the assay was repeated thrice. The cultured media were incubated in the dark or 20 µM/m2/s PPFD;16/8 light/dark regime at 25 ± 1 °C. The effects on embryo at various developmental stages and the light treatment on development of the embryos were evaluated after 4 weeks.
Maturation and germination
The fully developed cotyledonary embryos were subjected to desiccation to stimulate the maturation process. The embryos were transferred to empty petri dishes containing 2 ml of the MS medium without plant growth regulators (PGRs) dropped at the corner to avoid over desiccation. Each petri dish had at least 100 embryos and was kept in a laminar flow at 25 ± 1 °C in a dark condition for 2, 4, 6, 8 h without sealing by parafilm. After the expiration of the dedicated durations, the embryos were transferred to a ½ macro HF-MS medium to evaluate the effect of desiccation times on embryo germination. The same number of embryos was used as control (0 h) without any desiccation treatment and subjected directly to the ½ macro HF-MS medium. The cultures were incubated at 25 ± 1 °C under a 16/8 light/dark regime with 40 µM/m2/s light intensity of a fluorescent lamp. The percentage of germination of the embryos under different treatment was recorded after 4 weeks. This experiment was performed with 3 replicates. The germinated embryos were transferred to the soil after 2 weeks of acclimatization at 20 ± 1 °C and 85% relative humidity in the phytotron (100 µM/m 2/s PPFD; 16/8 light/dark regime) (Fig. H).
DNA extraction and AFLP assays
The gnomic DNA from hypocotyl of a single seed as a mother plant and leaves of 12 regenerated plantlets from cotyledonary leaves of the same seed were extracted using DNeasy Plant Mini Kit (Qiagen, USA) and 200 ng of DNA was subjected to a digestion step using EcoRI and MseI restriction enzymes (Thermo Fisher Scientific, USA). The ligations of adapters, preselective and selective amplification, were performed, according to the standard AFLP procedures (Vos et al. 1995). In the selective amplification step, 2 µl of 1:30 diluted pre-amplified products were added to the PCR tube with 12 µl of the final volume reaction of the PCR mix containing 0.5 unit Taq DNA polymerase (Thermo Fisher Scientific, USA), 2 mM MgCl2, 0.2 mM each dNTPs, and 0.5 μM each of reverse and forward primers. Amplification products were analyzed by electrophoresis in a 7% polyacrylamide gel and visualized using a silver staining method (Blum et al. 1987). The gel images were scanned with 300 dpi resolution using ImageScanner III (GE Healthcare, Sweden). The search for polymorph bands was performed visually.
Statistical analysis
All the experiments were performed in factorial using a CRD basal design with at least 3 replicates. The SAS ver. 9.2 was used for the analysis of variance. Comparisons of the mean values were made by Duncan’s multiple range test (P ≤ 0.05).
Results and discussion
Effect of explant type and PGRs on induction of embryogenic callus
The callus induction was begun after 12–14 days of the culture of both explant types. No callus was observed on explants incubated in the HF–MS medium; so this treatment was removed for further investigation and data analysis. According to ANOVA, significant differences (P ≤ 0.05)were observed among the different treatments for the induction of embryogenic callus on explants originating from in vitro germinated seeds. The maximum frequency of embryogenesis was observed on cotyledonary leaves incubated in the MS medium supplemented with 1 mg/l of 2,4-D + 0.25 mg/l Kin(Table 1; Fig. 1a). In this treatment, 17.67% of embryogenic callus was produced on the cotyledonary leaves (Table 1). Despite the callus formation on hypocotyls using these treatments, these explants proved to be incompetent to induct somatic embryos. The embryogenic callus generally showed a green, globular color and texture. The formation of embryogenic callus depended on the explant type and composition of culture media. The in vitro cultured somatic cells were able to differentiate into embryos using appropriate PGRs (Hand et al. 2016). The 2,4-D has been recorded as one of the most convenient treatments for the induction of SE on competent cells and tissues, partly through the induction of DNA hypermethylation (Altamura et al. 2016). The single-effect study of PGR indicated that 1 mg/l of 2,4-D + 0.25 mg/l of Kin produced 7.25% and 7.83% embryogenic callus, respectively. This concentration was significantly different (P ≤ 0.05) from 2,4-D (0.5 mg/l) + Kin (0.0 mg/l). Our findings are in agreement with other previous studies of 2,4-D mediated SE in medicinal plants (Martin 2003; Beena and Martin 2003; Dhir et al. 2014). Martin (2003) showed that 1 mg/l 2,4-D was the most effective growth regulator for the induction of embryogenic callus in Holostemma adakodien. The internode and leaf explants of Ceropegia Candelabrum L. showed SE on the MS medium containing 4.52 µM (~1 mg/l) of 2,4-D (Beena and Martin 2003). Same results have been reported on the induction of SE in Anethumgraveolens L., a medicinal herb, using 2,4-D with a lower concentration (Dhir et al. 2014). A combination of cytokinin with auxin was shown to improve SE in some plants. A successful induction of SE was reported by the culture of immature zygotic embryos of Acaciafarnesiana and A. schaffneriin in an MS medium supplemented with 9.05 μM(~2 mg/l) 2,4-D and 4.65 μM(1 mg/l)Kin(Ortiz et al. 2000). Similarly, the application of 8.8 μM(~2 mg/l) BA and 4.0 μM (~0.9 mg/l) 2,4-D was highly efficient for the induction of SE and embryogenic callus in Phellodendron amurense Rupr. (Azad et al. 2009).
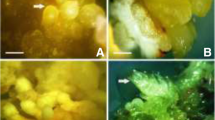
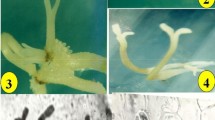
Somatic embryogenesis in K. odoratissima Mozaff. a induction of embryogenic callus on cotyledonary leaves, b embryogenic callus in proliferation step, c comparison of somatic embryo at globular stage in embryo development treatment; a dark and b: light conditions, d comparison of somatic embryo at heart stage in embryo development treatment; a: dark and b: light conditions, e comparison of somatic embryo at torpedo stage in embryo development treatment; a: dark and b: light conditions, f comparison of somatic embryo at cotyledonary stage in embryo development treatment; a: dark and b: light conditions, g germinated somatic embryos, h acclimatized plant from germinated somatic embryo 4 weeks after hardening
Proliferation of embryogenic callus
The proliferation of embryogenic callus was significantly (P ≤ 0.05) affected by different concentrations of 2,4-D, casein hydrolizate, carbon source, and light treatment. A single-effect study of 2,4-D on the proliferation of embryogenic callus indicated that in 1 mg/l of 2,4-D, 70.32% of calli remained embryogenic (Table 2). This was higher than 60.8% for 0.5 mg/l of 2,4-D. As demonstrated in Tables 2 and 3, following the application of casein hydrolizate (3 g/l), the average numbers of embryos in the globular, heart, torpedo and cotyledonary stages decreased significantly (P ≤ 0.05). This finding was in contradiction with the results of some of the other researchers such as Ageel and Elmeer (2011), who argued that the use of 3 g/l of casein hydrolizate improved the somatic embryogenesis in date palm. Although the casein hydrolizate was shown to provide a favorable nitrogen source during SE (Rybczynski and Zdunczyk 1986; Halperin 1995; Ageel and Elmeer 2011; Khierallah and Hussein 2013), its role remained controversial. It seems that the amount of nitrogen is more important than its type and form for the induction of SE (Reinert et al. 1967; Marques 1987; Fuentes-Cerda et al. 2001). As Ageel and Elmeer (2011) showed, the best result was obtained in ½ strength of the MS macro nutrients and 3 g/l of casein hydrolizate; so it is assumed that, in our experiment, the amount of nitrogen in the full MS macro nutrients was adequate to meet the requirement of the embryogenic tissues and the excessive nitrogen source had a deleterious effect on their proliferation.
The carbon source proved to be another determining factor in the maintenance of embryogenic callus. The dedicated parameters were significantly (P ≤ 0.05) higher in a medium containing a combination of 15 g/l of both sucrose and maltose (Tables 2, 3). The percent of embryogenic callus in this treatment was significantly higher (85.8%) than in the two other treatments with a carbon source. The source is a conventional carbon source for tissue culture of several plant species. The SE is suppressed with high concentrations of sucrose. The sucrose and nutrient starvation can induce embryogenic responses (Nic-Can et al. 2016). This stress can mediate for the induction of some stress related regulatory pathways involved in SE. Although the stress signal recognition and transduction pathway in explants to obtain the SE competency are not fully understood, the new findings indicate that these processes are controlled genetically. For example, the RWP-RK DOMAIN CONTAINING (RKD) and AGAMOUS-15 (AGL15) are two transcription factors with regulatory effects on the induction of cellular competency during SE. (Közsegui et al. 2011; Zheng and Perry 2014). The function of SERK1 (SOMATIC EMBRYOGENESIS RECEPTOR KINASE1) (Hecht et al. 2001) and LEAFY COTYLEDON (LEC1, LEC2) (Braybrook et al. 2006) have also been studied extensively for their involvement in the enhancement of cell competency in explants. Expression of these genes is correlated with the nutrient induced stresses under the in vitro condition (Nic-Can et al. 2016). Maltose is a reducing sugar with the same value or sometimes exceeding the sucrose in the SE of a number of species, such as carrot, alfalfa, wild cherry, Malus etc. This sugar is used as both osmoticum and a carbon source (George et al. 2008). Fuentes et al. (2000) showed that the substitution of fructose or maltose with sucrose increased the SE in Coffeacanephora (Fuentes et al. 2000).
In this study, light treatment had a positive effect on the growth of embryos (Fig. 1c–f). Although the percent of embryogenic callus and the average number of embryos at the heart stage were not affected by the light treatment, the average number of embryos at torpedo and cotyledonary stages were influenced (Table 2). The embryos at different developmental stages had a bigger and greener appearance under the light treatment compared to dark conditions. Light treatment promoted the development of embryos in the torpedo and cotyledonary stages (Table 3). It seems that the dark condition is preferable for the maintenance of embryogenic callus in globular stage. All of the calli remained embryogenic in the MS medium with 1 mg/l 2,4-D and 15 g/l of both sucrose and maltose under light and dark conditions, but the dark condition was preferable for its ability to produce 114 embryos in the globular stage per explant compared to light conditions with 77 embryos in the globular stage (Table 3). Light is one of the main physical parameters determining the fate of an embryogenic tissue. Meneses et al. (2005) indicated that one week of dark treatment of embryogenic calli in the pre-regeneration step of indica rice (Oryza sativa) followed by light can improve the regeneration of somatic embryos. In another attempt, Elmeer and Hennerty (2008) reported that five times more SEs were induced on calli in cucumber using a medium containing 2 mg/l of 2,4-D under dark conditions compared to a light regime (Cucumis sativus) (Elmeer and Hennerty 2008). This experiment indicated that the proliferation of embryogenic callus in K. odorotissima Mozaff. may not require light treatment, while further development of somatic embryos was enhanced by light.
Somatic embryo development
Although the general belief is that auxin removal is essential to allow the induction and further development of somatic embryos (Halperin 1964), here, we assayed the possibility that the somatic embryo development and maturation in K. odorotissima is influenced by light. To study the conversion rate of somatic embryo to the cotyledonary stage, the effects on embryo at different developmental stages and light conditions were evaluated. According to the single-effect analysis, the highest conversion rate (100%) was observed among embryos in the torpedo stage, which was significantly (P ≤ 0.05) more than the globular (15.5%)and heart(20.8%) stages. Light or dark conditions had a positive impact on the developmental behavior of embryos. The best conversion rate to the cotyledonary stage, which occurred under the light condition (51.4%), was significantly (P ≤ 0.05) higher than the dark treatment (39.4%). The Fig. 2 indicates the interaction effects of the embryo developmental stage and light treatment (0 and 20 µM/m2/s) on the development of embryos to the cotyledonary stage. As shown in the Fig. 2, light treatment improved the development of embryos at both the globular and heart stages. The morphological changes of embryos at different stages under light and dark regimes are presented in Fig. 1c–f. As mentioned earlier, the development of embryos in the torpedo and cotyledonary stages was promoted under light condition, and the embryos in different developmental stages were bigger and greener in light condition compared to the dark regime. The efficacy of light and dark conditions on the induction, development, and maturation of somatic embryos of various plant species were investigated. Our finding is consistent with Oh et al. (2013), demonstrating that light is an essential factor for the conversion of the globular embryos to the cotyledonary stage in Houttuyniacordata. Around 15% of the embryos at the globular stage successfully converted to the cotyledonary stage in the presence of light (Oh et al. 2013). The induction and development of somatic embryos in other plant species like squash (Cucurbita pepo L.), melon (Cucumis melo L.), pepper (Capsicum annum L.), gardenia (Gardenia jasminoides L.) and rose (Rosa hybrida L.) have been studied under different light intensity and duration (Kintzios et al. 1998). Although more somatic proembryos and globular embryos were induced on explants initially incubated in the dark or under a low PPFD, the development of embryo to the cotyledonary stage and the embryo maturation were significantly affected by the exposure of the cultures to light (Kintzios et al. 1998).
Effect of desiccation on maturation and germination of somatic embryos
Most of the medicinal plants are difficult to propagate through SE. They are mostly recalcitrant plants because of poor embryo maturation and conversion (Facchini et al. 2008). Treatment of somatic embryos with ABA and GA (0.1 and 0.2 mg/L) can promote the maturation process (Pathak et al. 2012). Partial desiccation is another practical treatment for the improvement of subsequent plant germination from somatic embryos (Othmani et al. 2009). In this experiment, the effects of four desiccation times (2, 4, 6 and 8 h) on the percentage of germination were examined and compared with control (0 h). The best result was observed by 4 hours’ treatment (Fig. 3) but was not significantly different from 2 h (P ≤ 0.05). These treatments significantly improved the percentage of germination compared to control (0 h). As shown in Fig. 3, the lowest percentage of germination occurred in the 8-hour desiccation treatment. The best germination rate of somatic embryos in our study occurred after 4 or 2 h of desiccation time. This finding is consistent with maturation of somatic embryos in date palm reported by Shareef et al. (2016). The maximum germination percentage was achieved when embryos were desiccated for 3 h. In the case of date palm, partial desiccation (0, 1, 2, 3 and 4 h) treatments were applied to boost the in vitro germination of somatic embryos. Like their zygotic counterparts, somatic embryos can remain dormant after development, and this can reduce embryo germination. It has been reported that the regulation of late embryogenesis abundant (LEA) proteins and the genes involved in biosynthesis and deposition of storage proteins are regulated by water stress and ABA treatment (Dodeman et al. 1997). These treatments can stimulate some important events such as starch depletion, raffinose occurrence, and sucrose and dehydrin accumulation to promote embryo maturation (Bomal and Tremblay 1999; Bomal et al. 2002). It is assumed that 2–4 h of desiccation treatment was enough for the maturation of somatic embryos in K. odorotissima, possibly through the inducement of these events.
AFLP assays
Among molecular markers, AFLP produces much more reproducible bands, making it a good option for detection of genetic variability of plants regenerated by in vitro culture and somaclonal variations (Aversano et al. 2011; Mehta et al. 2011; Mo et al. 2009; Prado et al. 2007). The genetic stability of the mother plant and 12 regenerated plantlets from the best treatment (MS + 1 mg/l 2,4-D + 15 g/l maltose + 15 g/l sucrose + 20 µM/m2/s illumination) was assayed using Ten selective primer combinations of AFLP (Table 4). Totally, 312 clear bands were amplified in the range of 100–600 bp (Fig. 4) all of which were monomorphic. This data shows that the propagation of K. odoratissima using in vitro embryogenesis produces true-to-type plantlets, which is important for mass production and conservation of this species. Depending on the species and genotype, in vitro regeneration of plants leads to genetic variation (Prado et al. 2007) or production of true-to-type regenerants (Mehta et al. 2011; Sebastiani and Ficcadenti 2016; Yadav et al. 2013). The effect of hormones and other treatments on the stimulation of somaconal variation is crucial. The AFLP analysis indicates that the method can be used for the protection and commercial true-to-type multiplication of Kelus.
Conclusion
Micropropagation of elite materials and genetic engineering-mediated improvements are the two main advantages of somatic embryogenesis in medicinal plants. The recent advances in transcriptome analysis have led to the elucidation of critical steps and regulatory mechanisms involved in secondary metabolism. However, the genetic manipulation of medicinal plants has been hampered by the fact that most of these plants are recalcitrant in matters of embryogenesis and transformation. Despite a successful primary embryogenesis, it is believed that embryo maturation and conversion are two limiting steps in the development of somatic embryo. In the present research, we developed a highly efficient protocol for the true-to-type micropropagation of an endangered medicinal plant through somatic embryogenesis. Some factors affecting the induction, proliferation, maturation, and conversion of somatic embryos in K. odoratissima have been studied. This method can be useful for germplasm conservation, mass propagation, and genetic engineering propocess in K. odoratissima Mozaff.
References
Abdollahi Sahlabadi E, Ranjbar GA, Babaeian Jelodar NA, Bagheri NA (2012) Study of in vitro germination of the indangered medicinal plant of mountain Celery (Kelussia Odoratissima Mozaff.). Biotechcongress, Tehran, Iran. http://www.biotechcongress.ir
Ageel S, Elmeer (2011) K effects of casein hydrolysates and glutamine on callus and somatic embryogenesis of date palm (Phoenix dactylifera L.). NY Sci J 4(7):121–125
Ahmadi F, Kadivar M, Shahedi M (2007) Antioxidant activity of Kelussia odoratissima Mozaff. in model and food systems. Food Chem 105:57–64
Altamura MM, Rovere FD, Fattorini L, D’Angeli S, Falasca G (2016) Recent advances on genetic and physiological bases of in vitro somatic embryo formation. In: Germanà MA, Lambardi Maurizio (eds), In vitro embryogenesis in higher plants, methods in molecular biology, vol. 1359, Springer, New York. doi:10.1007/978-1-4939-3061-6
Askari-Khorasgani O, Mortazaeinezhad F, Otroshy M, Golparvar AR (2013) Breaking seed dormancy of endangered medicinal plant kelussia odoratissima using zygotic embryo culture technique. Tech J Eng App Sci 3(15):1712–1718
Aversano R, Di Dato F, Di Matteo A, Frusciante L, Carputo D (2011) AFLP analysis to assess genomic stability in solanum regenerants derived from wild and cultivated species. Plant Biotechnol Rep 5:265–271
Azad MAK, Yokota S, Begum F, Yoshizawa N (2009) Plant regeneration through somatic embryogenesis of a medicinal plant, Phellodendron amurense Rupr. In Vitro Cell Dev Biol-Plant 45:441–449. doi:10.1007/s11627-008-9171-9
Basavaraj S, Rangaswamy K, Rao AM, Prameela H, Bhagyashree M (2016) Morphological and molecular characterisation of somaclonal variants in tissue culture banana variety grand naine. Adv Life Sci 5:1205–1210
Beena MR, Martin KP (2003) In vitro propagation of the rare medicinal plant Ceropegia Candelabrum L. through somatic embryogenesis. In Vitro Cell Dev Biol—Plant 39:510–513
Berthouly M, Michaux-Ferrière N (1996) High frequency somatic embryogenesis in Coffea canephora. induction conditions and histological evolution. Plant Cell Tiss Org 44:169–176. doi:10.1007/BF00048196
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Bomal C, Tremblay FM (1999) Effect of desiccation to low moisture content on germination, synchronization of root emergence, and plantlet regeneration of black spruce somatic embryos. Plant Cell Tiss Org Cult 56:193–200
Bomal C, Le VQ, Tremblay FM (2002) Induction of tolerance to fast desiccation in black spruce (Picea mariana) somatic embryos: relationship between partial water loss, sugars and dehydrins. Physiol Plant 115:423–530
Braybrook SA, Stone SL, Park S et al (2006) Genes directly regulated by leafy Cotyledon2 provide insight into the control of embryo maturation and somatic embryogenesis. Proc Natl Acad Sci (USA) 103:3468–3473. doi:10.1073/pnas.0511331103
Canter PH, Thomas H, Ernst E (2005) Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol 23(4):180–185
Chao W, Lin B (2012) Bioactivities of major constituents isolated from Angelica sinensis (Danggui). Chin Med 6:29
Choi J, Sung Z (1984) Two-dimensional gel analysis of carrot somatic embryonic proteins. Plant Mol Biol Rep 2:19–25. doi:10.1007/BF02885643
Dhir R, Shekhawat GS, Alam A (2014) Improved protocol for somatic embryogenesis and calcium alginate encapsulation in Anethum graveolens L.: a medicinal herb. Appl Biochem Biotechnol 173:2267–2278
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
Elmeer KMS, Hennerty MJ (2008) Observations on the combined effects of light, NAA and 2,4-D on somatic embryogenesis of cucumber (Cucumis sativus) hybrids. Plant Cell Tiss Organ Cult 95:381–384. doi:10.1007/s11240-008-9439-0
Facchini PJ, De Luca V (2008) Opium poppy and Madagascar periwinkle: model non-model systems to investigate alkaloid biosynthesis in plants. Plant J 54:763–784. doi:10.1111/j.1365-313X.2008.03438.x
Fuentes SRL, Calheiros MBP, Manetti J et al (2000) The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tiss Org 60:5–13. doi:10.1023/A:1006474324652
Fuentes-Cerda CFJ, Monforte-González M, Méndez-Zeel M et al (2001) Modification of the embryogenic response of Coffea arabica by nitrogen source. Biotechnol Lett 23:1341–1343. doi:10.1023/A:1010545818671
Fujimura T, Komamine A (1979) Involvement of endogenous auxin in somatic embryogenesis in a carrot cell suspension culture. Z Pflanzenphysiol 95(79):13–19. doi:10.1016/S0044-328X80023-9
George EF, Hall MA, De Klerk GJ (eds.) (2008) Plant propagation by tissue culture, 3rd Edn, pp 1–28. Springer, Dordrecht
Giri CC, Zaheer M (2016) Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult 126(1):1–18
Halperin W (1964) Morphogenetic studies with partially synchronized cultures of carrot embryos. Science 146(3642):408–410
Halperin W (1995) In vitro embryogenesis: some historical issues and unresolved problems. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer Academic Publishers, Dordrecht, pp 1–16
Hand ML, de Vries S, Koltunow AMG (2016) A comparison of in vitro and in vivo asexual embryogenesis. In: Germanà MA, Lambardi M (eds), In vitro embryogenesis in higher plants, methods in molecular biology, vol. 1359, Springer, New York. doi:10.1007/978-1-4939-3061-6
Haque SK, Ghossh B (2016) High-frequency somatic embryogenesis and artificial seeds for mass production of true-to-type plants in Ledebouria revoluta: an important cardioprotective plant. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-016-1030-5
Hecht V, Vielle-Calzada JP, Hartog MV et al (2001) The Arabidopsis somatic embryogenesis receptor kINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816. doi:10.1104/pp.010324
Hwang SC, Ko WH (2004) Cavendish banana cultivars resistant to Fusarium wilt acquired through somaclonal variation in Taiwan. Plant dis 88:580–588
Kato M (1996) Somatic embryogenesis from immature leaves of in vitro grown tea shoots. Plant Cell Rep 15:920–923
Khan MS, Ahmad D, Adnan M, Khan MA (2014) The effect of somaclonal variation on salt tolerance and glycoalkaloid content of potato tubers. Aust J Crop Sci 8:1597
Khierallah HS, Hussein NH (2013) The role of coconut water and casein hydrolysate in somatic embryogenesis of date palm and genetic stability detection using RAPD markers. Res Biotechnol 4(3):20–28
Kintzios SE, Hiureas G, Shortsianitis E, Sereti E, Blouhos P, Manos C, Makri O, Taravira N, Drossopoulos J, Holevas CD (1998) The Effect of light on the induction, development and maturation of somatic embryos from various horticultural and ornamental species. Acta Hortic 461:427–432
Közsegui D, Johnston AJ, Rutten T et al (2011) Members of the RKD transcription factor family induce an egg cell-like gene expression program. Plant J 67:280–291. doi:10.1111/j.1365-313X.2011.04592.x
Kumar V, Sheela C (2014) High frequency somatic embryogenesis and synthetic seed production of the endangered species Swertia chirayita. Biologia 69/2:186–192
Larkin PJ, Scowcroft WR (1981) Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Li D, Zhang J, Zhao J, Zhang Y, Chen F, Zhu J, Liu S, Yang Z (2006) Plant regeneration via somatic embryogenesis of Elymus sibiricus cv.‘chuancao No. 2. Plant Cell Tiss Organ Cult 84:285–292
Loyola-Vargas VM (2016) The History of somatic embryogenesis. In: Loyola-Vargas VM, Ochoa-Alejo N (eds), Somatic embryogenesis: fundamental aspects and applications, Springer, Cham. doi:10.1007/978-3-319-33705-0_25
Marques DV (1987) Study of some factors involved on in vitro callus growth and somatic embryogenesis of coffee tissues. In: Green CE, Somers DA, Hackett WP, Biesboer DD, Alan R (eds) Plant biology. vol 3. Plant tissue and cell culture, Liss Inc, New York, p 42
Martin KP (2003) Plant regeneration through somatic embryogenesis on Holostemma adakodien, a rare medicinal plant. Plant Cell Tiss Organ Cult 72:79–82
Martins M, Sarmento D, Oliveira M (2004) Genetic stability of micropropagated almond plantlets, as assessed by RAPD and ISSR markers. Plant Cell Rep 23:492–496
Mehta R, Sharma V, Sood A, Sharma M, Sharma RK (2011) Induction of somatic embryogenesis and analysis of genetic fidelity of in vitro-derived plantlets of Bambusa nutans wall., using AFLP markers. Eur J Forest Res 130:729–736
Meneses A, Flores D, Muñoz M et al (2005) Effect of 2,4-D, hydric stress and light on indica rice (Oryza sativa) somatic embryogenesis. Rev Biol Trop 53:361–368. doi:10.15517/rbt.v53i3-4.14598
Mo X, Long T, Liu Z, Lin H, Liu X, Yang Y, Zhang H (2009) AFLP analysis of somaclonal variations in Eucalyptus globulus. Biol plantarum 53:741–744
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:495–497
Naing AH, Min JS, Park KI, Chung MY, Lim SH, Lim KB, Kim CK (2013) Primary and secondary somatic embryogenesis in Chrysanthemum (Chrysanthemum morifolium) cv. ‘Baeksun’ and assessment of ploidy stability of somatic embryogenesis process by flow cytometry. Acta Physiol Plant. doi:10.1007/s11738-013-1328-4
Neuenschwander B, Baumann TW (1992) A novel type of somatic embryogenesis in Coffea arabica. Plant Cell Rep 10:608–612. doi:10.1007/BF00232380
Nic-Can GI, Avilez-Montalvo JR, Aviles-Montalvo RN, Márquez-López RE, Mellado-Mojica E, Galaz-Ávalos RM, Loyola-Vargas VM (2016) The relationship between stress and somatic embryogenesis. In: Loyola-Vargas VM, Ochoa-Alejo N (eds), Somatic embryogenesis: fundamental aspects and applications, Springer, Cham. doi: 10.1007/978-3-319-33705-0_25
Nosov AM (2012) Application of cell technologies for production of plant-derived bioactive substances of plant origin. Appl Biochem Microbiol 48(7):609–624
Oh MJ, Ahn MS, Jie EY, Liu JR, Min BW, Kim SW (2013) High-frequency plant regeneration from immature zygotic embryo cultures of Houttuynia cordata thunb via somatic embryogenesis. Plant Biotechnol Rep. doi:10.1007/s11816-013-0291-2
Ortiz BOC, Reyes MEP, Balch EPM (2000) Somatic embryogenesis and plant regeneration in Acacia farnesiana and A. schaffneri. In Vitro Cell Dev Biol-Plant 36:268–272. doi:10.1007/s11627-000-0049-8
Othmani A, Bayoudh C, Drira N, Marrakchi M, Trifi M (2009) Somatic embryo-genesis and plant regeneration in date palm Phoenix dactylifera L., cv. Boufeggousis significantly improved by fine chopping and partial desiccation of embryo-genic callus. Plant Cell Tiss Org 97:71–79
Pathak S, Mishra BK, Misra P et al (2012) High frequency somatic embryogenesis, regeneration and correlation of alkaloid biosynthesis with gene expression in Papaver somniferum. Plant Growth Regul 68:17–25. doi:10.1007/s10725-012-9689-z
Pedroso MC, Pais MS (1999) Direct somatic embryogenesis from leaves of Camellia japonica. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 5. Kluwer, London, pp 163–178
Peraza-Echeverria S, Herrera-Valencia VA, Kay AJ (2001) Detection of DNA methylation changes in micropropagated banana plants using methylation-sensitive amplification polymorphism (MSAP). Plant Sci 161:359–367
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci 91:5222–5226
Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A (1996) The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238
Prado M, Gonzalez M, Romo S, Herrera M (2007) Adventitious plant regeneration on leaf explants from adult male kiwifruit and AFLP analysis of genetic variation. Plant Cell Tiss Org 88:1–10
Raiesi S, Nadjafi F, Hadian J, Kanani MR, Ayyari M (2013) Autecological and phytochemical studies of Kelussia odoratissima Mozaff. An endangered ethnomedicinal plant of Iran. TBAP 3 (4) 285–294
Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O'Neal JM, Cornwell T, Pastor I, Fridlender B (2002) Plants and human health in the twenty-first century. Trends Biotechnol 20(12):522–531
Rathore NS, Rai MK, Phulwaria M, Rathore N, Shekhawat N (2014) Genetic stability in micropropagated Cleome gynandra revealed by SCoT analysis. Acta Physiol Plant 36:555–559
Razeghi L, Azizi M, Ziaratnia SM, Bagheri AR, Nemati SH (2015) Impact of hormonal combination on callus induction of Kelussia odoratissimia Mozaff. and evaluating its growth in broth. Iran J Med Aro Plants 30:6
Razeghi L, Azizi M, Ziaratnia SM, Bagheri AR, Nemati SH (2016) Evaluation in vitroculture of Kelussia odoratissima Mozaff and secondary metabolites production through suspension cultures. Pharm Innov J 5(1):74–80
Reinert J, Tazawa M, Semenoff S (1967) Nitrogen compounds as factors of the embryogenesis in vitro. Nature 216:1215–1216. doi:10.1038/2161215a0
Rybczynski JJ, Zdunczyk W (1986) Somatic embryogenesis and plantlet regeneration in the genus Secale. Theor Appl Genet 73:267–271
Sajjadi SE, Shokoohinia Y, Moayedi N (2012) Isolation and identification of ferulic acid from aerial parts of Kelussia odoratissima Mozaff. Jundishapur J Nat Pharm Prod 7(4):159–162
Sebastiani MS, Ficcadenti N (2016) In vitro plant regeneration from cotyledonary explants of Cucumis melo L. var. cantalupensis and genetic stability evaluation using RAPD analysis. Plant Cell Tiss Organ Cult 124:69–79
Shareef HJ, Khaun AM, Abdulrahman DA (2016) Improving the germination of somatic embryos in date palm Berhi cultivar in vitro. IJAAR 8 (1):17–23
Shojaei ZA, Ebrahimi A, Salimi M (2011) Chemical composition of three ecotypes of wild celery (Kelussia odoratissima). J Herbs Spices Med Plants 17(1):62–68
Slazak B, Sliwinska E, Saługa M, Ronikier M, Bujak J, Słomka A, Göransson U, Kuta E (2015) Micropropagation of Viola uliginosa (Violaceae) for endangered species conservation and for somaclonal variation-enhanced cyclotide biosynthesis. Plant Cell Tiss Org 120:179–190
Terryn N, Van Montagu M, Inzé D, Goosens A (2006) Functional genomic approaches to study and engineer secondary metabolites in plant cell cultures. In: Bogers LJ, Craker LE, Jange D (eds) Medicinal and aromatic plants. Springer, The Netherlands, pp 291–300
Vázquez-Flota FA, Monforte-González M, de Lourdes Miranda-Ham M (2016) Application of somatic embryogenesis to secondary metabolite-producing plants. In: Loyola-Vargas VM, Ochoa-Alejo N (eds), Somatic embryogenesis: fundamental aspects and applications, Springer, Cham. doi:10.1007/978-3-319-33705-0$425
Von Arlond S In: De Klerk GJ (eds) (2008) Somatic embryogenesis. In: George EF, Hall MA Plant propagation by tissue culture, 3rd edn. Springer, Dordrecht, pp 335–354
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Friters A, Pot J, Paleman J, Kuiper M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic acids res 23:4407–4414
Vroh-Bi I, Anagbogu C, Nnadi S, Tenkouano A (2011) Genomic characterization of natural and somaclonal variations in bananas (Musa spp.). Plant Mol Biol Rep 29:440–448
Wickramasuriya AM, Dunwell JM (2015) Global scale transcriptome analysis of Arabidopsis embryogenesis in vitro. BMC Genomics 16:301. doi:10.1186/s12864-015-1504-6 2015_BMCB_301_29152.
Wu YC, Hsieh C (2011) Pharmacological effects of Radix Angelica sinensis (Danggui) on cerebral in fraction. Chin Med 6:32. doi:10.1186/1749-8546-6-32
Yadav K, Aggarwal A, Singh N (2013) Evaluation of genetic fidelity among micropropagated plants of Gloriosa superba L. using DNA-based markers—a potential medicinal plant. Fitoterapia 89:265–270
Yantcheva A, Vlahova M, Antanassov A (1998) Direct somatic embryogenesis and plant regeneration of carnation (D ianthus caryophyllus L.). Plant Cell Rep 18:148–153
Zheng Q, Perry S (2014) Alterations in the transcriptome of soybean in response to enhanced somatic embryogenesis promoted by orthologs of AGAMOUS-like 15 and AGAMOUS-like 18. Plant Physiol 164:1365–1377. doi:10.1104/pp.113.234062
Acknowledgements
The project was supported a grant from the Agricultural Biotechnology Research Institute of Iran.
Author information
Authors and Affiliations
Contributions
ME is the project leader and this manuscript is a part of the results of a research project on Kelussiaodoratissima carried out at the ABRII-Isfahan Branch. AM and RA cooperated in the research.
Corresponding author
Additional information
Communicated by Sergio J Ochatt.
Rights and permissions
About this article
Cite this article
Ebrahimi, M., Mokhtari, A. & Amirian, R. A highly efficient method for somatic embryogenesis of Kelussia odorotissima Mozaff., an endangered medicinal plant. Plant Cell Tiss Organ Cult 132, 99–110 (2018). https://doi.org/10.1007/s11240-017-1314-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1314-4