Abstract
Cynanchum wilfordii is a traditional Chinese herb and has various pharmacological effects. However, its raw plant material is insufficient to meet the market demand. Adventitious roots (ARs) are a novel alternative material for producing plant products. Herein, C. wilfordii ARs were cultured in 5-L bioreactors to produce bioactive compounds and the effects of salt strength and sucrose concentration were investigated in this study. Furthermore, the kinetic study was implemented for confirming a suitable culture period. Finally, the culture efficiency of different size bioreactors was compared. The full salt strength of Murashige and Skoog medium supplemented with 30 g L−1 sucrose was most favorable for AR biomass and the production of flavonoids, ρ-hydroxyacetophenone, 2,5-dihydroxyacetophenone, and total acetophenone derivatives, whereas the media with 50 and 70 g L−1 sucrose were optimal for the production of 2,4-dihydroxyacetophenone and total polysaccharides, respectively. The accumulation of AR biomass and three acetophenone derivatives peaked on day 25, but those of total polysaccharide and flavonoids peaked on day 30, indicating that the selection of culture period should depend on the target compound to be produced. Among 5-, 10-, and 20 L-bioreactors, the 20-L bioreactor exhibited good culture efficiency. The findings suggest that bioactive compounds of C. wilfordii can be mass produced through AR culture in an established bioreactor system, and the produced ARs can be used as promising raw material in the production of plant products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cynanchum wilfordii, belonging to the Asclepiadaceae family, is a traditional Chinese herb that is widely distributed in China, Korea, and Japan. Its roots are used as herbal medicine for treating impotency, neurasthenia, lumbago, and abscesses (Tsiang and Li 1977) and possess antineoplastic, immunomodulatory, anti-inflammatory, hepatoprotective, vasodilating, and antioxidant activities (Lee et al. 1996; Chen et al. 2019). Acetophenones, polysaccharides, C21 steroidal glycosides, and flavonoids are the major bioactive compounds of this plant and directly associated with its pharmacological effects (Jiang et al. 2011; Cho et al. 2017; Chen et al. 2019). C. wilfordii has been registered as a New Medicinal Herbal in China (Xiao 2002) and the annual sales in Korea exceeded $100 million in 2014 (Jang et al.2017). However, at present, the wild resource of C. wilfordii has decreased because of the uncontrolled collection (Wang et al. 2012); the artificial cultivation cannot satisfy the rapid growing market demands because of the low quantity and quality of the plant material caused by long cultivation period, inefficient cultivation technology, and unstable environmental conditions (Ahn et al. 2018). Therefore, a novel method for the high production of plant materials has received considerable attention.
Cell and organ cultures have been well acknowledged as an important method for biosynthesizing phytochemicals in limited time and space (Jeong et al. 2009). Among these techniques, adventitious root (AR) culture in bioreactors has tremendous commercial potentialities for high proliferation rate and stable production of secondary metabolites (Jiang et al. 2015). Bioreactor AR culture systems have recently been established in various medicinal plant species for the production of bioactive compounds, such as Echinacea angustifolia for caffeic acid derivatives (Cui et al. 2013), Panax ginseng for ginsenosides (Sivakumar et al. 2005), Morinda citrifolia for anthraquinone (Baque et al. 2012), and Oplopanax elatus for polysaccharides (Jiang et al. 2015). The findings indicate that bioreactor culture technology can be used for the production of bioactive compounds. Different strategies including culture medium manipulation, environmental conditions, and elicitation have been applied for enhancing the production of secondary metabolites during AR culture (Baldi et al. 2009; Zhang et al. 2012). The salt strength and initial sucrose concentrations of the culture medium are the most important physicochemical factors influencing the culture efficiency. Therefore, efforts have been made to modify the levels of these factors in their culture systems (Wu et al. 2006, 2018; Rajesh et al. 2014).
As mentioned above, plant AR culture technology has been applied in the production of useful metabolites in numerous plant species (Sivakumar et al. 2005; Cui et al. 2013; Jiang et al. 2015). Unfortunately, AR culture has less been used on C. wilfordii. To date, only one study used C. wilfordii ARs in small vessels (flasks) and investigated the effect of indole butyric acid (IBA) concentration (Ahn et al. 2018), and no reports are available on bioreactor AR culture for the large-scale production of bioactive compounds. Thus, the present study used 5-L balloon-type airlift bioreactors to investigate the effects of salt strength and sucrose concentration of culture medium on the accumulation of AR biomass and several bioactive compounds (polysaccharides, flavonoids, and acetophenone derivatives) to improve culture efficiency. In addition, a kinetic study was implemented to select a suitable culture period. Finally, this study used 10- and 20-L bioreactors to culture ARs and compared the culture efficiency with the 5-L bioreactor to provide a reference for the pilot-scale production of C. wilfordii ARs in the future.
Materials and Methods
AR Induction and Maintenance
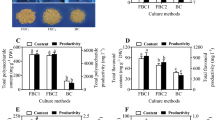
The C. wilfordii plant was collected from mountainous areas in Jinhu (39°4′ N, 121°98′ E), Dalian, China, and were identified by Professor Mei-Lan Lian of Agricultural College, Yanbian University. The leaves of C. wilfordii were cut into 1 cm × 1 cm size after sterilization and inoculated in petri dishes containing 0.75 × Murashige and Skong (MS) medium (Murashige and Skoog 1962) supplemented with 1 mg L−1 IBA (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China), 30 g L−1 sucrose, and 2.3 g L−1gelrite (pH 5.8) to induce ARs (Fig. 1A), which the medium was referred to that of Ahn et al. (2018) with slight modification. After 5 weeks, the induced ARs were cultured in flasks filled with 0.75 × MS medium supplemented with 1 mg L−1 IBA and 50 g L−1 sucrose (pH 5.8) at 100 rpm (Fig. 1B). All cultures were maintained at 25 ± 2 °C under the dark. After 20 days of flask culture, the ARs (Fig. 1C) were used in the experiments.
Experimental Design
The 5-L balloon-type airlift bioreactors with 4-L working volume were used in three sets of experiments, including the effects of MS medium salt strength and sucrose concentration of culture medium, and kinetic study. Each bioreactor was inoculated with 2.5 g L−1 of 16-day-old fresh ARs (approximately 1-cm length). In the first experiment, the effect of MS medium salt strength was investigated. The bioreactor was added with different salt strengths of MS medium (0.5 × , 0.75 × , 1 × , and 1.5 × MS) supplemented with 1 mg L−1 IBA and 30 g L−1 sucrose. The medium pH was adjusted to 5.8. In the second experiment, 1 × MS medium was selected according to the result of MS medium strength experiment, and the effect of sucrose concentrations was examined. The sucrose concentrations were adjusted to 10, 30, 50, and 70 g L−1 and added to MS medium supplemented with 1 mg L−1 IBA. The medium pH was adjusted to 5.8. In both sets of experiments, the AR biomass (fresh weight (FW) and dry weight (DW)) and contents of bioactive compounds (polysaccharides, flavonoids, and acetophenone derivatives including ρ-hydroxyacetophenone, 2,5-dihydroxyacetophenone, and 2,4-dihydroxyacetophenone) was determined after 25 d of bioreactor culture; the bioactive compound productivities were calculated (DW × content) and used as the evaluating indexes. Furthermore, a kinetic study was performed to confirm a suitable culture period. The ARs were cultured in MS medium supplemented with 1 mg L−1 IBA and 30 g L−1 sucrose (pH 5.8). The ARs were then harvested, and the culture medium was collected at 5-d intervals until day 40. The biomass and bioactive compound contents of each AR sample were determined, and the values of electrical conductivity (EC) and contents of soluble sugars in each medium sample were also measured.
Finally, to explore the feasibility of AR growth and biosynthesis in larger bioreactors, 10-L (8-L working volume) and 20-L (16-L working volume) bioreactors were used to culture ARs under the same culture conditions as the 5-L bioreactor culture, and the culture efficiency was compared among different bioreactor sizes (5, 10, and 20 L). Each bioreactor was inoculated with 2.5 g L−1 (FW) of 16-d-old ARs, and MS medium supplemented with 1 mg L−1 IBA and 30 g L−1 sucrose was added according to their working volume. After 25 days of culture, the AR biomass and contents of bioactive compounds were determined.
All bioreactors were aerated at 0.1 vvm (air volume/culture volume/min) and maintained at 25 ± 2 °C under the dark. Three independent experiments were repeated in the same conditions during each experiment.
Biomass Determination
The harvested ARs were rinsed twice with tap water and the FW was measured after draining the surface water. Then, the ARs were dried in a drying oven (Tianjin North China Experimental Instrument Co., Ltd., Tianjin, China) at 60 °C for 48 h to achieve a constant weight, and AR DW was recorded. AR growth ratio was calculated as follows:
Determination of Total Polysaccharide and Total Flavonoid Contents
Total polysaccharides were extracted using the method of Li et al. (1990). Briefly, dried ARs (0.1 g) were soaked in 90% (v/v) ethanol for 6 h at room temperature to adequately remove the interferential compounds including monosaccharides, oligosaccharides, and glycosides. The filter residue was added with 20 mL of distilled water and extracted with ultrasonic treatment (THC; Tianhua Ultrasonic Electronic Instrument Co., Ltd., Jining, China) for 30 min, and the supernatant was collected after centrifugation. Polysaccharides were quantitatively assessed with a UV–visible spectrophotometer (UV-2600; Shimadzu Corporation, Kyoto, Japan) at 490 nm according to a phenol–sulfuric acid assay with glucose (Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China) as the reference standard.
To determine the content of flavonoids, the dry ARs (0.1 g) were soaked in 10 mL of 70% (v/v) ethanol for 3 h at 60 °C and filtered through a filter paper; the filtrate was added with 70% (v/v) ethanol to a constant volume of 25 mL, and the absorbance was recorded on a spectrophotometer (UV-2600; Shimadzu Corporation, Kyoto, Japan) at 510 nm by using the aluminum chloride colorimetric method (Cui et al. 2010b); rutin (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) was used as the reference standard for the preparation of the calibration curve.
Determination of the Contents of Acetophenone Derivatives
Acetophenone derivatives (ρ-hydroxyacetophenone, 2,5-dihydroxyacetophenone, and 2,4-dihydroxyacetophenone) were measured in accordance with the methods of Sun et al. (2013) with slight modifications. In brief, 0.2 g of dry samples was soaked in 10 mL of 70% ethanol with ultrasonic extraction (THC; Tianhua Ultrasonic Electronic Instrument Co., Ltd., Jining, China) for 1 h. After filtering with a filter paper (Hangzhou Whatman-Xinhua Filter Paper Co., Ltd., Hangzhou, China), the filtrate and residue were collected, and the latter was extracted using the same extraction procedure. All filtrates were mixed and used in high-performance liquid chromatography (HPLC) analysis after filtering through a 0.22-µm membrane filter. The HPLC system (Waters Corporation, Milford, MA) was equipped with an XTerra RP 18 column (150 × 3 mm, 3.0 μM; Waters Corporation). The mobile phases were water (A) and acetonitrile (Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China) (B), and the flow rate was 1.0 mL min−1. The gradient elution for the separation of acetophenone fractions was modified as follows: initial 10% B for 10 min, 50% B for the next 10 min, recycle to initial condition for 10 min. Acetophenone derivatives were detected at 330 nm (Fig. S1). The total content of acetophenones was the sum of ρ-hydroxyacetophenone, 2,5-dihydroxyacetophenone, and 2,4-dihydroxyacetophenone contents.
Determination of EC in Culture Medium
The collected medium samples were filtered through a 0.2-µm membrane filter prior to the measurement of the EC. The EC value was measured by using a conductivity meter (Leici DDS-30; Shanghai Precision & Scientific Instrument Co., Ltd., Shanghai, China).
Determination of Soluble Sugars in Culture Medium
The sucrose, glucose, and fructose concentrations in culture medium were determined using the method described by Jeong et al. (2006) with slight modifications. The medium samples were filtered through a 0.2-µm filter and diluted 20-fold. An HPLC system with high-performance carbohydrate column (300 × 7.8 mm; Waters Corporation, Milford, MA, USA) and a refractive index detector (Refract meter Differential; Waters Corporation) was used to measure the contents of soluble sugars. Then, 75% acetonitrile was used as the mobile phase with a flow rate of 1.0 mL min−1.
Statistical Analysis
Three biological replicates were performed in all experiments, data were the mean values from three replicates, and the results were presented as mean ± standard error. To identify the significant differences, Duncan’s multiple range test (Duncan 1955) and Student’s t-test (Student 1908) by using SPSS17.0 program (IBM Institute, Armonk, NY) were conducted to analyze the mean values. A probability of p < 0.05 was considered significant.
Results and Discussion
In this study, ARs were induced in leaves of C. wilfordii plants and the proliferated ARs were cultured in bioreactors to investigate several factors affecting the accumulation of AR biomass and bioactive compounds for mass and rapid production of useful metabolites of C. wilfordii.
Effect of Medium Salt Strength on AR Biomass and Bioactive Compound Accumulation
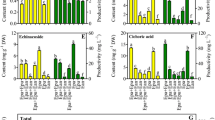
The medium salt strength is a critical determinant in controlling AR biomass and secondary metabolite accumulation (Cui et al. 2013). Table 1 shows that C. wilfordii AR biomass increased with increasing salt strengths from 0.5 × to 1 × and reached the maximum at 1 × MS. At this salt strength, the AR FW or DW was more than 1.4-fold higher than that of the lowest salt strength group (0.5 × MS), and the AR growth ratio reached up to 41.8. However, MS medium with salt strength higher than 1 × was not suitable for AR biomass accumulation. The MS medium salt strength also affected bioactive compound accumulation (Fig. 2); the effects on polysaccharide and flavonoid contents had a similar pattern. That is, the contents of total polysaccharides, flavonoids and acetophenone derivatives (ρ-hydroxyacetophenone, 2,5-dihydroxyacetophenone, and 2,4-dihydroxyacetophenone) reached the maximum at 1 × MS (37.1, 16.8, and 2.2 mg g−1 DW, respectively).
Effect of medium salt strength on bioactive compound accumulation of adventitious roots of Cynanchum wilfordii after 25 days culture in bioreactors. Each medium was supplied 1 mg L−1 IBA and 50 g L−1 sucrose. Data are the mean ± standard error (n = 3). The different letters within the same color column indicate significant difference by Duncan’s multiple range test at p < 0.05.
Studies have indicated that osmotic stress and lipid peroxidation initiated by high salt strength of medium exacerbate water deprivation and toxicity in cells, thereby inhibiting AR growth and biosynthesis; on the contrary, the low salt strength of medium results in nutrient deficiency, which impedes the normal biological activities of cultures (Lee and Paek 2012; Cui et al. 2013). For instance, Baque et al. (2010) indicated that AR biomass and anthraquinone accumulation of Morinda citrifolia were elevated at 0.25 × MS. However, Echinacea angustifolia AR culture efficiency for phenolic production increased at 0.5 × MS (Wu et al. 2006). Cui et al. (2010b) found that the AR biomass of Hypericum perforatum was enhanced at 0.5 × or 1 × MS, and phenolic and flavonoid accumulation was promoted at 0.25 × or 0.5 × MS. These findings indicate that the optimal salt strength of an AR culture system varies among plant species and for the production of different target compounds. To date, reports on C. wilfordii AR culture are limited. Only one study used 0.5 × MS medium to culture C. wilfordii AR in a small vessel (flask) (Ahn et al. 2018). Unfortunately, the effect of salt strength has not been investigated previously. The present study found that the maximum AR biomass of C. wilfordii and the synthesis of bioactive compounds, including polysaccharide, flavonoids, and acetophenone derivatives, were determined at 1 × MS, indicating that the standard salt strength of MS medium was appropriate for the AR bioreactor culture of C. wilfordii.
Effect of Sucrose Concentration on AR Biomass and Bioactive Compound Accumulation
AR FW, DW, and growth ratio reached the maximum values at 30 g L−1 sucrose and decreased when the sucrose concentrations were higher than 30 g L−1 (Table 2). The effect of sucrose concentration on the contents of different bioactive compounds varied. Figure 3 shows that the polysaccharide content increased with increasing sucrose concentrations and peaked at 70 g L−1 sucrose (93.08 mg g−1 DW). However, the flavonoid and total acetophenone derivatives contents both reached the highest values at 30 g L−1 sucrose (17.18 and 2.73 mg g−1 DW). The contents of three acetophenone derivatives were differently affected by sucrose concentration. That is, the highest contents of ρ-hydroxyacetophenone (0.98 mg g−1 DW) and 2,5-dihydroxyacetophenone (0.76 mg g−1 DW) were observed at 30 g L−1 sucrose, but the highest content of 2,4-dihydroxyacetophenone (1.22 mg g−1 DW) was found at 50 g L−1 sucrose. This result indicated that the effects of sucrose concentration on AR biomass and contents of different bioactive compounds was not similar. Furthermore, the highest polysaccharide productivity was found at 50 and 70 g L−1 sucrose. However, no significant difference (p < 0.05) was found between the two sucrose concentrations. The productivities of flavonoid, ρ-hydroxyacetophenone, 2,5-dihydroxyacetophenone, and total acetophenone derivatives, reached the highest values at 30 g L−1 sucrose, whereas that of 2,4-dihydroxyacetophenone was found at 50 g L−1 sucrose.
Effect of sucrose concentration in full strength MS medium on bioactive compound accumulation of adventitious roots of Cynanchum wilfordii after 25 days culture in bioreactors. Data are the mean ± standard error (n = 3). The different letters within the same color column indicate significant difference by Duncan’s multiple range test at p < 0.05.
Sucrose is the most used carbon source in plant tissue culture. It plays an essential role in the regulation of important metabolic activities and is a signaling molecule involved in plant development (Tognetti et al. 2013). However, a high sucrose concentration can induce osmotic stress, leading to the production of a large amount of reactive oxygen species (Al-Khayri and Al-Bahrany 2002) and cell cycle arrest, which retard culture growth (Cui et al. 2013). By contrast, a low sucrose concentration can induce poor growth and metabolic synthesis of the culture, which might be associated with the limitation of nutrient uptake due to inadequate energy and osmotic pressure (Wu et al. 2018). At an appropriate sucrose concentration, metabolite accumulation can be promoted, which may be attributed to the changes of carbon metabolic pathway from catabolism to anabolism (He et al. 2007).
The amount of sucrose required by various plant cells or organs in culture medium varies. Thus, different sucrose concentrations are used in each culture system to achieve a high culture efficiency (Jiang et al. 2015). For example, Yin et al. (2013) indicated that MS culture medium supplemented with 40 g L−1 sucrose is favorable for Pseudostellaria heterophylla AR biomass and saponin or polysaccharide accumulation. Cui et al. (2010a) demonstrated that the maximum values of AR fresh and dry weight of Hypericum perforatum were determined at 30 g L−1 sucrose, whereas those of chlorogenic acid and total hypericin contents at 70 and 90 g L−1 sucrose, respectively. Rajesh et al. (2014) revealed that 20 g L−1 sucrose was beneficial for Podophyllum hexandrum AR biomass, but podophyllotoxin accumulation was enhanced at 60 g L−1 sucrose. In the present study, 30 g L−1 sucrose had positive effects on AR biomass and accumulation of flavonoids and acetophenone derivatives (ρ-hydroxyacetophenone and 2,5-dihydroxyacetophenone), 50 g L−1 sucrose was beneficial for 2,4-dihydroxyacetophenone accumulation, and 70 g L−1 sucrose was most suitable for polysaccharide accumulation. The findings suggest that the selection of sucrose concentration should depend on the target compound production in the culture system. In addition, plant metabolic synthesis is a complex process. The detailed mechanism behind the effect of sucrose concentration in culture medium on compound biosynthesis is unknown. Thus, the mechanism regulating biosynthesis needs to be determined in future studies to maximize production.
Kinetics of AR Growth and Bioactive Compound Accumulation
The changes of AR biomass and bioactive compound accumulation and medium were investigated to confirm a suitable culture period. Figure 4 shows that AR FW and DW had a similar change pattern and reached their maximum values on day 25 of culture. AR biomass exerted a typical lag phase from initial culture today 5, an exponential phase from day 5 today 25, and a slight decrease in the following days.
The changes of polysaccharide, flavonoid, and acetophenone derivative contents exhibited a similar pattern (Fig. 5). From initial culture to 10 d of culture, the bioactive compound contents decreased with the extension of culture days, indicating that the catabolism of the inoculated 20-d-old roots dominated in these culture days, which provides a basis for the further proliferation and biomass accumulation of ARs. By contrast, after 10 d of culture, bioactive compound contents began to increase. The contents of polysaccharides and flavonoids peaked on day 30, and those of the three acetophenone derivatives (ρ-hydroxyacetophenone, 2,5-dihydroxyacetophenone, and 2,4-dihydroxyacetophenon), peaked on day 25. The highest productivities of polysaccharides (502.35 mg L−1) and flavonoids (206.37 mg L−1) were obtained on day 30, whereas those of ρ-hydroxyacetophenone (6.56 mg L−1), 2,5-dihydroxyacetophenone (8.6 mg L−1), and 2,4-dihydroxyacetophenone (15.34 mg L−1) were determined on day 25, at which day the total acetophenone derivative productivity reached 30.5 mg L−1.
Changes of bioactive compound contents and productivity in adventitious roots of Cynanchum wilfordii during culture period in bioreactor. Culture medium was full strength MS medium supplemented with 1 mg L−1 IBA and 30 g L−1 sucrose. Data are the mean ± standard error (n = 3). The corresponding color letters in each color curve indicate significant difference by Duncan’s multiple range test at p < 0.05.
In general, the EC value and sugar concentration in culture medium exhibited a close inverse correlation with the culture’s biomass, and thus the biomass can be estimated depending on information from the EC and sugar sensor attached to a bioreactor (McDonald and Jackman 1989; Ryu et al. 1990; Cui et al. 2011). In this study, the changes of EC and sugar concentration were examined to prove the above viewpoint for the industrial production of C. wilfordii ARs in the future. Figure 6A shows that the EC gradually decreased from initial culture to day 5, sharply decreased from day 5 to day 25 and remained stable in the following days. The EC value from 4.83 mS cm−1 at initial culture decreased to 0.94 mS cm−1 at the end of culture.
Changes of electrical conductivity (A) and sugar concentration (B) in culture medium, correlation of EC (C) and sugar concentration with dry weight (D), respectively. Data are the mean ± standard error (n = 3). The corresponding color letters in each color curve indicate significant difference by Duncan’s multiple range test at p < 0.05.
The consumption of sugar in culture medium is shown in Fig. 6B. The sucrose concentration rapidly decreased before 10 d of culture, but fructose and glucose concentrations increased. This was attributed to the decomposition of sucrose to fructose and glucose by the sucrose invertase secreted from the inoculated ARs (Jeong et al. 2009). The reduction of sucrose concentration continued until day 15, at which day the sucrose was not identified. From day 10 to day 20 of culture (the days of AR vigorous growth), the fructose and glucose concentrations sharply declined because of the large uptake by ARs, followed by a gradual decrease until both sugars were depleted on day 40. The total sugar concentration sharply decreased from day 5 to day 20 and gradually decreased in the following days until reaching zero on day 40.
The correlation of EC and AR DW and that of total sugar concentration and AR DW were analyzed, and a close negative correlation was found (Fig. 6C, D).This finding indicated that AR biomass can be estimated by the EC value or total sugar concentration in culture medium, which is consistent with the viewpoints of Ryu et al. (1990) and Cui et al. (2011).
Comparison of AR Culture Efficiency in Different Sizes of Bioreactors
The 10- and 20-L bioreactors were used to culture ARs, and their culture efficiency was compared with that of the 5-L bioreactor for further large-scale AR bioreactor culture. Table 3 shows the increase of AR FW and DW in the larger size bioreactors (10 L and 20 L), and the AR growth ratio reached approximately 50 in the 20-L bioreactor, which was significantly (p < 0.05) higher than that of the 5-L bioreactor. By contract, the contents of polysaccharides, flavonoids, ρ-hydroxyacetophenone, and 2,5-dihydroxyacetophenone were not affected by the bioreactor size. However, the contents of 2,4-dihydroxyacetophenoneand and total acetophenone derivatives in the 10-L and 20-L bioreactors were significantly (p < 0.05 and p < 0.01) higher than those in the 5-L bioreactor (Table 4). The finding indicated that the experimental results at the laboratory level can be used as a reference for controlling the culture conditions in the establishment of an optimal system for a large-scale bioreactor culture. Furthermore, the interesting results of increased biomass and acetophenone derivative content in the larger bioreactors (10 L and 20 L) encourage implementing future studies from various aspects, such as physiology and micromorphology, to elucidate the mechanism of C. wilfordii AR bioreactor culture. At present, the ARs of many plant species have been successfully cultured in large-scale bioreactors for commercial applications (Cui et al. 2013; Yin et al. 2014; Wu et al. 2017). In the present study, the possibility of scaling up C. wilfordii AR culture was clarified, which is helpful for further culturing C. wilfordii ARs in industrial production.
In addition, this study found that the contents of the main bioactive compounds of C. wilfordii in the cultured ARs were higher than those in the field-grown roots. For instance, the contents of ρ-hydroxyacetophenone, 2,5-dihydroxyacetophenone, and 2,4-dihydroxyacetophenone were 0.33, 0.1, and 1.3 mg g−1 in the natural roots (Li et al. 2013), which were 0.7-, 6.3-, and 1.95-fold lower than ARs, respectively. Consequently, the AR culture is the promising approach for mass production of useful metabolites of C. wilfordii.
Conclusion
Medium salt strength and sucrose concentration obviously affected AR biomass and bioactive compound accumulation of C. wilfordii. The full-strength MS medium was most beneficial for increasing AR biomass and contents of polysaccharides, flavonoids, and acetophenone derivatives (ρ-hydroxyacetophenone, 2,5-dihydroxyacetophenone, and 2,4-dihydroxyacetophenone) compared with 0.5 × , 0.75 × and 1.5 × salt strengths. Among the sucrose concentrations (10 to 70 g L−1), 30 g L−1 sucrose increased AR biomass and flavonoid, ρ-hydroxyacetophenone, and 2,5-dihydroxyacetophenone accumulation, but 50 and 70 g L−1 sucrose was better for 2,4-dihydroxyacetophenone and polysaccharide accumulation, respectively. The kinetic study indicated that the culture period of 25 days was the most favorable for AR biomass and acetophenone derivative accumulation, whereas that of 30 d was beneficial for polysaccharide and flavonoid accumulation. The comparison study revealed that a large bioreactor size (5 to 20 L) is beneficial for the biomass and bioactive compound accumulation, indicating good applicability of the AR culture system established in a 5-L bioreactor. The findings of the present study suggest that AR bioreactor culture is an efficient route for the production of C. wilfordii raw material, which contains various bioactive compounds.
References
Ahn MS, So EJ, Jie EY, Choi SY, Park SU, Moon BC, Kang YM, Min SR, Kim SW (2018) Metabolic comparison between standard medicinal parts and their adventitious roots of Cynanchum wilfordii (Maxim.) Hemsl. using FT-IR spectroscopy after IBA and elicitor treatment. J Plant Biotechnol 45:250–256
Al-Khayri JM, Al-Bahrany AM (2002) Callus growth and proline accumulation in response to sorbitol and sucrose-induced osmotic stress in rice. Biol Plant 45:609–611
Baldi A, Srivastava AK, Bisaria VS (2009) Fungal elicitors for enhanced production of secondary metabolites in plant cell suspension cultures. In: Varma A, Kharkwal AC (eds) Symbiotic fungi, Soil biology 18. Springer-Verlag, Berlin, Heidelberg, pp 373–380
Baque MA, Elgirban A, Lee EJ, Paek KY (2012) Sucrose regulated enhanced induction of anthraquinone, phenolics, flavonoids biosynthesis and activities of antioxidant enzymes in adventitious root suspension cultures of Morinda citrifolia (L.). Acta Physiol Plant 34:405–415
Baque MA, Lee EJ, Paek KY (2010) Medium salt strength induced changes in growth, physiology and secondary metabolite content in adventitious roots of Morinda citrifolia: the role of antioxidant enzymes and phenylalanine ammonia lyase. Plant Cell Rep 29:685–694
Chen WH, Zhang ZZ, Ban YF, Rahman K, Ye BZ, Sun XL, Tan HY, Zheng XH, Liu HY, Xu LC, Yan B, Han T (2019) Cynanchum bungei Decne and its two related species for “Baishouwu”: A review on traditional uses, phytochemistry, and pharmacological activities. J Ethnopharmacol 243:112110
Cho CW, Ahn S, Lim TG, Hong HD, Rhee YK, Yang DC, Jang M (2017) Cynanchum wilfordii polysaccharides suppress dextran sulfate sodium-induced acute colitis in mice and the production of inflammatory mediators from macrophages. Mediat Inflamm 2017:3859856
Cui HY, Baque MA, Lee EJ, Paek KY (2013) Scale-up of adventitious root cultures of Echinacea angustifolia in a pilot-scale bioreactor for the production of biomass and caffeic acid derivatives. Plant Biotechnol Rep 7:297–308
Cui XH, Chakrabarty D, Lee EJ, Paek KY (2010b) Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresour Technol 101:4708–4716
Cui XH, Murthy HN, Jin YX, Yim YH, Kim JY, Paek KY (2011) Production of adventitious root biomass and secondary metabolites of Hypericum perforatum L. in a balloon type airlift reactor. Bioresour Technol 102:10072–10079
Cui XH, Murthy HN, Wu CH, Paek KY (2010a) Sucrose-induced osmotic stress affects biomass, metabolite, and antioxidant levels in root suspension cultures of Hypericum perforatum L. Plant Cell Tiss Org 103:7–14
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
He TG, Yang LT, Li YR, Wang CQ, Su J (2007) Effect of sucrose on growth of protocorms of Dendrobium candidum and accumulation of polysaccharide. J Anhui Agric Sci 35:3817–3819
Jang HS, Jeong B, Choi SY, Jang GH, Park KC, Kwon YC, Yang HJ (2017) Conduritol F, the discriminant marker between C. wilfordii and C. auriculatum by 1HNMR spectroscopy. Microchem J 135:153–157
Jeong CS, Chakrabarty D, Hahn EJ, Lee HL, Paek KY (2006) Effects of oxygen, carbon dioxide and ethylene on growth and bioactive compound production in bioreactor culture of ginseng adventitious roots. Biochem Eng J 27:252–263
Jeong JA, Wu CH, Murthy HN, Hahn EJ, Paek KY (2009) Application of an airlift bioreactor system for the production of adventitious root biomass and caffeic acid derivatives of Echinacea purpurea. Biotechnol Bioproc E 14:91–98
Jiang Y, Choi HG, Li Y, Park YM, Lee JH, Kim DH, Lee JH, Son JK, Na M, Lee SH (2011) Chemical constituents of Cynanchum wilfordii and the chemotaxonomy of two species of the family Asclepiadacease, C. wilfordii and C. auriculatum. Arch Pharm Res 34:2021–2027
Jiang YJ, Piao XC, Liu JS, Jiang J, Lian ZX, Kim MJ, Lian ML (2015) Bioactive compound production by adventitious root culture of Oplopanax elatus in balloon-type airlift bioreactor systems and bioactivity property. Plant Cell Tiss Org 123:413–425
Lee DU, Shin US, Huh K (1996) Inhibitory effects of gagaminine, a steroidal alkaloid from Cynanchum wilfordi, on lipid peroxidation and aldehyde oxidase activity. Planta Med 62:485–487
Lee EJ, Paek KY (2012) Enhanced productivity of biomass and bioactive compounds through bioreactor cultures of Eleutherococcus koreanum Nakai adventitious roots affected by medium salt strength. Ind Crop Prod 36:460–465
Li M, Hirata Y, Xu GJ, Niwa M (1990) Determination of polysaccharide contents in the drugs of Dendrobium. Chin Tradi Herb Drugs 21:10–12
Li Y, Piao D, Zhang H, Woo MH, Lee JH, Moon DC, Lee SH, Chang HW (2013) Son JK (2013) Quality assessment and discrimination of the roots of Cynanchum auriculatum and Cynanchum wilfordii by HPLC–UV analysis. Arch Pharm Res 36:335–344
McDonald KA, Jackman AP (1989) Bioreactor studies of growth and nutrient utilization in alfalfa suspension cultures. Plant Cell Rep 8:455–458
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Rajesh M, Sivanandhan G, Arun M, Vasudevan V, Theboral J, Girija S, Manickavasagam M, Selvaraj N, Ganapathi A (2014) Factors influencing podophyllotoxin production in adventitious root culture of Podophyllum hexandrum Royle. Acta Physiol Plant 36:1009–1021
Ryu DDY, Lee SO, Romani RJ (1990) Determination of growth rate for plant cell cultures: comparative studies. Biotechnol Bioeng 35:305–311
Sivakumar G, Yu KW, Paek KY (2005) Production of biomass and ginsenosides from adventitious roots of Panax ginseng in Bioreactor Cultures. Eng Life Sci 5:333–342
Student (1908) The probable error of a mean. Biometrika 6:1–25
Sun Y, Liu Z, Wang J, Yang S, Li B, Xu N (2013) Aqueous ionic liquid based ultrasonic assisted extraction of four acetophenones from the Chinese medicinal plant Cynanchum bungei Decne. Ultrason Sonochem 20:180–186
Tognetti JA, Horacio P, Martinez-Noel G (2013) Sucrose signaling in plants: a world yet to be explored. Plant Signal Behav e23316
Tsiang Y, Li P (1977) Asclepiadaceae. In: Editorial committee of flora of Chinese academy of sciences (ed.) Flora of China, vol 63. Science Press, Beijing, pp 309
Wang QQ, Li DX, Gu QY, Xu N, Jiang CY (2012) Study on clone establishment from tender stems of Cynanchum wilfordii. Jiangxi Sci 30:603–606
Wu CH, An D, Sun LN, Wang M, Chang GN, Zhao CY, Lian ML (2017) A novel co-culture system of adventitious roots of Echinacea species in bioreactors for high production of bioactive compounds. Plant Cell, Tiss Org 130:301–311
Wu CH, Dewir YH, Hahn EJ, Paek KY (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol 49:193
Wu CH, Tang J, Jin ZX, Wang M, Liu ZQ, Huang T, Lian ML (2018) Optimizing co-culture conditions of adventitious roots of Echinacea pallida and Echinacea purpurea in air-lift bioreactor systems. Biochem Eng J 132:206–216
Xiao PG (2002) New Chinese Medicinal Herbal. Chemical Industry Press, Beijing, China, p 525
Yin SS, Gao WY, Liang YY, Wang J, Liu H, Wei CL, Zuo BM (2013) Influence of sucrose concentration and phosphate source on biomass and metabolite accumulation in adventitious roots of Pseudostellaria heterophylla. Acta Physiol Plant 35:1579–1585
Yin SS, Zhang Y, Gao WY, Wang J, Man S, Liu H (2014) Effects of nitrogen source and phosphate concentration on biomass and metabolites accumulation in adventitious root culture of Glycyrrhiza uralensis Fisch. Acta Physiol Plant 36:915–921
Zhang J, Gao WY, Wang J, Li XL (2012) Effects of sucrose concentration and exogenous hormones on growth and periplocin accumulation in adventitious roots of Periploca sepium Bunge. Acta Physiol Plant 34:1345–1351
Funding
This research was supported by the National Natural Science Foundation of China (31370388).
Author information
Authors and Affiliations
Contributions
MW and CHW designed and conducted experiments; MW maintained plant materials; DA conducted data analysis; GL, WW, and XSZ detected bioactive compound contents; and MLL wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Wang, M., An, D. et al. Establishment of adventitious root culture system of Cynanchum wilfordii in air-lift bioreactors for the efficient production of bioactive compounds. In Vitro Cell.Dev.Biol.-Plant 59, 216–226 (2023). https://doi.org/10.1007/s11627-022-10325-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-022-10325-1