Abstract
We investigated the influence of ammonia/nitrate ratio and phosphate concentration on biomass and accumulation of metabolites in adventitious roots of Glycyrrhiza uralensis Fisch. A NH4 +/NO3 − ratio of 10:20 was optimal for the production of biomass (0.30 g dry weight) as well as polysaccharide (18.98 mg g−1) and glycyrrhetinic acid (0.31 mg g−1). The content of glycyrrhizic acid (0.47 mg g−1) and flavonoid (8.11 mg g−1) reached the optimum at an ammonia/nitrate ratio of 15:15 and 20:10, respectively. In case of phosphate concentration, a higher growth rate (9.18) and content of polysaccharide (15.66 mg g−1) was obtained at 1.25 mM phosphate concentration. However, 0.625 mM phosphate was favorable for the content of flavonoid (7.54 mg g−1) and glycyrrhizic acid (0.57 mg g−1). The content of glycyrrhetinic acid (0.32 mg g−1) reached the peak when treated with 0.3125 mM phosphate. A scale-up culture of adventitious roots was established using a balloon-type bubble bioreactor (BTBB). Maximum growth rates of 25.95- and 22.44-fold were obtained in 3 and 5 L BTBBs, respectively, which was higher than that in 0.5 L shake flask (18.36). The contents of flavonoid (7.60 mg g−1) and polysaccharide (15.09 mg g−1) reached the peak in 5 L and 3 L BTBB, respectively. The contents of glycyrrhizic and glycyrrhetinic acid were a little higher than that in BTBBs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glycyrrhiza uralensis Fisch has been widely used for over 3,000 years as a traditional oriental herb (Zhang et al. 2009). In China, it was used in a large number of traditional Chinese medicinal prescriptions for the treatment of cancer, hepatitis as well as detoxication (Li et al. 2011). Glycyrrhizic and glycyrrhetinic acid, as main saponins, are the most important pharmacologically active component in G.uralensis, exhibiting anti-inflammation, anti-virus and anti-HIV properties (Wang et al. 2012). Flavonoids, secondary metabolites of the plant, are widely used for anti-oxidant (Man et al. 2013) and anti-tumor (Zhang et al. 2009). The polysaccharide, primary metabolites in G. uralensis has drawn the attention of researchers due to its physical and functional properties (Wan and Cheng 2009).
In recent years, G. uralensis has been increasingly used as a health additive formulated into kinds of commercial products such as food, health products, cosmetics (Man et al. 2013). Recently, natural sources of wild G. uralensis are very limited because of over-exploitation (Dong et al. 2012). The current supply of G. uralensis mainly depends on field culture, which is an extremely time-consuming and labor-intensive process. However, there is a great demand and scant supply for G. uralensis. As a result, cell, tissue, and organ culture has been exploited as an alternative for more efficient and controllable production of G. uralensis and its active constituents.
Nitrogen source has been an essential factor that influences the quality of cell and root growth in a species-dependent manner (Gorret et al. 2004). The significant role of the nitrogen source has been well demonstrated in many cultures such as Panax ginseng (Kim et al. 2005), Echinacea angustifolia (Wu et al. 2006), Eleutherococcus koreanum Nakai (Lee and Paek 2012) and so on. Phosphate source is another important element that constitutes nucleotide, phosphatides and adenosine triphosphate and so on (Wang et al. 2009; Zhong and Zhu 1995). Moreover, it has an important influence on the growth and active components synthesis which has been proved by many reports (Liu and Zhong 1998; Jiang et al. 2006; Huang et al. 2010).
Callus induction and suspension cell culture systems as well as hairy root culture have been studied to obtain flavonoids in G. uralensis (Guo et al. 2012; Yang et al. 2008; Zhang et al. 2009). Wang et al. (2012) made content comparison with regards to seeding, callus, cell and adventitious root in G. uralensis. However, to our knowledge, there have been few reports on adventitious root culture of G. uralensis with regard to optimization of culture conditions. The objective of this study was to optimize the ratio of ammonium to nitrate and phosphate concentration for the large-scale productivity of biomass and bioactive compounds in adventitious roots of G. uralensis.
Materials and methods
Plant material
Seeds of G. uralensis were supplied by Beijing materia herbal medicine technology Co. (Beijing, China).
Seeds’ surface were washed under running tap water for 2 h and then they were further sterilized with 75 % (v/v) ethanol for 30 s, immersed in 2 % (v/v) NaClO solution for 30 min and rinsed with sterile distilled water. The sterilized seeds were inoculated into conical flasks containing 50 mL Murashige and Skoog (MS) medium supplemented with 30 g L−1sucrose and 6.5 g L−1 agar. Cultures were maintained at 23 ± 2 °C with 54–72 μmol m−2 s−1 light intensity under a 16/8 h (day/night) photoperiod. After 4 weeks, they grew into plantlets and the root explants were cut into 1 cm length for the subsequent experiments.
Induction of adventitious roots and their maintenance
Adventitious roots of G. uralensis were induced from root explants on half-strength MS medium supplemented with 30 g L−1 sucrose, 1 mg L−1 IBA and 6.5 g L−1 agar. Those induced adventitious roots were inoculated into 250 mL Erlenmeyer flasks containing 100 mL half-strength MS medium supplemented with 1 mg L−1 IBA and 30 g L−1sucrose. Cultures were maintained at 23 ± 2 °C in the dark on gyratory shakers at 120 rpm and were sub-cultured every 30 days.
Effects of nitrogen source and phosphate concentration on adventitious root growth and metabolite accumulation
Adventitious roots (6 g L−1) were inoculated into 1/2 MS medium supplemented with 30 g L−1 sucrose, 1 mg L−1 IBA and various ratios of NH4 +/NO3 − (0/30, 10/20, 15/15, 20/10, 30/0, using NH4Cl and KNO3) at the constant nitrogen source level of 30 mM, which was basically the level of that in a half-strength MS medium. Adventitious roots (6 g L−1) were inoculated into 1/2 MS medium supplemented with 0, 0.3125, 0.625, 1.25, 1.875 mM phosphate. The ratio of NH4 +/NO3 − was 10:20 and the culture conditions were the same as described above. Each treatment was repeated three times.
Scale-up culture of adventitious root from shake flask to bioreactor
Adventitious roots were further proliferated in 0.5 L Erlenmeyer flasks, 3 and 5 L balloon-type bubble bioreactors (BTBBs) with 0.2, 2 and 3 L working volume, respectively. The half-strength MS medium was supplemented with 1 mg L−1 IBA and 30 g L−1 sucrose. Root inoculum was adjusted to a density of 1.0 g L−1 and the air volume was adjusted to constant flow rate of 0.4 air volume/culture volume/min (vvm) during BTBBs culture according to previous experimental optimization. Cultures were maintained at 23 ± 2 °C in the dark. Each treatment was repeated three times.
Determination of root biomass
After 30 days culture, the harvested roots were washed with running water twice, and then rinsed with distilled water three times. Fresh weight (FW) was determined after blotting the washed roots on filter paper. The fresh roots were then dried in vacuum at 50 °C for 2 days to constant dry weight (DW). The growth rate was calculated as (harvested dry weight−inoculated dry weight)/inoculated dry weight.
Determination of total flavonoid and polysaccharides
Samples of adventitious roots were ground to a fine powder and extracted twice with 30 mL of 75 % ethanol for 30 min at 60 °C in an ultrasonic bath (Kunshan, China). After filtration, the extract was evaporated to dryness, and then dissolved in 2 mL 75 % ethanol. The content of flavonoid was determined as described in the paper (Man et al. 2013) with Liquiritin as a reference standard (A = 0.0065C − 0.0046, r = 0.9976).
In terms of the extraction of polysaccharides, the residue after filtration was drying and extracted three times with 25 mL distilled water for 1 h at 100 °C. The extract was diluted to 50 mL for quantification. The content of polysaccharide was analyzed using the method of sulphuric acid—anthrone reported by Chen et al. (2005) with glucose as a reference standard (A = 0.0073C + 0.0213, r = 0.9962).
Determination of the content of glycyrrhizic and glycyrrhetinic acid
Extracts of ethanol were analyzed by HPLC (Agilent1100, PaloAlto, CA) using a promosil C18 column (4.6 mm × 250 mm, 5 μm; Agela, Tianjin, China) to determine the content of glycyrrhizic and glycyrrhetinic acid. Mobile phase was composed of A-formic acid (0.04 %, v/v) and B-acetonitrile. Gradient elution profile (A:B) was 0–4 min, 80:20; 20 min, 62:38; 25 min, 45:55; 38–40 min, 10:90. The detection wavelength was set at 250 nm. The flow rate was 1.0 mL min−1 and the column temperature was maintained at 30 °C.
Statistical analysis
The statistical analysis was performed according to the V 17.0 SPSS system. Mean and standard errors were used throughout, and the statistical significance between the mean values was assessed applying a Duncan’s multiple range tests. A probability of P < 0.05 was considered significant.
Results and discussion
Effects of nitrogen source on biomass production and metabolites accumulation
Nitrogen source significantly influenced the biomass accumulation of G. uralensis adventitious roots. The optimum biomass of 5.08 g flask−1 FW and 0.30 g flask−1 DW were obtained when the NH4 +/NO3 − ratio was 10:20 (Table 1). The highest polysaccharide content (18.98 mg g−1) was also obtained under a NH4 +/NO3 − ratio of 10:20; whereas, the content of total flavonoid reached the peak at a NH4 +/NO3 − ratio of 20:10. In case of saponin, glycyrrhizic acid was optimum when the NH4 +/NO3 − ratio was 15:15 and the content of glycyrrhetinic acid reached the peak at a NH4 +/NO3 − ratio of 10:20. These results suggest that the NH4 +/NO3 − ratio of 10:20 was favorable to generate the optimum biomass and accumulation of polysaccharide and glycyrrhetinic acid. It was a general trend that a lower NH4 +/NO3 − ratio is more beneficial for plant cell growth (Panda et al. 1992; Kaul and Hoffman 1993; Yu et al. 2001), and our study also supported this point (Table 1). It may be because ammonium diffuses easily and accumulates into the cell which becomes toxic if not immediately metabolized, so the ammonium must control to a low concentration (Zhang et al. 2011).
With ammonium as the sole N source (i.e. NH4 +/NO3 − = 30/0), the root scarcely grew in the culture (Table 1). Zhong and Wang (1998) demonstrated that high ammonium concentration had an inhibitory effect on cell growth in culture of Panax quinquefolium. Kaul and Hoffman (1993) also reported that ammonium as the sole N source inhibited callus growth of Pinus strobus. We can suppose that the sole nitrogen source of ammonia was unfavorable for root growth. In the case of nitrate as the sole N source, the root grew a little better than that with totally ammonium in the medium. In the other case where ammonium and nitrate were both supplied into the medium, the root grew well compared with nitrate or ammonium as the sole N source in the medium. The existence of ammonium could suppress nitrate assimilation which would result in medium acidification. Generally speaking, the plant cell utilized the ammonium priority in the medium. However, excessive ammonium has toxic effects on plant cell and root growth (Wang et al. 2009). Although nitrate is safe for plant, over addition may result in over-acidification of the medium which is unfavorable to root growth (Zhang et al. 2011).
Nitrogen source significantly affects the plant cell and tissue growth and metabolite formation (Cui et al. 2010b). Ammonium and nitrate as nitrogen source have different effects on cell and tissue growth and active component production (Zhang et al. 1996). It was apparent that nitrate was more necessary than ammonium for root growth and metabolites accumulation. Similar observations were also reported by Nagella and Murthy (2011) who concluded that biomass and withanolide-A were larger, when the NO3 − was higher than that of NH4 + concentration in cell suspension cultures of Withania somnifera (L.) Dunal. In adventitious shoot cultures of Bacopa monnieri (L.), the number of adventitious shoot biomass and bacoside A content were optimum at a lower NH4 +/NO3 − ratio (Naik et al. 2011). Pan et al. (2004) indicated that cell dry weight was improved under lower NH4 +/NO3 − ratio in cell suspension cultures of Camptotheca acuminate. Our results were similar to the literatures. Therefore, we can draw a conclusion that most of the plants need a lower NH4 +/NO3 − ratio.
Effects of phosphate concentration on biomass production and metabolites accumulation
The biomass accumulation of G. uralensis adventitious roots was highly affected by phosphate concentration. In the range of phosphate level from 0 mM to 1.875 mM, the maximum root dry weight (0.34 g·flask) and the highest growth rate (9.18) were observed in the culture treated with 1.25 mM phosphate (Table 2). When phosphate concentration exceeded 1.25 mM, the biomass of adventitious roots showed a drop in growth rate (7.52). We can infer that higher phosphate over 1.25 mM phosphate concentration was detrimental to the root growth. However, excessively lower phosphate was also harmful to adventitious root growth which cannot absorb enough phosphate to metabolite. According to Table 2, we can see that it was unfavorable to adventitious root proliferation in the phosphate-free medium (i.e., 0 mM phosphate) whose growth ratio was only 2.49. We can conclude that adventitious root growth was unfavorable in the medium without phosphate source. The similar phenomenon was also reported in rice cells (Wen and Zhong 1997). In terms of Catharanthus roseus (L.), Sakano et al. (1995) reported that cells could not proliferate at all in medium lacking phosphate source. Phosphate source plays an important role in the production of biomass and accumulation of metabolites. It also participates in various kinds of energy metabolism and material biosynthesis (Huang et al. 2010).
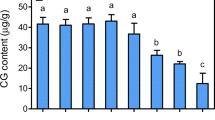
The results exhibited in the culture treated with 1.25 mM phosphate, the content of polysaccharide reached the maximum i.e. 15.66 mg g−1. The highest amount of flavonoid (7.54 mg g−1) and glycyrrhizic acid (0.57 mg g−1) was accumulated with 0.625 mM phosphate. However, 0.3125 mM phosphate was optimum for the accumulation of glycyrrhetinic acid (0.32 mg g−1). We can see that the accumulation of polysaccharide required higher phosphate (1.25 mM), however the accumulation of flavonoid and saponin (glycyrrhizic and glycyrrhetinic acid) required lower phosphate (0.625 and 0.3125 mM). We can suppose that it may relate to the metabolic pathways of primary metabolites (polysaccharide) and secondary metabolites (flavonoid and saponin) (Table 3).
Phosphate is an essential nutrient which participates in metabolite formation and energy metabolism and biosynthesis. In adventitious root culture of P. ginseng CA (Huang et al. 2010), it was found that the root growth ratio reached its peak at the concentration of 0.625 mM phosphate, however, the maximum ginsenoside content was achieved at 1.25 mM. Zhong and Zhu (1995) concluded that the highest production and yield of ginsenosides were obtained at 1.25 mM of medium phosphate in suspension cell culture of Panax notoginseng which was somewhat similar with our results. This phenomenon showed that the effect of phosphate concentration on adventitious root was very complicated, which depended on both species and kinds of secondary metabolites.
Scale-up of adventitious root in G. uralensis
The growth of adventitious root in Erlenmeyer flasks and BTBB was shown in Fig. 1. Adventitious root growth differed significantly between Erlenmeyer flasks and bioreactor cultures. Roots began to differentiate after 7 d and 12 d in the liquid cultures of Erlenmeyer flask and bioreactor, respectively. The growth ratio of 25.95 and 22.44 was obtained in 3 L (53.9 g FW, 2.52 g DW) and 5 L BTBB (90.22 g FW, 4.22 g DW) which were significantly more than Erlenmeyer flasks cultures of 18.36 (9.68 g FW, 0.68 g FW). Gradually scale-up culture of adventitious roots increased the root biomass as well as the contents of polysaccharide and flavonoid. However, there was no significant change in the content of glycyrrhizic and glycyrrhetinic acid in bioreactor compared with the Erlenmeyer flask.
The biomass increase might be attributed to the culture conditions in bioreactor which can be optimized by real-time manipulation of temperature, pH and oxygen in the medium (Wang et al. 2012). We supposed that the culture condition may have an influence on the active component synthesis of G.uralensis. In adventitious root culture of P. ginseng, Choi et al. (2000) demonstrated that the content of total saponin in the pilot-scale culture was similar to that obtained in the small-scale bioreactor. Our result regarding the content of saponin was similar to the viewpoint.
The use of bioreactors has resulted in the development of a technology for large-scale biomass of adventitious roots culture (Cui et al. 2010a). Similar observation was also reported in A. membranaceus adventitious root culture (Wu et al. 2011). The maximum growth rate of A. membranaceus in 5 L bioreactors was tenfold after 40 days of culture higher than that cultivated in flasks. This is also partially supported by Echinacea purpurea adventitious roots (Jeong et al. 2009).
Conclusion
In conclusion, the adventitious root culture system of G. uralensis was successfully established for the metabolites accumulation. We investigated the effects of nitrogen source and phosphate concentration on root growth and metabolites accumulation. The optimal culture condition we obtained in experiments was an ammonia/nitrate rate of 10:20 and 1.25 mM phosphate concentration. In scale-up culture of adventitious root, the growth rate in 5 L BTBB reached 22.44 which indicated a potential manner for the large-scale production of biomass and bioactive compounds of G. uralensis.
Author contribution
All authors contributed extensively to the work presented in this paper. Shuangshuang Yin and Yao Zhang designed the experiment and wrote the paper under the guidance of WenYuan Gao. Juan wang and Shuli Man were responsible for viability tests and statistical analysis. Hui Liu was in charge of culturing adventitious roots.
References
Chen JH, Xie MY, Nie SP, Wang YX, Peng RH (2005) Determination of polysaccharides in Panax quinquefolium L. J Food Sci Biotechnol 24:72–76
Choi SM, Son SH, Yun SR, Kwon OW, Seon JH, Paek KY (2000) Pilot-scale culture of adventitious roots of ginseng in a bioreactor system. Plant Cell Tiss Org 62:187–193
Cui XH, Chakrabarty D, Lee EJ, Paek KY (2010a) Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresource Technol 101:4708–4716
Cui XH, Murthy HN, Wu CH, Paek KY (2010b) Adventitious root suspension cultures of Hypericum perforatum: effect of nitrogen source on production of biomass and secondary metabolites. In Vitro Cell Dev Biol Plant 46:437–444
Dong YY, Gao WY, Zhang JZ, Zuo BM, Huang LQ (2012) Quantification of four active ingredients and fingerprint analysis of Licorice (Glycyrrhiza uralensis Fisch.) after spaceflight by HPLC–DAD. Res Chem Intermed 38:1719–1731
Gorret N, Rosli SK, Oppenheim SF, Willis LB, Lessard PA, Rha C, Sinskey AJ (2004) Bioreactor culture of oil palm (Elaeis guineensis) and effects of nitrogen source, inoculum size, and conditioned medium on biomass production. J Biotechnol 108:253–263
Guo SB, Man SL, Gao WY, Liu H, Zhang LM, Xiao PG (2012) Production of flavonoids and polysaccharide by adding elicitor in different cellular cultivation processes of Glycyrrhiza uralensis Fisch. Acta Physiol Plant 35:679–686
Huang T, Gao WY, Wang J, Cao Y, Zhao YX, Haung LQ, Liu CX (2010) Selection and optimization of a high-producing tissue culture of Panax ginseng C.A. Meyer. Acta Physiol Plant 32:765–772
Jeong CS, Murthy HN, Hahn EJ, Lee HL, Paek KY (2009) Inoculum size and auxin concentration influence the growth of adventitious roots and accumulation of ginsenosides in suspension cultures of ginseng (Panax ginseng C.A. Meyer). Acta Physiol Plant 31:219–222
Jiang ST, Wei M, Luo JP (2006) Effect of phosphate on growth and polysaccharide production by suspension cultures of protocorm-like bodies of Dendrobium huoshanense. J Chin Biotech 22:613–618
Kaul K, Hoffman SA (1993) Ammonium ion inhibition of Pinus strobus L. callus growth. Plant Sci 88(2):169–173
Kim JH, Chang EJ, Oh HI (2005) Saponin production in submerged adventitious root culture of Panax ginseng as affected by culture conditions and elicitors. Asia Pac J Mol Biol Biotech 13:87–91
Lee EJ, Paek KY (2012) Effect of nitrogen source on biomass and bioactive compound production in submerged cultures of Eleutherococcus koreanum Nakai adventitious roots. Biotechnol Prog 28:508–514
Li YJ, Chen J, Li Y, Li Q, Zheng YF, Fu Y, Li P (2011) Screening and characterization of natural antioxidants in four Glycyrrhiza species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A 45:8181–8191
Liu S, Zhong JJ (1998) Phosphate effect on production of ginseng saponin and polysaccharide by cell suspension cultures of Panax ginseng and Panax quinquefolium. Process Bioechem 33:69–74
Man SL, Wang J, Gao WY, Guo SB, Li YY, Zhang LM, Xiao PG (2013) Chemical analysis and anti-inflammatory comparison of the cell culture of Glycyrrhiza with its field cultivated variety. Food Chem 136:513–517
Nagella P, Murthy HN (2011) Effects of macro elements and nitrogen source on biomass accumulation and withanolide-A production from cell suspension cultures of Withania somnifera (L.) Dunal. Plant Cell Tiss Organ Cult 104:119–124
Naik PM, Manohar SH, Murthy HN (2011) Effects of macro elements and nitrogen source on biomass accumulation and bacoside A production from adventitious shoot cultures of Bacopa monnieri (L.). Acta Physiol Plant 33:1553–1557
Pan XW, Xu HH, Liu X, Gao X, Lu YT (2004) Improvement of growth and camptothecin yield by altering nitrogen sourcesupply in cell suspension cultures of Camptotheca acuminate. Biotechnol Lett 26:1745–1748
Panda AK, Mishra S, Bisaria VS (1992) Alkaloid production by plant cell cultures of Holarrhena antidysentericaIeffects of major nutrients. Biotechnol Bioeng 39:1043–1051
Sakano K, Matsumoto M, Yazaki Y, Kiyota S, Okihara K (1995) Inorganic phosphate as a negative conditioning factor in plant cell culture. Plant Sci 107:117–124
Wan FC, Cheng AW (2009) Polysaccharide isolated from Glycyrrhiza uralensis Fisch induces intracellular enzyme activity of macrophages. Mediterr J Nutr Metab 1:165–169
Wang J, Gao WY, Cao Y, Huang T (2009) Effects of carbon, nitrogen and phosphate sources on biomass and active components of suspension cells in Panax quinquefolium L. Chin Pharm J 44:815–818
Wang J, Gao WY, Zhang LM, Huang LQ (2012) Establishment and quality assessment of tissue cultures in Glycyrrhiza uralensis Fisch. Appl Biochem Biotechnol 169:588–594
Wen ZY, Zhong JJ (1997) Effects of initial phosphate concentration on physiological aspects of suspension cultures of rice cells: a kinetic study. J Fermen Bioeng 83:381–385
Wu CH, Dewir YH, Hahn EJ, Paek KY (2006) Optimization of culturing conditions for the production and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol 49:193–199
Wu SQ, Lian ML, Gao R, Park SY, Piao XC (2011) Bioreactor application on adventitious root culture of Astragalus membranaceus. In Vitro Cell Dev Biol Plant 47:719–724
Yang Y, He F, Yu LJ (2008) Dynamics analyses of nutrients consumption and flavonoids accumulation in cell suspension culture of Glycyrrhiza inflate. Biol plantarum 52:732–734
Yu KW, Gao WY, Hahn EJ, Paek KY (2001) Effects of macro elements and nitrogen source on adventitious root growth and ginsenoside production in Ginseng (Panax ginseng C. A. Meyer). J Plant Biol 44:179–184
Zhang YH, Zhong JJ, Yu JT (1996) Effects of nitrogen source on cell growth and production of ginseng saponin and polysaccharide in suspension cultures of Panax notoginseng. Biotechnol Prog 12:567–571
Zhang HC, Liu JM, Lu HY, Gao SL (2009) Enhanced flavonoid production in hairy root cultures of Glycyrrhiza uralensis Fisch by combining the over-expression of chalcone isomerase gene with the elicitation treatment. Plant Cell Rep 28:1205–1213
Zhang J, Gao WY, Wang J, Li XL, Xiao PG (2011) Improvement of growth and periplocin yield of Periploca sepium adventitious root cultures by altering nitrogen source supply. Chin Herb Med 3:226–231
Zhong JJ, Wang SL (1998) Effects of nitrogen source on the production ginseng saponin and polysaccharide by cell cultures of Panax quinquefolium. Process Biochem 33:671–675
Zhong JJ, Zhu QX (1995) Effect of initial phosphate concentration on cell growth and ginsenoside saponin production by suspended cultures of Panax notoginseng. Appl Biochem Biotech 55:241–247
Acknowledgments
This research was funded by central significant increase or decrease program, China (ID: 20603020302) and Tianjin University innovation fund, China (ID: 2013XQ-0046).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K.-Y. Paek.
Yao Zhang is the co-first author.
Rights and permissions
About this article
Cite this article
Yin, S., Zhang, Y., Gao, W. et al. Effects of nitrogen source and phosphate concentration on biomass and metabolites accumulation in adventitious root culture of Glycyrrhiza uralensis Fisch. Acta Physiol Plant 36, 915–921 (2014). https://doi.org/10.1007/s11738-013-1470-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1470-z