Abstract
This study aimed at investigating the expression of osteoblast and chondrocyte-related genes in mesenchymal stem cells (MSCs), derived from rabbit adipose tissue, under mechanical vibration. The cells were placed securely on a vibrator’s platform and subjected to 300 Hz of sinusoidal vibration, with a maximum amplitude of 10 μm, for 45 min per day, and for 14 consequent days, in the absence of biochemical reagents. The negative control group was placed in the conventional culture medium with no mechanical loading. The expression of osteoblast and chondrocyte-related genes was investigated using real-time polymerase chain reaction (real-time PCR). In addition, F-actin fiber structure and alignment with the help of actin filament fluorescence staining were evaluated, and the level of metabolic activity of MSCs was determined by the methyl thiazolyl tetrazolium assay. The real-time PCR study showed a significant increase of bone gene expression in differentiated cells, compared with MSCs (P < 0.05). On the other hand, the level of chondrocyte gene expression was not remarkable. Applying mechanical vibration enhanced F-actin fiber structure and made them aligned in a specific direction. It was also found that during the differentiation process, the metabolic activity of the cells increased (P < 0.05). The results of this work are in agreement with the well-accepted fact that the MSCs, in the absence of growth factors, are sensitive to low-amplitude, high-frequency vibration. Outcomes of this work can be applied in cell therapy and tissue engineering, when regulation of stem cells is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advantage of MSCs, i.e., their multipotency, low immunogenicity, ease of isolation—especially for adipose derived MSCs—and expansion, made them a promising source for cell-based therapy and tissue engineering (Barry and Murphy 2004; Orbay et al. 2012; Tan et al. 2013). The regulation of stem cell differentiation is one of the challenges in tissue engineering. Although various studies have been done on the effect of chemical stimuli on MSCs, recent studies have emphasized on the inevitable role of mechanical signals on stem cell behavior (Eslaminejad and Taghiyar 2007; Litwack 2011; Macqueen et al. 2013; Oconor et al. 2013). The induction of mechanical differentiation in MSCs at the molecular level requires a specific process with strong programming. Various environmental conditions have been introduced as key features responsible for MSC differentiation (Steward and Kelly 2015). It has been confirmed that cells and tissues are exposed to various types of mechanical loads, such as compressive, tensile, and shear forces in their innate environment, which affect their development and natural functions (Atala et al. 2010; Litwack 2011). Currently, mechanical loads are used as an efficient tool in tissue engineering for the formation of cartilage (Safshekan et al. 2014), ligament (Altman et al. 2002), muscle (Ghazanfari et al. 2009; Haghighipour et al. 2012; Amin et al. 2014), and bone (Ozcivic et al. 2010).
Among different loading types affecting stem cells, mechanical vibration is a non-invasive and biophysical stimulus. Moreover, the anabolic effects of mechanical vibration on tissue and cellular levels have been validated by experimental evidence (Rubin et al. 2001; Hooshiar et al. 2008; Lau et al. 2011; Edwards and Reilly 2015) and were compared with other medical interventions, such as the laser therapy, to evaluate the anabolic effectiveness (Jafarabadi et al. 2016). According to some studies performed on the effects of mechanical vibration on MSCs, it can be concluded that MSCs are mechanosensitive to mechanical vibration, which results in an increase in the bone gene expression besides being responsive to micro-vibration at gene and protein level (Judex et al. 2005; Zhou et al. 2011; Kim et al. 2012). Prè et al. have fabricated a device that provides mechanical vibration signal for the pools of cells, and the enhancement of osteogenesis gene expressions by the mechanical vibration was reported in their studies (Pre et al. 2008; Pre et al. 2011; Pre et al. 2013). In addition, some researchers have reported that mechanical vibrations are responsible for the inhibition of osteoclast formation and adipogenesis suppression (Dumas et al. 2010; Lau et al. 2010; Sen et al. 2011; Kulkarni et al. 2013). It was reported that the mechanical vibration can not only enhance bone formation but also stimulate the synthesis of chondrocyte matrix proteins, such as chondroitin, collagen type II, and proteoglycan (Liu et al. 2001; Takeuchi et al. 2006).
Reviewing the trends of recent articles on MSCs, few studies reported the evaluation of the mechanical vibration’s effect on chondrocyte- related gene. For instance, Cashion et al. suggested that low-frequency vibration (1 Hz) would result in cartilage phenotype (Cashion et al. 2014). Maryczy et al. developed a prototype device that induces low-magnitude (0.3 g), low-frequency (25, 35, and 45 Hz) vibrations and showed that signal with the frequency of 35 Hz leads to stable cartilaginous tissue(Marycz et al. 2016).
The present study aimed at evaluating changes in the expression of osteoblast- and chondrocyte-related genes following the application of low-amplitude, high-frequency mechanical vibrations (LAHF), i.e., frequency of 300 Hz and 10 μm displacement, in the absence of chemical cues under in vitro conditions. The transformation mechanism of a stimulus from mechanical signals to biological signal is not fully known yet, so in this work, in order to address the question of whether or not the F-actin fibers are changing during stem cell differentiation, cytoskeleton alteration was also investigated.
Materials and Methods
Cell culture and preparation
Rabbit adipose derived mesenchymal stem cells were obtained from the National Cell Bank of Iran, Tehran and were then cultured in a free of growth factor medium, Dulbecco’s Modified Eagle Media-high glucose (DMEM-hg) (Gibco, Grand Island, NY) containing Penicillin-Streptomycin (Gibco) antibiotics and supplemented with 10% fetal bovine serum. After reaching sub-confluent state, stem cells at passage 3 were cultured at a density of 3 × 104 cells/well and incubated at 37°C for 24 h.
Mechanical vibrator to oscillate the cultured cells
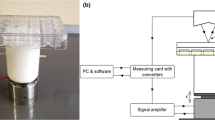
A mechanical vibration device was designed and fabricated in this study (Fig. 1) in order to evaluate the effects of mechanical vibration on MSC response (Safavi et al. 2013). The device designed for this project has two main “mechanical” and “electrical” units, which can produce vertical mechanical vibration and generate harmonic sine waves of a given frequency and amplitude. The mechanical part mainly made up of a vibrator panel that has the ability to move up and down vertically. The electrical unit contains an electric motor that rotates with the angular velocity of up to 20,000 rpm to create rotational forces for mechanical shaking of the multiwall plate and transferring the mechanical vibrations through the PMMA (poly methyl methacrylate) platform to the cells.
(a) The mechanical vibrator (device) to oscillate the cultured cells, designed and fabricated in this study (Safavi et al. 2013). (b) Schematic representation of vertical mechanical vibration.
In this vibrator, the vibration frequency is tunable from 50 to 350 Hz with vertical movement, and the vibrator can be housed inside a CO2 incubator, at the temperature of 37°C. The information of the mechanical vibration, registered acceleration with a sample rate of 800 per second, is recordable through using a MATLAB (R2013a) program.
Experimental protocol
MSCs, passage 3, were cultured at a density of 3 × 104 cells/well. Vibration loading was started 24 h after plating the cells. The plates were placed securely on the vibrator’s platform and subjected to 300 Hz of sinusoidal vibrations, and a maximum amplitude of 10 μm for 45 min per d, for 14 consequent d (Tirkkonen et al. 2011). The control cells in the no-vibration group were placed on the same, but on a stationary plate. Three biological replicates were performed for each experiment during osteogenic, and chondrogenic differentiation.
MTT assay for metabolic activity
In order to study the metabolic activity changes in response to LAHF mechanical vibration, the methyl thiazolyl tetrazolium (MTT) assay was utilized (Wang et al. 2010). The cells were seeded in 24-well plates at a density of 2 × 103 cells/well. To determine the metabolic activity of the MSCs, at the end of the loading period, 14th day of the test, two groups of mechanical vibration, i.e., 300 Hz and 10 μm displacement, and control groups were used. For MTT, the medium was replaced with a 5:1 ratio of medium and MTT solution (5 mg/ml in phosphate buffer saline PBS). Then, the cells were incubated for 4 h in an incubator at 37°C in an atmosphere containing 5% CO2 to allow for optimum formazan production. In the next step, MTT solution (Sigma, St. Louis, MO) was removed, the cells were lysed, and the formazan crystals were dissolved by adding isopropanol (Sigma). The optical density of the solubilized formazan in each well was then quantified. The absorbance of the colored solution was determined at a wavelength of 545 nm, using an ELISA reader.
Alizarin red staining
The differentiation of MSCs into bone phenotype can be observed by the formation of the calcium nodes. Calcium nodes were confirmed by alizarin red staining on day 14. Cells were washed twice with PBS, fixed with a 4% paraformaldehyde for 20 min, rinsed with 9% NACL, and stained by alizarin red solution for 30 min at 37°C. After washing four times with PBS, the stained cells were photographed.
Alician blue staining
The cartilaginous structure of the mesenchymal stem cells was verified by alician blue staining of proteoglycans on day 14. Cells were washed twice with PBS, fixed with 3% glutaraldehyde (Sigma) for 2 h, rinsed three times with PBS, and stained by the alician blue (Sigma) solution for 3 h. After washing with a 3% acetic acid, the stained cells were photographed.
Actin filament staining
The cytoskeleton provides an important structural framework for cell shape. The cytoskeleton is made up of three kinds of protein filaments such as actin filament, intermediate filament, and microtubules. Actin filament has the main role in cell structure and functions (Fletcher and Mullins 2010). Actin stains and probes are used in determining the structure and function of the cytoskeleton in living and fixed cells. Phalloidin is a highly selective bicyclic peptide that is used for staining actin filaments (also known as F-actin). In order to stain actin filaments, cells were washed twice with a 10% PBS, and then fixed with 3.7% formaldehyde diluted in PBS for the fixation. After 5 min, the cells were washed with PBS again and permeabilized with a 1% Triton X-100 (Merck, Darmstadt, Germany). The permeabilized cell samples in both control and test groups were incubated with phalloidin (Sigma) for 45 min in the dark room. The stained F-actin filaments were visible in green (513 nm wavelength) after exposure to UV (495 nm).
Gene expression analysis
Immediately after 14 d, the total RNA was prepared using the TRIzol reagent (Invitrogen, Carlsbad, CA) in conjunction with the manufacturer’s single-step chloroform extraction protocol. cDNA was generated by reverse transcription of 1 μg total RNA using random hexamer primers (100 μM) and RevertAid™ M-MuLV Reverse Transcriptase (Fermentas Opelstrasse, Germany) at 25°C for 5 min and at 42°C for 1 h in a total reaction volume of 20 μl. A total of 25 ng cDNA was amplified using specific primers and Power SYBR® Green PCR Master Mix in ABI (Applied Biosystems, Warington, United Kingdom). Reaction parameters were 95°C for 10 min, followed by 95°C for 10 s and 60°C for 1 min for 30 cycles. Finally, to confirm PCR specificity, the PCR products were subjected to a melting curve analysis. The relative gene expression levels were estimated with the 2−ΔΔCT method (Livak and Schmittgen 2001).
The sequences of the primers and probes were designed by the Beacon Designer (BD, Palo Alto, CA) software and checked by primer express gene runner software in this study (Table 1). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) marker was used as the housekeeping gene. The PCR tests were carried out in triplicate in two groups of negative control and mechanically stimulated.
Statistical analysis
The data collected in this study were presented as mean ± SD from independent experiments performed in triplicate. The results were then compared with those of control group, with the mean values with the t test, and the significance was set at (P < 0.05).
Results
The bioreactor developed for these experiments successfully generated the desired waveform parameters. The cells were cultured in a basal medium and were then subjected to vertical mechanical vibration at the frequency of 300 Hz and maximum amplitude of 10 μm displacement, for 45 min per day for 14 d.
Cell metabolic activity assay
The metabolic activity of the cells was analyzed on day 14 of mechanical vibration, as well as for the non-vibrational control conditions. MTT assay on day 14 of the loading duration indicated that the absorption rate has been increased indicating that the growth of the cells’ metabolic activity. The obtained data showed that cell metabolic activity on day 14 increased by 1.49-fold, compared with non-vibrational control group (Fig. 2), (P < 0.05). The results of this study showed that during the loading period, the metabolic activity of the differentiated cells increases. This increment can be attributed to the metabolic activity of the cells protein synthesis in the differentiation process (Zhang et al. 2007).
Alizarin red and alician blue staining
The red parts in Fig. 3b shows calcified matrix in the MSCs under mechanical vibration. The cartilaginous structure of the MSCs was not detected by alician blue staining (Fig. 3d).
Alizarin red and alician blue staining. (a) The control group. (b) Alizarin red staining shows a significant increase in mineralization after mechanical vibration (f = 300 Hz, amplitude = 10 μm, 45 min/d, for 14 consecutive days). (c) The control group. (d) Alician blue staining has no presentation of proteoglycans due to mechanical vibration (inverted microscope, ×400).
Actin filament staining
Whereas F-actin fibers in control groups were thinner and distracted randomly in different directions (Fig. 4a), mechanical vibration not only increases actin fiber thickness but also aligns them in a specific direction.
Gene expression analysis
The results of this work showed that the groups treated with a high-frequency mechanical vibration, f = 300 Hz, amplitude = 10 μm, caused a significant increase in osteogenic markers at day 14 (Fig. 5a). The mRNA level of RUNX2, COLI, and ALP in the test group showed a significant increase after 14 d of mechanical vibration, f = 300 Hz, amplitude = 10 μm, 45 min/d, compared with the control group (Fig. 5a) (P < 0.05). mRNA level of osteocalcin as a latest osteogenic marker did not show any significant increase on day 14 in the vibration group compared with the control group (Fig. 5a) (P < 0.05). Moreover, there was no significant change in the expression of chondrogenic markers’ elements in the vibration group compared with the control group. mRNA level of COLII in the vibration group showed a 1.77-fold increase, compared with the control group (Fig. 5b).
The expression level of mRNA in vibration and control groups. (a) RUNX, COLI, ALP, and OCN genes on day 14 of mechanical vibration (f = 300 Hz, amplitude = 10 μm, 45 min/d). (b) SOX9, COLII, and ACAN genes on day 14 of mechanical vibration (f = 300 Hz, amplitude = 10 μm, 45 min/d). *P < 0.05, ** P < 0.01, and *** P < 0.001.
Discussion
Mechanical regulation of mesenchymal stem cell’s fate is starting to gain interest with the early results indicating that mechanical cues can influence the differentiation process and are capable of upregulating key differentiation markers (Butler et al. 2000; Guilak et al. 2001; Zhang et al. 2012; Meier and Lam 2016). Nonetheless, a few studies investigated the effects of high-frequency mechanical vibration on chondrocyte-related genes. In this research, stem cells isolated from rabbit fat tissues were exposed to mechanical vibration, i.e., 300 Hz and 10 μm, for 45 min per day for 14 d, to see if LAHF vibrations in the absence of growth factors can noticeably alter bone and cartilage gene expression.
The results of this study showed that during the mechanical induction, the metabolic activity of differentiated cells has a considerable increase (Fig. 2). This increment can be due to the metabolic activity of the cell protein synthesis, which is involved in the process of differentiation (Mohajeri et al. 2010). The results of alizarin red staining showed that there is a formation of calcium nodule in cell matrix (Fig. 3b), which can be deemed as an advanced osteogenic differentiation (Zhang et al. 2012). The positive result of alizarin red staining indicates that there is an increase in bone gene expression in vibration group, compared with the control group, which is in agreement with the previous published study (Zamini et al. 2008). On the other hand, alician blue staining has no sign of the presence of proteoglycans caused by the LAHF mechanical vibration (Fig. 3d). The alterations of the cytoskeleton components during the period of differentiation were confirmed in various previous studies (Steward and Kelly 2015). The most important structural components of MSC which responds to the mechanical forces are their actin fibers. The results of this study showed that MSCs respond to mechanical vibration by aligning their F-actin fibers in a specific direction (Fig. 4b).
In this study, RUNX, ALP, and COLI for the osteoblast-related gene (Wang et al. 2015), and SOX9, COL2, and aggrecan for the chondrocyte-related gene were selected (Estes et al. 2010). The stem cell differentiation is a controlled process that involves several factors and messengers. In this work, with the removal of media differentiation, only the effect of mechanical loading on gene expression was evaluated. RUNX2 and ALP were commonly regarded as early markers of osteogenic differentiation. According to the real-time PCR results, expression levels of RUNX2, ALP, and COLI showed a strong increase in vibration group compared with the control group (Fig. 5a), and ALP was the first gene that was expressed and possessed the highest level according to (Fig. 5a). The osteocalcin gene expression is a time-dependent phenomenon, which is disclosed in the final phase of induction which indicates lack of gene expression on the 14th day of mechanical vibration (Fig. 5a), in agreement with the finding of Wang et al. (2015),who reported that exposure to osteogenic differentiation medium less than 21 d could not significantly induce osteocalcin expression. Moreover, the mRNA level of cartilage markers, such as Collagen type II, SOX9, and aggrecan, was determined to investigate the cartilage differentiation process, due to mechanical vibration, as well as in control group. SOX9 is an important transcription factor during chondrogenesis and cartilage formation, which plays major roles in the expression of chondrogenic genes, such as COLLII and aggrecan (Wescoe et al. 2008). The results of this work also showed that the mRNA expression level was zero for SOX9 and aggrecan, and 1.77-fold for collagen type II (Fig. 5b) in comparison with the negative control group. This study confirmed that the expression of chondrogenic markers due to the application of high-frequency, low-amplitude mechanical vibration, in the absence of chondroinductive growth factor, was not remarkable (see Fig. 5b), which may suggest that LAHF mechanical vibration is only capable of triggering osteogenic differentiation, in agreement with the results of a recent work by Marycz et al. (Cashion et al. 2014; Marycz et al. 2016)
It is well known that mechanical stimulation can increase cells differentiation (Delaine-Smith and Reilly 2012; Meier and Lam 2016), but the main reasons and mechanisms are not yet entirely clear. Presumably, the influence of mechanical forces might be transduced via cell binding sites, leading to enhanced rates or extent of differentiation (Li et al. 2011). It is also reasonable to speculate that mechanical signals may trigger cell-surface stretch receptors and adhesion sites, resulting in a cascade of events that involve activation of genes response for the synthesis and secretion of key molecular components of the matrix (Steward and Kelly 2015).
Even though, the mechanical vibrator, which was designed and fabricated in this study, could successfully generate controlled vibration and stimulated MSCs in the absence of chemical reagents. Similar with other scientific works, there were several limitations in this study. First, the use of only one frequency, one specific direction of vibration, and single amplitude, which are not what MSCs experience in in vivo condition, can be seen as some limitations of this work. Secondly, cells were grown on a single layer, which is not compatible with the bone and cartilage tissues’ 3D structure. Further investigation, compared with this work, is needed to shed more light on the effects of mechanical vibration on MSCs differentiation, in the absence of growth factor.
Conclusion
In conclusion, findings of this work suggest that LAHF vibration acts differently on chondrogenic and osteogenic differentiation of MSCs (see Fig. 5a, b). Another key finding of this study was that mechanical vibration can be employed as a proper method to accelerate development of bone engineered tissues with functional cells.
References
Atala A, Lanza R, Thomson J et al (2010) Foundation of regenerative medicine. Elsevier, San Diego
Altman G, Horan R, Martin I et al (2002) Cell differentiation by mechanical stress. The FASEB J 16:270–272
Amin S, Shadpour MT et al (2014) Comparing the effect of uniaxial cyclic mechanical stimulation on GATA4 expression in adipose-derived and bone marrow-derived mesenchymal stem cells. Cell Biol Int 38:219–227
Barry FP, Murphy M (2004) Mesenchymal stem cells: clinical applications and biological characterization. IJBCB 36:568–584
Butler D, Goldstein S, Guilak F (2000) Functional tissue engineering: the role of biomechanics. J Biomech Eng122:570–575
Cashion AT, Caballero M, Halevi A, Pappa A, Dennis RG, van Aalst JA (2014) Programmable mechanobioreactor for exploration of the effects of periodic vibratory stimulus on mesenchymal stem cell differentiation. Bioresearch 3:19–28
Delaine-Smith RM, Reilly GC (2012) Mesenchymal stem cell responses to mechanical stimuli. MLTJ 2:169–180
Dumas V, Ducharne B, Perrier A, Fournier C, Guignandon A, Thomas M, Peyroche S, Guyomar D, Vico L, Rattner A (2010) Extracellular matrix produced by osteoblasts cultured under low-magnitude, high-frequency stimulation is favorable to osteogenic differentiation of mesenchymal stem cells. Calcif Tissue Int J 87:351–364
Edwards JH, Reilly GC (2015) Vibration stimuli and the differentiation of musculoskeletal progenitor cells: review of results in vitro and in vivo. WJSC 7:568–582
Eslaminejad MRB, Taghiyar L (2007) Mesenchymal stem cell purification from the articular cartilage cell culture. IJBMS 10:146–153
Estes BT, Diekman BO, Gimble JM, Guilak F (2010) Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. J Nat Protoc 5:1294–1311
Fletcher DA, Mullins RD (2010) Cell mechanics and the cytoskeleton. Nature 463:485–506
Ghazanfari S, Shadpour MT et al (2009) Effects of cyclic stretch on the proliferation of mesenchymal stem cells and their differentiation to smooth muscle cells. J BBRC 388:601–605
Guilak F, Butler DA, Goldstein SA (2001) Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop Relat Res 391:s295–s305
Haghighipour N, Heidaryan S et al (2012) Differential effects of cyclic uniaxial stretch on human mesenchymal stem cell into the skeletal muscle cell. Cell Biol Int36:669–675
Hooshiar S A, Rouhi G, Arshi A et al. ( 2008) An investigation on the effects of low-amplitude, high-frequency (LAHF) mechanical stimuli on matrix-extracellular fluid-osteocyte complex. 56th Annu Meet Orthop Res Soc.
Jafarabadi MR, Rouhi GR et al (2016) The effects of photobiomodulation and low-amplitude high-frequency vibration on bone healing process: a comparative study. J Lasers Med Sci 31:1827–1836
Judex S, Zhong N, Squire ME, Ye K, Donahue LR, Hadjiargyrou M, Rubin CT (2005) Mechanical modulation of molecular signals which regulate anabolic and catabolic activity in bone tissue. J Cell Biochem 94:982–994
Kim IS, Song YM, Lee B, Hwang SJ (2012) Mesenchymal stromal cells are mechanosensitive to vibration stimuli. J Dent Res 91:1135–1140
Kulkarni RW, Voglewede PA, Liu D (2013) Mechanical vibration inhibits osteoclast formation by reducing DC-STAMP receptor expression in osteoclast precursor cells. Bone 57:493–498
Lau E, Al-Dujailis S, Guuenther A et al (2010) Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone 46:1508–1515
Lau E, Lee WD, Li J, Xiao A, Davies JE, Wu Q, Wang L, You L (2011) Effect of low-magnitude, high-frequency vibration on osteogenic differentiation of rat mesenchymal stromal cells. J Orthop Res 29:1075–1080
Li D, Zhou J, Chowdhury F, Cheng J, Wang N, Wang F (2011) Role of mechanical factors in fate decisions of stem cells. Regen Med 6:229–240
Liu J, Sekiya I, Asai K, Tada T, Kato T, Matsui N (2001) Biosynthetic response of cultured articular chondrocytes to mechanical vibration. Res Exp Med 200:183–193
Litwack G (2011) Stem cell regulators. Gwendolen R, Reilly C The effects of mechanical loading on mesenchymal stem cell differentiation and matrix production. Elsevier, San Diego
Livak K, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods 25:402–408
Macqueen L, Sun Y, Simmons CA (2013) Mesenchymal stem cell mechanobiology and emerging experimental platforms. J R Soc Interface 10:1–19
Marycz K, Lewandowski D, Tomaszewski KA, Henry BM, Golec EB, Marędziak M (2016) Low-frequency, low-magnitude vibrations (LFLM) enhances chondrogenic differentiation potential of human adipose-derived mesenchymal stromal stem cells (hASCs). Peer J 4:e1637
Meier E, Lam MT (2016) Role of mechanical stimulation in stem cell differentiation. JSM Biotechnol Biomed Eng 3:1060–1072
Mohajeri M, Hosseinkhani H, Ebrahimi NG et al (2010) Proliferation and differentiation of mesenchymal stem cell on collagen sponge reinforced with polypropylene/polyethylene terephthalate blend fibers. J Tissue Eng Part A 16:3821–3830
Orbay H, Tobita M, Mizuno H (2012) Mesenchymal stem cells isolated from adipose and other tissues: basic biological properties and clinical applications. SCI 2012:1–9
Oconor C, Case N, Guilak F (2013) Mechanical regulation of chondrogenesis. Stem Cell Res Ther 4:1–13
Ozcivic E, Luu YK, Adler B et al (2010) Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol 6:50–59
Pre D, Ceccarelli G, Gastaldi G et al (2011) The differentiation of human adipose-derived stem cells (hASCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment. J Bone 49:295–303
Pre D, Magenes G, Ceccarelli G et al ( 2008) A high-frequency vibrating system to stimulate cells in bone tissue engineering, In Bioinformatics and Biomedical Engineering, ICBBE The 2nd International Conference on 884–887
Pre D, Magnese G, Ceccarelli G et al (2013) High-frequency vibration treatment of human bone marrow stromal cells increases differentiation toward bone tissue. J Bone Marrow Res 2013:1–13
Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K (2001) Low mechanical signals strengthen long bones. J Nat 412:603–604
Safavi AS, Haghighipour N, Ghomi M (2013) IR Patent# 81111
Safshekan F, Shadpour MTT et al (2014) Effects of short-term cyclic hydrostatic pressure on initiating and enhancing the expression of chondrogenic genes in human adipose-derived mesenchymal stem cells. J Mech Med Biol 14:1–14
Sen B, Xie Z, Case N, Syner M, Rubin CT, Rubin J (2011) Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech 44:593–599
Steward AJ, Kelly DJ (2015) Mechanical regulation of mesenchymal stem cell differentiation. J Anat 227:717–731
Tan S-L, Ahmad ST, Selvaratnam L et al (2013) Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J Anat 222:437–450
Takeuchi R, Saito T, Ishikawa H, Takigami H, Dezawa M, Ide C, Itokazu Y, Ikeda M, Shiraishi T, Morishita S (2006) Effects of vibration and hyaluronic acid on activation of three-dimensional cultured chondrocytes. Arthritis Rheum 54:1897–1905
Tirkkonen L, Halonen H, Hyttinen J et al (2011) The effects of vibration loading on adipose stem cell number, viability, and differentiation towards bone-forming cells. J R Soc Interface 8:1736–1747
Wang CZ, Wang G-J, Ho M-L et al (2010) Low-magnitude vertical vibration enhances myotube formation in C2C12 myoblasts. J Appl Psychol 109(3):840–848
Wang L, Li Z, Wang Y, Wu ZH, Yu B (2015) Dynamic expression profiles of marker genes in osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Chin Med Sci J 30:108–113
Wescoe KE, Schugar RC, Chu CR, Deasy BM (2008) The role of the biochemical and biophysical environment in chondrogenic stem cell differentiation assays and cartilage tissue engineering. Cell Biochem Biophys 52:85–102
Zhou Y, Guan X, Zhu Z et al (2011) Osteogenic differentiation of bone marrow-derived mesenchymal stromal cells on bone-derived scaffolds: effect of micro-vibration and role of ERK1/2 activation. J Eur Cell Mater 22:12–25
Zhang A et al (2007) Proteomic identification of differently expressed proteins responsible for osteoblast differentiation from human mesenchymal stem cells. Mol Cell Biochem 304:167–179
Zhang C, Li J, Zhang L, Zhou Y, Hou W, Quan H, Li X, Chen Y, Yu H (2012) Effects of mechanical vibration on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Arch Oral Biol 57:1395–1407
Zamini A, Ragerdi KA, Barbarestani M et al (2008) Melatonin influences the proliferative and differentiative activity of rat adipose-derived stem cells. Cell J (Yakhteh) 10:25–32
Acknowledgments
The authors would like to express their gratitude to the Amirkabir University of Technology and the Pasteur Institute of Iran for their kind assistance, and are also grateful to Mahdi Rajaei for his critical thoughts.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Safavi, A.S., Rouhi, G., Haghighipour, N. et al. Efficacy of mechanical vibration in regulating mesenchymal stem cells gene expression. In Vitro Cell.Dev.Biol.-Animal 55, 387–394 (2019). https://doi.org/10.1007/s11626-019-00340-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-019-00340-9