Abstract
Cementum is a calcified, avascular connective tissue that laminates the root of a tooth and plays a pivotal role in the development, homeostasis, and regeneration of a periodontal tissue. As a potential treatment for periodontal tissue defects in the patient with chronic periodontitis, much attention has been paid to tissue engineering combined with mesenchymal stem cells for regenerating periodontal tissues including cementum. However, limited information is available for the molecular factors that have impacts on the differentiation of mesenchymal stem cells into cementoblasts. Here, we focus on the effect of Wnt3a as a potential inducer and tested the effect of this protein in vitro using human bone marrow-derived mesenchymal stem cells. It was found that, when cells were cultured in an osteogenic medium containing Wnt3a, cementoblast-specific genes, such as cementum protein 1 and cementum attachment protein, as well as bone-related genes were significantly upregulated. These results suggest that Wnt3a promotes differentiation of the cells into cementoblast-like cells. Further experiments were carried out using inhibitors to gain deeper insights into molecular mechanisms underlying the observed differentiation. As a result, we conclude that Wnt3a-triggered differentiation into cementoblast-like cells is the consequence of the activation of the canonical Wnt signaling pathway with possible involvement of the non-canonical pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cementum is a calcified, avascular connective tissue that laminates the root of a tooth in the form of a thin layer with a thickness of approximately 30–200 μm. This layer is formed by cementoblasts of mesenchymal origin as a part of periodontium (Arzate et al. 2015). Cementum plays a pivotal role in the development, homeostasis, and regeneration of a periodontal tissue (Komaki et al. 2012; Han et al. 2015). Once cementum is destroyed together with the alveolar bone and the periodontal ligament through gum inflammation, these tissues cannot regenerate by themselves due to their poor regenerative potential. Therefore, a new therapeutic strategy is absolutely needed for a growing number of patients with chronic periodontitis (Liu et al. 2014).
Tissue engineering combined with stem/progenitor cells is regarded as a promising approach for regenerating periodontal tissues including cementum, especially for large horizontal tissue defects where a regenerative capability is quite limited due to insufficient supply of endogenous precursor cells from the surrounding host tissues. Therefore, much attention has been paid to the utilization of multipotent stem cells for compensating the shortage of tissue-forming cells (Mitsiadis and Orsini 2016; Hu et al. 2017; Shang et al. 2017; Takewaki et al. 2017). The availability of processing stem cells in vitro prior to their transplantation further adds an advantage to their potentials.
Among several stem cell sources, mesenchymal stem cells have been extensively studied as a promising source for regenerating various tissues including periodontium (Kawaguchi et al. 2004; Matyas et al. 2017; Miao et al. 2017). Mesenchymal stem cells (MSCs) can be isolated from various tissues such as bone marrow, dental pulp, periodontal ligament, and exfoliated deciduous tooth canal, although evidences showed that cells of different origins are not always identical in their capacity of self-renew and multi-lineage differentiation. To date, numerous studies have been conducted for demonstrating an ability of MSCs to differentiate into multiple lineages to form various tissues such as the alveolar bone and the periodontal ligament.
On the other hand, limited information is available for the differentiation of human bone marrow-derived mesenchymal stem cells (hBMSCs) into cementoblasts, though, in hBMSC-based periodontium regeneration, the formation of new cementum as well as bone and ligament is required for the structural and functional recovery of the composite tissue. Therefore, the present study is directed to gain a deeper insight into the factors that have impacts on the differentiation of hBMSCs into cementoblasts.
As a potential activator for the differentiation of hBMSCs into cementoblasts, we focused here wingless-type MMTY integration site family member 3A (Wnt3a). This protein is known as one of the Wnt ligands that activate the canonical Wnt/β-catenin signaling pathway. It is known that Wnt3a is expressed in vivo in Hertwig’s epithelial sheath and Malasse epithelial rests (Nemoto et al. 2016). Since these elements are known to promote cementoblast differentiation through epithelial-mesenchymal interactions during tooth development (Jung et al. 2011; Sonoyama et al. 2007), it may be expected that Wnt3a plays an important role in cementoblast differentiation.
However, to our best knowledge, no study has been made to elucidate the direct effect of Wnt3a on the differentiation of hBMSCs into cementoblasts. Here, we investigated the effect of Wnt3a using immortalized hBMSCs, analyzing gene expression to assess differentiation of these cells into cementoblast-like cells under osteogenic conditions. To gain insights into signaling pathways involved in the Wnt3a-mediated alterations of gene expression patterns, the effect of Wnt3a was examined in the presence of inhibitors for the canonical and non-canonical Wnt signaling pathways.
Materials and Methods
Cells
A hBMSC line, UE6E7T-3, was obtained from JCRB Cell Bank, Osaka, Japan. The cells were immortalized by transformed with hTERT gene as well as human papillomavirus-derived E6 and E7 genes. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; Gibco, Buffalo, NY), 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 ng/mL fibroblast growth factor-2 (FGF-2, Life Technologies, Waltham, MA) in a 10-cm polystyrene tissue culture dish (Corning, Durham, NC) at 37°C under 5% CO2 atmosphere with medium exchange every 3 d. A cementoblast cell line (Kitagawa et al. 2006) was received from Dr. Takashi Takata, Department of Oral and Maxillofacial Pathobiology, Graduate School of Biomedical & Health Sciences, Hiroshima University, and maintained in minimum essential medium Eagle, alpha modification (αMEM; Sigma-Aldrich) supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C under 5% CO2 atmosphere.
Differentiation culture
hBMSCs were harvested by 0.25% trypsin-EDTA treatment and suspended in DMEM plus 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 1 ng/mL FGF-2. The cells were seeded to each well of a 12-well plate at a density of 2 × 104 cells/cm2 and incubated at 37°C under 5% CO2 atmosphere. One day after cell seeding, medium was changed with αMEM containing 10% FBS, 0.1 μM dexamethasone (Sigma-Aldrich), 10 mM β-glycerophosphate (Wako Pure Chemical Industries, Osaka, Japan), 50 μg/mL ascorbic acid (Wako Pure Chemical Industries), 100 U/mL penicillin, and 100 μg/mL streptomycin. This medium is referred to as osteogenic induction medium (OIM). Then, the cells were cultured for additional 6 d at 37°C under 5% CO2 atmosphere. After 6 d, the medium was changed with a fresh medium without FBS but containing 200 ng/mL human recombinant Wnt3a (R&D, Minneapolis, MN). The cells were additionally incubated for 1 d to 14 d and then harvested by trypsinization.
To examine signaling pathways involved in Wnt3a-activated differentiation, several synthetic or polypeptide inhibitors for the canonical or non-canonical Wnt signaling pathways were added to a medium 30 min before the addition of 200 ng/mL Wnt3a. The inhibitors for the Wnt/β-catenin signaling cascade included recombinant human Dickkopf-related protein 1 (DKK-1, 8 nM, Peprotech, Rocky Hill, NJ) and ICG-001 (20 μM, Adooq Bioscience, Irvine, CA). The inhibitors for the MAPK pathways included PD98059 [extracellular signal-regulated kinase (ERK) inhibitor, 50 μM, EMD Millipore, Billerica, MA], SB203580 (p38 inhibitor, 10 μM, EMD Millipore), and SP600125 [c-Jun N-terminal kinase (JNK) inhibitor, 5 μM, EMD Millipore].
Gene expression analysis
After washing cells once with phosphate buffered saline (PBS), total RNA was extracted from the cells using SV Total RNA Isolation System (Promega, Madison, WI) and subjected to complementary DNA (cDNA) synthesis using Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). The cDNA was mixed with KAPA SYBR Fast qPCR Kit (Kapa Biosystems, Wilmington, MA), and the relative amounts of mRNA specific for various genes were determined by quantitative polymerase chain reactions (qPCR). The genes analyzed here included cementum protein 1 (CEMP1), cementum attachment protein (CAP), alkaline phosphatase (ALP), osteocalcin (OCN), dentin sialophosphoprotein (DSPP), β-catenin, axis inhibition protein 2 (Axin2), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The sequence of gene-specific primers used for amplification is shown in Table 1. The primers for DSPP were the gift from Dr. Sigeki Suzuki, Department of Biological Endodontics, Graduate School of Biomedical & Health Sciences, Hiroshima University. Reactions were performed using Eco Real-Time PCR System (Illumina, San Diego, CA) under the thermal cycling condition of denature at 95°C for 3 s and annealing/extension at 60°C for 30 s. Data were analyzed by the ΔΔCt method (Livak and Schmittgen 2001) using a result from housekeeping gene, GAPDH, as an internal control.
Alizarin red S staining
Calcium deposition was assessed by alizarin red S staining, according to the method by others (Nørgaard et al. 2006). Briefly, hBMSCs were seeded to each well of a 12-well plate at a density of 2 × 104 cells/cm2 and cultured until cells reached confluence. Then, Wnt3a was added to a medium to a concentration of 200 ng/mL and cultured for 20 d in an incubator at 37°C under 5% CO2 atmosphere. The cells were fixed by treating with 99.5% ethanol for 10 min and then washed with PBS. The fixed samples were added with 10% alizarin red S solution (Sigma-Aldrich, adjusted to pH 6.4 using 2.8% ammonium solution) and allowed to incubate for 1 h to stain deposited calcium. After rinsed with water and then air-dried, the samples were observed with a microscope (IX73, Olympus, Tokyo, Japan). To quantify the amount of an alizarin red S dye bound to samples, 200 μL of 10% cetylpyridium chloride (Sigma-Aldrich) was added to the samples and allowed to incubate for 20 min at room temperature to extract the dye. Optical density at 550 nm was measured on the supernatant using a microplate reader (Thermo Scientific, Waltham, MA).
Western blotting
hBMSCs were cultured for 12 h in the presence of Wnt3a and washed three times with PBS. The cells were lysed with tris-HCl sample buffer containing 2% sodium dodecyl sulfate (SDS), pulverized for 10 s with a homogenizer (Tomy Seiko, Tokyo, Japan) on ice, and heated at 100°C for 5 min. Then, the homogenized lysate was electrophoresed in 15% SDS-polyacrylamide gel. The separated proteins were blotted to a poly(vinylidene fluoride) membrane (Bio-Rad, Hercules, CA) using Mini Trans-Blot Cell operated at 100 V for 1 h. After blotting, the membrane was blocked with tris-HCl buffer solution containing 0.05% Tween-20 (TBS-T) and 5% skim milk for 1 h at room temperature. Then, the membrane was exposed to a solution of anti-CEMP1 antibody (1:1000, polyclonal, Abcam, Cambridge, UK) diluted with Can Get Signal Solution 1 (Toyobo, Osaka, Japan) and allowed to react for 1 d at 4°C. The membrane was washed three times with TBS-T for each 5 min and then reacted with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G antibody (1:6000, R&D) in TBS-T for 1 h at 4°C. After washing the membrane three times with TBS-T for each 15 min, the membrane was treated with Clarity Western ECL Substrate (Bio-Rad). Finally, chemiluminescence signals were detected with a luminescent image analyzer (ChemiDoc XRS Plus, Bio-Rad).
Statistical analysis
The results of gene expression analysis and alizarin red S staining were statistically analyzed, and Student’s t test was performed at a significance level of p < 0.01 or 0.05.
Results
The effect of Wnt3a on the expression of CEMP1
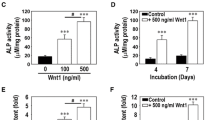
As shown in Fig. 1, the expression of CEMP1 gene was upregulated by Wnt3a addition to OIM in both dose- (Fig. 1A) and time-dependent (Fig. 1B) manners. The relative amount of CEMP1 gene expressed in cells cultured under OIM containing 200 ng/mL Wnt3a was significantly larger than that under OIM with no Wnt3a at the same time point (Fig. 1A). On the other hand, an increase in CEMP1 gene expression was seen after 6 h of incubation and gradually increased during 24-h culture.
The effect of Wnt3a on the expression of CEMP1 in hBMSCs cultured in OIM. The relative expression of CEMP1 gene is shown as a function of (A) Wnt3a concentration (culture period 6 d) and (B) a culture period (Wnt3a concentration 200 ng/mL). Data are expressed as the mean ± standard deviation (n = 3). Statistical significance of difference between data was analyzed by Student’s t test (*p < 0.05, **p < 0.01).
Figure 2 shows the results of gene expression analysis performed for various genes related to bone/cementum formation (CAP, ALP, OCN, and DSPP) and the canonical Wnt3a/β-catenin signaling pathway (Axin2 and β-catenin). It can be seen that the expression level of all genes studied here was higher in hBMSCs cultured under OIM containing Wnt3a than those under OIM with no Wnt3a added, indicating that Wnt3a enhanced the expression of genes related to bone/cementum formation and the Wnt/β-catenin signaling.
The effect of Wnt3a on the expression of genes related to bone and cementum formation (CEMP1, CAP, ALP, OCN, and DSPP) and the canonical Wnt/β-catenin signaling pathway (Axin2 and β-catenin). hBMSCs were cultured in OIM for 7 d, while 200 ng/mL Wnt3a was added to the medium 12 h before RNA isolation. Data are expressed as the mean ± standard deviation (n = 3). Statistical significance of difference compared to the control was analyzed by Student’s t test (*p < 0.05, **p < 0.01).
In order to study changes in gene expression during much longer time periods, hBMSCs were cultured in the presence of Wnt3a for 14 d. As shown in Fig. 3, the relative amount of CEMP1, CAP, and OCN mRNAs increased with time, whereas that of ALP mRNA reached maximum at day 1 and markedly decreased on days 7 and 14.
The expression of genes related to bone and cementum formation, including CEMP1, CAP, ALP, and OCN, in hBMSCs cultured in the presence of 200 ng/mL Wnt3a. Total RNA was extracted from cells cultured for 0, 1, 7, or 14 d and subjected to reverse transcription and then qPCR. Data are expressed as the mean ± standard deviation (n = 3). Statistical significance of difference between data was analyzed by Student’s t test (*p < 0.05, **p < 0.01).
Calcium deposition
In order to examine the effect of Wnt3a on calcium deposition, hBMSCs were cultured in the presence or the absence of Wnt3a for 20 d and then stained with alizarin red S. As shown in Fig. 4A and B, negligible staining was seen in a culture under growth medium regardless of the presence or absence of Wnt3a. In contrast, cells cultured under OIM were strongly stained with alizarin red S (Fig. 4C and D). As shown in Fig. 4E, quantitative analysis revealed that the amount of alizarin red S bound was significantly larger in the case of cells under OIM containing Wnt3a than under control OIM. These results clearly indicate that Wnt3a promotes calcium deposition in the osteogenic environment.
Alizarin red S staining of hBMSCs incubated in the presence or the absence of Wnt3a for 20 d. hBMSCs were cultured in growth medium or OIM with or without Wnt3a. After 20 d, cells were stained with alizarin red S. Culture medium: (A) growth medium without Wnt3a, (B) growth medium with 200 ng/mL Wnt3a, (C) OIM without Wnt3a, and (D) OIM with 200 ng/mL Wnt3a. (E) Quantification of alizarin red S bound to cells cultured in OIM with or without 200 ng/mL Wnt3a. Data are expressed as the mean ± standard deviation (n = 3). Statistical significance of difference between data was analyzed by Student’s t test (*p < 0.05).
Inhibition experiments
Polypeptide and chemical inhibitors for the canonical Wnt/β-catenin signaling pathway, DKK-1 or ICG-001, were added to hBMSC culture under OIM 30 min before an addition of 200 ng/mL Wnt3a. Western blot analysis for the expression of CEMP1 protein was performed for the cells. The results are shown in Fig. 5, together with those from control experiments. It is seen that an addition of Wnt3a enhanced CEMP1 expression, though the blots of CEMP1 are faint (Fig. 5A and B). On the other hand, an addition of Wnt3a together with DKK-1 (Fig. 5A) or ICG-001 (Fig. 5B) impaired CEMP1 expression. It is known that DKK-1 is low-density lipoprotein receptor-related proteins 5/6 (LRP5/6) antagonist and that ICG-001 binds to histone acetyltransferase CREB binding protein (CBP) to disrupt its interaction with β-catenin, leading to the attenuation of Wnt-target gene expression. The results of our inhibition experiments indicate that the canonical Wnt/β-catenin signaling pathway is involved in the upregulation of CEMP1 expression by Wnt3a.
The effect of inhibitors specific for the Wnt/β-catenin signaling pathway on the expression of CEMP1 in hBMSCs cultured under OIM in the presence of 200 ng/mL Wnt3a. (A) DKK-1 or (B) ICG-001 were added to the medium to a concentration of, respectively, 8 nM and 20 μM, 30 min before addition of Wnt3a.
Further experiments were carried out to examine the effect of three inhibitors for the MAPK signaling pathways. The inhibitors included MEK1 inhibitor PD98059, p38 inhibitor SB203580, and JNK inhibitor SP600125. As shown in Fig. 6, the Wnt3a-activated expression of CEMP1 was inhibited by PD98059 (Fig. 6A) and SB203580 (Fig. 6B). To the contrary, an addition of SP600125 with Wnt3a rather promoted CEMP1 expression (Fig. 6C).
The effect of inhibitors specific for the MAPK signaling pathways on the expression of CEMP1 at the mRNA (A–C) and protein levels (D–F). hMSCs were cultured under OIM in the presence of 200 ng/mL Wnt3a. (A, D) PD98059, (B, E) SB203580, and (C, F) SP600125 were added to the medium to a concentration of, respectively, 50, 10, and 5 μM, 30 min before addition of Wnt3a. Data of relative mRNA expression are expressed as the mean ± standard deviation (n = 3). Statistical significance of difference between data was analyzed by Student’s t test (*p < 0.05, **p < 0.01).
Discussion
Differentiation of cementoblasts is crucial for the functional restoration of periodontium via MSC-based regenerative therapy. However, limited information has been available with regard to molecular factors that have impacts on the differentiation of MSCs into cementoblasts. In the present study, we focused on the effect of Wnt3a on the expression of cementoblast-related genes, such as CEMP1 and CAP, in hBMSCs cultured under an osteogenic condition. Here, we demonstrate that Wnt3a promotes differentiation of hBMSCs into cementoblast-like cells. According to our results, the effect of Wnt3a is most likely through activation of the canonical Wnt/β-catenin signaling pathway. In addition, our results indicate the possibility that non-canonical Wnt signaling is also involved in cementoblast differentiation promoted by Wnt3a.
In order to examine the effect of Wnt3a on cementoblast differentiation, we first consider the specific marker that serves to simply report differentiation of cells into the cementoblast lineage. According to previous studies (Alvarez-Pérez et al. 2006; Carmona-Rodríguez et al. 2007), CEMP1 is known to be expressed in cementoblasts and progenitors in the paravascular zone of the periodontal ligament, and therefore can be regarded as a specific marker for cementoblasts and their precursor cells. In our preliminary study, we found that CEMP1 gene was expressed in fact in the cementoblast cell line (data not shown).
It was further reported that overexpression of CEMP1 served to reduce the expression of periodontal ligament-related protein-1 (PLAP-1)/asporin in periodontal ligament cells, while enhancing the expression of cementum-related proteins, such as CAP and bone sialoprotein (BSP) (Komaki et al. 2012). In addition, a similar experiment using human gingival fibroblasts resulted in elevated expression of ALP, OPN, OCN, BSP, and CAP (Carmona-Rodríguez et al. 2007). These results suggest that CEMP1 is involved in the regulation of downstream processes toward bone and cementum formation. It was further shown that CEMP1 promoted proliferation and migration of periodontal ligament cells (Paula-Silva et al. 2010; Hoz et al. 2012). These observations suggest that CEMP1 mediates several cellular processes including cementoblast differentiation as well as wound hearing and regeneration of periodontal tissues. Consequently, we focus here on the expression of CEMP1 in hBMSCs to assess their differentiation into cementoblast-like cells.
The major aim of the present study is to elucidate the effect of Wnt ligands, namely the most representative family member Wnt3a, on the induction of CEMP1-expressing cementoblast-like cells from hBMSCs. It is known that Wnt signaling plays significant roles in the proliferation, polarity, differentiation, and morphogenesis of various cell types. Among diverse functions, we have been inspired by the effect that canonical Wnt/β-catenin signaling is involved in the differentiation of cementoblasts in vitro and the formation of a cementum tissue in vivo (Kim et al. 2011; Han et al. 2012; Zhang et al. 2013; Han et al. 2015).
The canonical Wnt/β-catenin pathway is the most representative for signal transduction triggered by Wnt ligands. In the absence of Wnt stimuli, cytosolic β-catenin is phosphorylated in axis inhibition protein (Axin) complex with glycogen synthase kinase 3β (GSK-3β), adnomatous polyposis coli gene product (APC), and casein kinase 1 (CK1), which then leads to the ubiquitination and proteosomal degradation of β-catenin (MacDonald et al. 2009). In contrast, the binding of Wnt with G-protein coupled receptor, frizzled (Fz), initiates to form a protein complex consisting of Wnt, Fz, and LRP5/6, which subsequently activates intracellular disheveled (Dvl) to recruit the Axin complex, leaving free β-catenin in the cytosol. The stabilized β-catenin is accumulated and translocated into the nucleus to function as a coactivator with a transcription factor, T cell factor (TCF), to promote the expression of target genes (Komiya and Habas 2008; Koch 2017).
In this study, we observed that an addition of Wnt3a promoted the expression of cementum- and bone-related genes including ALP, OCN, and DSPP. Importantly, the expression of cementoblast-specific CEMP1 and CAP genes was also upregulated. In addition, it was found that an addition of Wnt3a into OIM enhanced the mineralization of hBMSCs (Fig. 4). These results strongly suggest that the activation of the canonical Wnt/β-catenin signaling pathway leads to cementogenic differentiation of hBMSCs as well. Quantitative comparison for the expression level of these genes in the cementoblast cell line with that in Wnt3a-treated hBMSCs will reinforce our conclusion. Nevertheless, we speculate that the paracrine signaling of Wnt3a is involved in the epithelial-mesenchymal interactions during tooth development (Jung et al. 2011; Sonoyama et al. 2007) and that Wnt3a-enhanced cementoblast differentiation of hBMSCs partly mimics the natural process of tooth development.
The involvement of the Wnt/β-catenin pathway is substantiated by the observation that the expression of Axin2 gene, known as a direct target gene of Wnt/β-catenin signaling (Han et al. 2015), was promoted. We have analyzed here neither the expression of TCF-responsive genes nor the translocation of β-catenin into nucleus after treatment with Wnt3a. These analyses will provide further evidences for the activation of Wnt/β-catenin signaling pathway by Wnt3a. A previous report has shown that β-catenin upregulates osterix (Osx) expression via direct binding to the promoter region of Osx, resulting in the promotion of cementogenesis in vivo and in vitro (Choi et al. 2017). A further study on the effect of Wnt3a on Osx expression under our experimental conditions will help mechanistically understand the Wnt3a functions in hBMSCs. The observation that CEMP1 expression was attenuated by DKK-1 and ICG-001 inhibitors further confirms the significance of the canonical Wnt/β-catenin signaling pathway in cementogenic differentiation of hBMSCs. Additional experiments showing downregulation of CEMP1 and Axin2 mRNA expression as well as β-catenin target genes upon addition of DKK-1 and ICG-001 will supplementary support our conclusion. Strikingly, β-catenin gene expression was also upregulated, even though the expression of this protein itself seems to be independent from the pathway. The upregulation of β-catenin expression was also reported by others for the case of lithium ion-treated periodontal ligament cells (Han et al. 2012). Our observations that mineralization was enhanced by Wnt3a and that the expression of cementoblast-specific genes was upregulated upon addition of Wnt3a suggest cementoblast differentiation of hBMSCs. Since it is not easy to distinguish between cementum and bone in vitro, further in vivo experiments will serve to strengthen our conclusion.
In literatures, there are several evidences showing that Wnt/β-catenin signaling promotes cementoblast differentiation. Han et al. reported that in in vitro culture of periodontal ligament cells, lithium ions released from biomaterials into a medium served to activate the Wnt/β-catenin signaling, leading to the enhanced expression of cementoblast-related genes including CEMP1, CAP, ALP, and OCN (Han et al. 2012; Han et al. 2015). On the other hand, cementoblast differentiation from periodontal ligament stem cells and periapical follicular stem cells was observed under hypoxia conditions through the activation of Wnt/β-catenin signaling (Choi et al. 2014; Li et al. 2016). In addition, constitutive stabilization of β-catenin is reported to cause excessive cementum formation in vivo (Kim et al. 2011), whereas conditional knockout of β-catenin gene is shown to disrupt cementoblast formation in mice (Zhang et al. 2013). A similar effect of Wnt stimuli was further reported for mouse mesenchymal stem cell line, C3H10T1/2 (Hu et al. 2005). All these reports together with our observation with hBMSCs suggest an essential role of the canonical Wnt/β-catenin signaling in cementoblast differentiation and cementum formation. Since CHIR99021, a glycogen synthase kinase 3 (GSK3) inhibitor, is known to efficiently activate the Wnt/β-catenin signaling more than natural ligands (Naujok et al. 2014), experiments using CHIR99021 would provide a robust evidence that supports the promotive effect of Wnt/β-catenin signaling on cementoblast differentiation of hBMSCs. However, we did not perform such experiments because our major focus was to elucidate the effect of Wnt3a.
According to a report by Nemoto et al. (Nemoto et al. 2009), the expression of cementoblast-related genes is rather downregulated by the activation of Wnt/β-catenin pathway in immortalized cementoblasts. This observation leads us to speculate that the promotive effect of the canonical Wnt/β-catenin pathway has an impact particularly on the early stage of cementoblast differentiation. On the other hand, a previous report showed that the Wnt/β-catenin signaling pathway inhibited cementogenic differentiation of adipose tissue-derived stem cells (Liu et al. 2014). In their study, Wnt3a was added to a conditioned medium prepared using dental follicle cells. This means that the condition employed in their study may be different from that we did here. Therefore, we think that our finding is not necessarily contradictory to their observation.
Earlier studies (Zhang et al. 2014) demonstrated that the non-canonical Wnt signaling pathways have also impacts on the Wnt-sensitive regulation of intracellular β-catenin levels and thus the transcription of target genes. Here, we examined the involvement of three major MAPKs including ERK, p38, and JNK in the upregulation of CEMP1 expression due to activation of the canonical pathway by Wnt3a. As shown in Fig. 6A and B, Wnt3a-induced CEMP1 upregulation was suppressed by inhibitors specific for ERK (PD98059) and p38 (SB203580) signaling cascades, suggesting the cross-talk of these cascades with the canonical Wnt/β-catenin pathway.
It was reported that Wnt stimulates phosphorylation of Raf-1, MEK, and ERK in fibroblasts (Yun et al. 2005). The phosphorylated ERK is translocated and accumulated in the nucleus to effect the β-catenin-dependent transcription. Our results suggest that ERK signaling pathway is involved in the upregulation of CEMP1 expression as a consequence of Wnt/β-catenin signaling in hBMSCs.
Similarly, a cross-talk could be presumed for the case of p38 signaling in hBMSCs, as demonstrated in Fig. 6B. Wnt stimuli were reported to activate p38 MAPKs in totipotent mouse teratocarcinoma cell line F9, while inactivating GSK3β in the Axin complex (Bikkavilli et al. 2008). It was further shown that the activation of p38 MAPK was mediated by Dvl. These observations suggest that p38 MAPK signaling intimately interacts with the canonical Wnt/β-catenin pathway. Our results show that such interactions take place in the case of hBMSCs upon stimulation with Wnt3a. Similar results were reported for mouse mesenchymal cell line C3H10T1/2 (Caverzasio and Manen 2007).
In contrast to the above two cases, an addition of a JNK inhibitor, SP600125, together with Wnt3a in hBMSC culture resulted in further upregulation of CEMP1 gene compared to the case with Wnt3a alone (Fig. 6C). It is known that non-canonical Wnts such as Wnt5a and Wnt11a activate JNK via the non-canonical Wnt/JNK pathway. Although detailed mechanisms remain unknown, it is considered that there is a link between the canonical Wnt/β-catenin and non-canonical Wnt/JNK pathways (Qiu et al. 2011). Interestingly, the non-canonical Wnt5a was reported to attenuate the canonical Wnt/β-catenin signaling by destabilizing β-catenin [Caverzasio and Manen 2007], while the expression of endogenous Wnt5a is enhanced by Wnt3a stimuli in murine dental follicle cells (Sakisaka et al. 2015). These facts led us to assume that attenuated signaling through the canonical Wnt/β-catenin pathway by endogenously secreted non-canonical Wnt5a is moderated by the JNK specific inhibitor SP600125 in hBMSCs, leading to the upregulation of Wnt3a-induced CEMP1 expression, as seen in Fig. 5C.
Taken together, it is suggested that Wnt3a-triggered upregulation of CEMP1 expression in hBMSCs, thus induction of cementoblast-like cells, may be the consequence of close interactions between the canonical and non-canonical pathways, although the present set of data are not necessarily sufficient to confirm the involvement of non-canonical Wnt signaling pathway. It is unclear whether the MAPK inhibitors used here affect the activation of β-catenin signaling during Wnt3a-induced promotion of cementoblast differentiation. In order to clarify this, we further need to analyze, for instance, the influence on the phosphorylation of Axin2 or the nuclear translocation of β-catenin.
Conclusions
This study demonstrates that Wnt3a stimuli serve to upregulate the expression of cementoblast-related genes in hBMSCs likely through the activation of the canonical Wnt signaling pathway with possible involvement of the non-canonical pathway.
References
Alvarez-Pérez MA, Narayanan S, Zeichner-David M, Rodríguez Carmona B, Arzate H (2006) Molecular cloning, expression and immunolocalization of a novel human cementum-derived protein (CP-23). Bone 38:409–419
Arzate H, Zeichner-David M, Mercado-Celis G (2015) Cementum proteins: role in cementogenesis, biomineralization, periodontium formation and regeneration. Periodontol 2000 67: 211–233
Bikkavilli RK, Feigin ME, Malbon CC (2008) p38 mitogen-activated protein kinase regulates canonical Wnt-beta-catenin signaling by inactivation of GSK3β. J Cell Sci 121: 3598–3607
Carmona-Rodríguez B, Alvarez-Pérez MA, Narayanan AS, Zeichner-David M, Reyes-Gasga J, Molina-Guarneros J, García-Hernández AL, Suárez-Franco JL, Chavarría IG, Villarreal-Ramírez E, Arzate H (2007) Human cementum protein 1 induces expression of bone and cementum proteins by human gingival fibroblasts. Biochem Biophys Res Commun 358:763–769
Caverzasio J, Manen D (2007) Essential role of Wnt3a-mediated activation of mitogen-activated protein kinase p38 for the stimulation of alkaline phosphatase activity and matrix mineralization in C3H10T1/2 mesenchymal cells. Endocrinology 148:5323–5330
Choi H, Jin H, Kim JY, Lim KT, Choung HW, Park JY, Chung JH, Choung PH (2014) Hypoxia promotes CEMP1 expression and induces cementoblastic differentiation of human dental stem cells in an HIF-1-dependent manner. Tissue Eng Part A 20:410–423
Choi H, Kim TH, Yang S, Lee JC, You HK, Cho ES (2017) A reciprocal interaction between β-catenin and osterix in cementogenesis. Sci Rep 7: 8160
Han P, Ivanovski S, Crawford R, Xiao Y (2015) Activation of the canonical Wnt signaling pathway induces cementum regeneration. J Bone Miner Res 30:1160–1174
Han P, Wu C, Chang J, Xiao Y (2012) The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/β-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials 33:6370–6379
Hoz L, Romo E, Zeichner-David M, Sanz M, Nuñez J, Gaitán L, Mercado G, Arzate H (2012) Cementum protein 1 (CEMP1) induces differentiation by human periodontal ligament cells under three-dimensional culture conditions. Cell Biol Int 36:129–136
Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F (2005) Sequential roles of hedgehog and Wnt signaling in osteoblast development. Development 132:49–60
Hu L, Liu Y, Wang S (2017) Stem cell-based tooth and periodontal regeneration. Oral Dis (in press). https://doi.org/10.1111/odi.12703
Jung HS, Lee DS, Lee JH, Park SJ, Lee G, Seo BM, Ko JS, Park JC (2011) Directing the differentiation of human dental follicle cells into cementoblasts and/or osteoblasts by a combination of HERS and pulp cells. J Mol Histol 42:227–235
Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H, Takata T, Kato Y, Kurihara H (2004) Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol 75:1281–1287
Kim TH, Lee JY, Baek JA, Lee JC, Yang X, Taketo MM, Jiang R, Cho ES (2011) Constitutive stabilization of β-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem Biophys Res Commun 412:549–555
Kitagawa M, Tahara H, Kitagawa S, Oka H, Kudo Y, Sato S, Ogawa I, Miyaichi M, Takata T (2006) Characterization of established cementoblast-like cell lines from human cementum-lining cells in vitro and in vivo. Bone 39:1035–1042
Koch S (2017) Extrinsic control of Wnt signaling in the intestine. Differentiation 97:1–8
Komaki M, Iwasaki K, Arzate H, Narayanan AS, Izumi Y, Morita I (2012) Cementum protein 1 (CEMP1) induces a cementoblastic phenotype and reduces osteoblastic differentiation in periodontal ligament cells. J Cell Physiol 227:649–657
Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organ 4:68–75
Li S, Shao J, Zhou Y, Friis T, Yao J, Shi B, Xiao Y (2016) The impact of Wnt signalling and hypoxia on osteogenic and cementogenic differentiation in human periodontal ligament cells. Mol Med Rep 14:4975–4982
Liu N, Gu B, Liu N, Nie X, Zhang B, Zhou X, Deng M (2014) Wnt/β-catenin pathway regulates cementogenic differentiation of adipose tissue-deprived stem cells in dental follicle cell-conditioned medium. PLoS One 9:e93364
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods 25:402–408
MacDonald BT, Tamai K, He X (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26
Matyas JJ, Stewart AN, Goldsmith A, Nan Z, Skeel RL, Rossignol J, Dunbar GL (2017) Effects of bone-marrow-derived MSC transplantation on functional recovery in a rat model of spinal cord injury: comparisons of transplant locations and cell concentrations. Cell Transplant 26:1472–1482
Miao C, Lei M, Hu W, Han S, Wang Q (2017) A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res Ther 8:242
Mitsiadis TA, Orsini G (2016) Editorial: a new era in dentistry: stem cell-based approaches for tooth and periodontal tissue regeneration. Front Physiol 7:357
Naujok O, Lentes J, Diekmann U, Davenport C, Lenzen S (2014) Cytotoxicity and activation of the Wnt/beta-catenin pathway in mouse embryonic stem cells treated with four GSK3 inhibitors. BMC Research Notes 7:273
Nemoto E, Koshikawa Y, Kanaya S, Tsuchiya M, Tamura M, Somerman MJ, Shimauchi H (2009) Wnt signaling inhibits cementoblast differentiation and promotes proliferation. Bone 44:805–812
Nemoto E, Sakisaka Y, Tsuchiya M, Tamura M, Nakamura T, Kanaya S, Shimonishi M, Shimauchi H (2016) Wnt3a signaling induces murine dental follicle cells to differentiate into cementoblastic/osteoblastic cells via an osterix-dependent pathway. J Periodontal Res 51:164–174
Nørgaard R, Kassem M, Rattan SI (2006) Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells. Ann N Y Acad Sci 1067:443–447
Paula-Silva FW, Ghosh A, Arzate H, Kapila S, da Silva LA, Kapila YL (2010) Calcium hydroxide promotes cementogenesis and induces cementoblastic differentiation of mesenchymal periodontal ligament cells in a CEMP1- and ERK-dependent manner. Calcif Tissue Int 87:144–157
Qiu W, Chen L, Kassem M (2011) Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem cells. Biochem Biophys Res Commun 413:98–104
Sakisaka Y, Tsuchiya M, Nakamura T, Tamura M, Shimauchi H, Nemoto E (2015) Wnt5a attenuates Wnt3a-induced alkaline phosphatase expression in dental follicle cells. Exp Cell Res 336:85–93
Shang F, Liu S, Ming L, Tian R, Jin F, Ding Y, Zhang Y, Zhang H, Deng Z, Jin Y (2017) Human umbilical cord MSCs as new cell sources for promoting periodontal regeneration in inflammatory periodontal defect. Theranostics 7:4370–4382
Sonoyama W, Seo BM, Yamaza T, Shi S (2007) Human Hertwig’s epithelial root sheath cells play crucial roles in cementum formation. J Dent Res 86:594–599
Takewaki M, Kajiya M, Takeda K, Sasaki S, Motoike S, Komatsu N, Matsuda S, Ouhara K, Mizuno N, Fujita T, Kurihara H (2017) MSC/ECM cellular complexes induce periodontal tissue regeneration. J Dent Res 96:984–991
Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY (2005) Both ERK and Wnt/beta-catenin pathways are involved in Wnt3a-induced proliferation. J Cell Sci 118:313–322
Zhang R, Yang G, Wu X, Xie J, Yang X, Li T (2013) Disruption of Wnt/beta-catenin signaling in odontoblast and cementoblasts arrests tooth root development in postnatal mouse teeth. Int J Biol Sci 9:228–236
Zhang Y, Pizzute T, Pei M (2014) A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res 358:633–649
Acknowledgements
The part of this study was supported by Strategic Promotion of Innovative Research and Development (S-Innovation) program, Japan Science and Technology Agency, FY2012. The authors thank Dr. Takashi Takata, Department of Oral and Maxillofacial Pathobiology, Graduate School of Biomedical & Health Sciences, Hiroshima University, for the cementoblast cell line and Dr. Sigeki Suzuki, Department of Biological Endodontics, Graduate School of Biomedical & Health Sciences, Hiroshima University, for the DSPP-specific primers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Aida, Y., Kurihara, H. & Kato, K. Wnt3a promotes differentiation of human bone marrow-derived mesenchymal stem cells into cementoblast-like cells. In Vitro Cell.Dev.Biol.-Animal 54, 468–476 (2018). https://doi.org/10.1007/s11626-018-0265-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-018-0265-3