Abstract
Objectives
This study aimed to investigate the functions of 19 types of Wnt ligands during the process of osteogenic differentiation in human periodontal ligament stem cells (hPDLSCs), with particular attention to WNT3A and WNT4.
Materials and Methods
The expression levels of 19 types of Wnt ligands were examined using real-time quantitative polymerase chain reaction (real-time qPCR) during hPDLSCs osteogenic differentiation at 7, 10, and 14 days. Knockdown of WNT3A and WNT4 expression was achieved using adenovirus vectors, and conditioned medium derived from WNT3A and WNT4 overexpression plasmids was employed to investigate their roles in hPDLSCs osteogenesis. Osteogenic-specific genes were analyzed using real-time qPCR. Alkaline phosphatase (ALP) and alizarin red S activities and staining were employed to assess hPDLSCs' osteogenic differentiation ability.
Results
During hPDLSCs osteogenic differentiation, the expression of 19 types of Wnt ligands varied, with WNT3A and WNT4 showing significant upregulation. Inhibiting WNT3A and WNT4 expression hindered hPDLSCs' osteogenic capacity. Conditioned medium of WNT3A promoted early osteogenic differentiation, while WNT4 facilitated late osteogenesis slightly.
Conclusion
Wnt ligands, particularly WNT3A and WNT4, play an important role in hPDLSCs' osteogenic differentiation, highlighting their potential as promoters of osteogenesis.
Clinical Relevance.
Given the challenging nature of alveolar bone regeneration, therapeutic strategies that target WNT3A and WNT4 signaling pathways offer promising opportunities. Additionally, innovative gene therapy approaches aimed at regulating of WNT3A and WNT4 expression hold potential for improving alveolar bone regeneration outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The alveolar bone destruction is highly common in oral diseases and the reconstruction and restoration of the lost-bone is paramount to treat them [1,2,3]. Alveolar bone, the most metabolically active and modified part of the skeletal system, consists mainly mineralized tissue, organic matrix and water. The orthodontic treatment principle for patients with malocclusion is the remodeling and resorption of alveolar bone. In patients with severe periodontal disease, a major pathological change is the resorption and destruction of the alveolar bone, which ultimately leads to tooth loss. And it is equally important for the patients with jawbone destruction invaded by carcinoma or the patients with implant placement for tooth loss. Therefore, it is urgent to achieve alveolar bone regeneration [4, 5]. Human periodontal ligament stem cells (hPDLSCs) represent a crucial promising seed cells in periodontal tissue regeneration, characterized by strong clonogenicity, highly proliferation rate, and multi-lineage differentiation potential [6, 7].
Since it is discovered in 2004, hPDLSCs have ushered in a new era in alveolar bone regeneration for its strong osteogenic capacity [6,7,8]. The introduction of hPDLSCs into collagenous porcine bone blocks, comprising both natural cancellous and cortical bone, has been demonstrated to trigger the robust generation of newly mineralized osseous tissue, as documented in previous research [9]. Moreover, a pivotal role in the vascularization of Calcium Phosphate Cement (CPC) scaffolds is played through the co-cultivation of periodontal stem cells with umbilical vein endothelial cells [10]. Simultaneously, the interaction of hPDLSCs with osteogenic differentiation factors, such as bone morphogenetic protein-9 (BMP-9) or specialized bioactive materials designed for bone regeneration, substantially contributes to the effective repair of periodontal, cranial, and various other bone tissue defects [11,12,13]. This remarkable differentiation potential of hPDLSCs, guiding their commitment towards the mesenchymal stem cells (MSCs) lineage, is intricately linked with multiple biological processes. These encompass the secretion of essential paracrine growth factors, active participation in the remodeling of the extracellular matrix, immunomodulatory functions, facilitation of angiogenesis, and engagement with critical signaling pathways, including those governed by BMP and the wingless and int-1 (Wnt) /β-catenin pathway [13,14,15,16].
Previous studies have shown that the Wnt/β-catenin signaling pathway plays an irreplaceable role in the proliferation and osteogenic differentiation of hPDLSCs for the repair of bone defects [7, 8, 17]. Activating the Wnt/β-catenin signaling pathway may contribute to the osteogenic differentiation of hPDLSCs via compounds such as asiaticoside [18], baicalein [19], and luteolin [20]. Kim et al. [21] found that the overexpression of the classical Wnt signaling molecules of Wnt3A and β-catenin could enhance the osteogenic activity of hPDLSCs. And local activation of classical Wnt signaling leds to significant deposition of new cellular dental bone and the well formation of periodontal membrane fibers [22]. However, some studies found that the osteogenic capacity of hPDLSCs can be diminished when Wnt/β-catenin is activated [23, 24]. Presently, the involvement of the Wnt signaling pathway in periodontal stem cell osteogenesis is a topic of debate in the scientific community.
The Wnt signaling pathway, one of the most important pathways in osteogenic differentiation, is composed of Wnt ligands, Wnt receptors, dishevelled (Dsh/Dvl) proteins, β-catenin, and glycogen synthase kinase 3β (GSK-3β). It is also can be classified into classical pathway (β-catenin dependent) and non-classical pathway (not β-catenin dependent) according to whether it is β-catenin dependent or not [25]. Till now, 19 kinds of Wnt ligands have been identified and current studies have shown that multiple Wnt ligands may be involved in the balance between osteogenesis and osteolysis in the same cell, and that even if the same pathway is activated, the different ligands activated often produce different results; and that the same ligands may play different roles in different cells, which differs from the early view that the classical Wnt signaling pathways tended to promote osteogenesis and as for the non-classical ones may promote osteoclast activity [26,27,28]. Currently, there is too little research to study the role of these 19 types of Wnt ligands in the osteogenic differentiation of hPDLSCs.
In this study, we explored the role of 19 types of Wnt ligands in the process of osteogenic differentiation of hPDLSCs and focus on the molecular mechanisms of the significant upregulation of WNT3A and WNT4.
Methods and Materials
Sample preparation, hPDLSCs isolation, culture, and identification
Healthy permanent teeth from individuals aged 12–18 years extracted for orthodontic treatment reason were collected for hPDLSCs culture, following approval from the Medical Ethics Committee of the Affiliated Stomatological Hospital of Fujian Medical University (2018-IRB-28). The culture of hPDLSCs was performed using enzymatic digestion combined with tissue blocks, as described in previous literature [29]. Immunohistochemical staining of vimentin and keratin (Rabbit anti-human vimentin/ keratin protein polyclonal antibody, 1:200 dilution, Maixin, Fuzhou, China) proteins was conducted on the third passage of hPDLSCs to confirm their mesenchymal nature. The trilineage differentiation potential of hPDLSCs was assessed through alizarin red staining (Alizarin Red Staining Kit G8550-25, Solarbio, Beijing, China) for osteogenic differentiation, oil red O staining (O0625-25G, Sigma, USA) for lipogenic differentiation, and alcian blue staining (Alcian Blue Kit G2541-2, Solarbio, Beijing, China) for chondrogenic differentiation. For this study, hPDLSCs from the 3–5 passages were used.
Detection of Wnt ligand expression during hPDLSCs osteogenic differentiation using Real-time qPCR
hPDLSCs were exposed to osteogenic induction media (referred to as the DM group) or non-induction media (referred to as the CM group) for 7, 10, and 14 days to induce osteogenic differentiation. The culture media were changed with fresh eluates every 3 days. Total RNA was extracted from the cells at the designated time points using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Reverse transcription was performed using the PrimeScriptTM RT reagent kit (Takara Bio, Inc.) to produce cDNA from 1 μg of total RNA (20 μL volume). The mRNA expression levels were determined using the SYBR Premix ExTaq kit (Takara Bio, Inc.) with β-actin as the internal reference. Data analysis was performed using the 2−ΔΔCt method. The primer sequences used for mRNA analysis are provided in supplementary Table 1–3.
Construction of WNT3A and WNT4 short hairpin (sh) RNA expression lentivirus
The lentiviral vector of shRNA targeting WNT3A and WNT4 was packaged by GenePharma (Shanghai, China), combining the GV lentiviral vector series, the pHelper1.0 vector, and the pHelper2.0 vector with three plasmids. The constructed shRNA expression vectors were named as follows: LV-WNT3A-RNAi (62,405–1), LV-WNT3A-RNAi (62,406–1), LV-WNT3A-RNAi (62,407–1), LV-WNT4-RNAi (82,225–1), LV-WNT4-RNAi (82,226–2), LV-WNT4-RNAi (82,227–1). They were abbreviated as shRNA-62405, shRNA-62406, shRNA-62407, shRNA-82225, shRNA-82226, and shRNA-82227, respectively. A negative control shRNA with no homology to WNT3A and WNT4 sequences was synthesized and named shRNA-NC. The primer sequences for these constructs are provided in supplementary Table 3.
A puromycin sensitivity assay was conducted to determine the minimum concentration of puromycin that completely killed all cells for subsequent experiments. hPDLSCs were seeded in 24-well plates at a density of 15,000 cells/well and incubated for 24 h. The culture medium was then replaced with complete medium containing puromycin at concentrations of 0, 0.5, 1, 1.5, 2, 2.5, 3, and 3.5 μg/ml. Each group consisted of 3 wells, and cell death was evaluated after 48 h. Flow cytometric analysis was performed to determine the suitable multiplicity of infection (MOI) and the type of transfection enhancement reagent. The silencing effect of WNT3A and WNT4 was verified using real-time qPCR and western blotting following the respective manufacturer's instructions.
Detection of hPDLSCs osteogenic differentiation with silencing of WNT3A and WNT4
The osteogenic differentiation ability of hPDLSCs was assessed through alkaline phosphatase (ALP) and alizarin red S activities, staining, and the expression of osteogenesis-related genes.
hPDLSCs were cultured in osteogenic media for 7 days. ALP staining was performed on fixed cells using a previously reported method, and the OD values were obtained by cleaving the cells with 1% Triton X-100 for 2 h on ice. The ALP quantification kit was used to assess the ALP activity.
After 14 days of osteogenic induction, alizarin red S staining was performed on hPDLSCs, and their activities were evaluated using the Alizarin Red Semi-Quantitative Assay Kit. Real-time qPCR was conducted to detect the expression of osteogenic genes, including ALP, osteocalcin (OCN), osteopontin (OPN), and collagen I (COL1), in hPDLSCs induced for 14 days.
Effect of WNT3A/WNT4 conditioned medium on hPDLSCs osteogenic differentiation
Exogenous WNT3A and WNT4 proteins were obtained from the supernatant of HEK293T cells transfected with WNT3a and WNT4 plasmids, respectively. An equal concentration of supernatant from HEK293T cells transfected with the pEnter (Empty Vector) plasmid was used as a negative control.
For transfection, HEK293T cells were digested and seeded at a density of 10 × 105 cells in 10 cm dishes. After 16–18 h, transfection was performed following the instructions of the PEI Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Firstly, the PEI solution was heated on a 55 °C water bath for 5 min. Simultaneously, 10 μg of pEnter/WNT3A/WNT4 plasmids (purchased from Shandong Weizhen Biological Company) and 1 μg of eGFP DNA (co-transfected with eGFP) were diluted in 1 ml of 150 mM NaCl solution and mixed thoroughly. Secondly, 30 μg of PEI was thoroughly diluted in 1 ml of 150 mM NaCl solution. Finally, the PEI/NaCl solution was added to the DNA/NaCl solution, mixed well, and incubated at room temperature for 20 min. The resulting mixture was then added to the culture dish. After 24 h of transfection, the original culture medium was replaced with fresh osteogenic induction medium. And 48 h of culture later, the supernatant was collected, filtered through a 0.22 μm filter sieve, dispensed into 1.5 ml sterile EP tubes, and stored at -20 °C. Prior to use, the supernatant was thawed at 4 °C.
The successful transfection of the WNT3A and WNT4 plasmids into HEK293T cells was confirmed by fluorescence microscopy, and the collected supernatant proteins were subjected to Western Blotting using an affinity tag Flag.
To assess the effect of WNT3A/WNT4 conditioned medium on the proliferation of hPDLSCs, the cell counting kit (CCK-8 kit, Dojindo, Kumamoto, Japan) method was employed. hPDLSCs were seeded at a density of 2500 cells per well in a 96-well plate. After 24 h, the culture medium was replaced with 10% WNT3A/WNT4-conditioned medium separately. The absorbance at a wavelength of 450 nm was measured daily for 5 consecutive days using a CCK-8 assay kit. The average values of each group's optical density (OD) were obtained, and the data were plotted as a line graph.
For the osteogenic differentiation experiments, hPDLSCs were cultured in osteogenic solutions containing 10% exogenous WNT3A/WNT4 protein, respectively. After 7 days of culture, changes in ALP activity and the expression of osteogenesis-related genes were examined, while on day 14, alizarin red staining was performed to evaluate calcified nodule formation.
Statistical analysis
Each experiment was repeated three times, and the results are presented as mean ± standard deviation (x̅ ± SD). Statistical analysis was performed using SPSS 20.0 software (Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to analyze the data, as they met the assumptions of normality and homogeneity. Dunnett's post hoc test was conducted to compare each transfected group with the negative control group (shRNA-NC). Statistical significance was set at P < 0.05.
Results
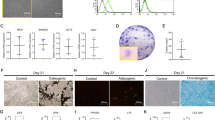
The hPDLSCs exhibit trilineage differentiation potential (Fig. 1 )
Isolation, culture, and identification of human periodontal ligament stem cells (hPDLSCs). (A). Primary culture of hPDLSCs for 7 days (magnification × 100). (B). Primary culture of hPDLSCs for 14 days (magnification × 100). (C). Immunohistochemical staining showing Vimentin-positive expression of hPDLSCs (magnification × 200). (D). Immunohistochemical staining showing Keratin-negative expression of hPDLSCs (magnification × 200). (E). Alizarin Red staining after 14 days of osteogenic induction, demonstrating the presence of insoluble calcium salts (indicated by black arrows) (magnification × 100). (F). Oil Red O and Hematoxylin staining of hPDLSCs after 21 days of lipogenic induction, showing the presence of lipid droplets (indicated by black arrows) (magnification × 200). (G). Alcian Blue staining after 21 days of cartilage formation induction, indicating the presence of endo-acid mucopolysaccharides in cartilage tissue (indicated by the arrow) (magnification × 200).
Spindle-shaped hPDLSCs were successfully isolated from the periodontal tissue mass after 7 days of incubation (Fig. 1A). After approximately 14 days, the hPDLSCs exhibited robust proliferation with a swirling pattern (Fig. 1B). Immunohistochemical staining showed positive expression of vimentin (Fig. 1C) and negative expression of keratin (Fig. 1D) in hPDLSCs, confirming their mesenchymal origin and absence of epithelial cell contamination. Upon trilineage differentiation induction, hPDLSCs demonstrated the formation of numerous alizarin red-positive mineralized nodules (Fig. 1E), red-stained lipid droplets (Fig. 1F), and blue-stained endoacidic mucopolysaccharides and cartilage stroma (Fig. 1G). These findings indicate the stemness and multi-directional differentiation potential of hPDLSCs, thus justifying their designation as hPDLSCs.
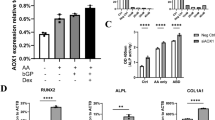
Differential expression of Wnt ligands during hPDLSCs osteogenic differentiation (Fig. 2 )
During the process of hPDLSCs osteogenic differentiation, the expression levels of Wnt ligands were analyzed (Fig. 2). Among the analyzed Wnt ligands, only WNT3A and WNT4 exhibited a significant increase in expression in the DM group (Fig. 2D and 2E). WNT5A and WNT7B showed a slight decrease in expression (Fig. 2F and 2H) and WNT5B exhibited only a marginal increase in expression on the 10th day. However, WNT2, WNT2B, WNT3, WNT9A, WNT10B, WNT11, and WNT16 did not show any significant changes in expression (Fig. 2A-C, G, I-L). The expression levels of WNT1, WNT6, WNT7A, WNT8A, WNT8B, WNT9B, and WNT10A could not be determined as they were below the detection limit in our study.
Successful silencing of WNT3A and WNT4 (Fig. 3
The puromycin sensitivity assay demonstrated that a concentration of 2 μg/mL was optimal for screening lentiviral stable transfected cells. Flow cytometry analysis confirmed that a multiplicity of infection (MOI) of 20, along with the addition of the transfection enhancement reagent hitransG P, resulted in high transfection efficiency (Supplemental Fig. 1 ). The lentivirus-mediated green fluorescent protein (GFP) expression indicated a high infection efficiency of the lentiviral vector (Supplemental Fig. 2).
At the mRNA level, the expression of WNT3A and WNT4 was significantly reduced with the introduction of their respective shRNA interference (Fig. 3A and 3B). Furthermore, at the protein level, shRNA-62406 and shRNA-62407 effectively inhibited the expression of WNT3A protein, while shRNA-62405 had no inhibitory effect (Fig. 3C). Similarly, shRNA-82225 and shRNA-82227 demonstrated effective inhibition of WNT4 protein expression in hPDLSCs, whereas shRNA-82226 did not show inhibitory effects (Fig. 3D). Therefore, for further functional experiments, shRNA-62406, shRNA-62407, shRNA-82225, and shRNA-82227 were selected.
Silencing of WNT3A and WNT4 resulted in decreased osteogenic differentiation of hPDLSCs (Fig. 4 )
Effects of WNT3A and WNT4 silencing on hPDLSCs' osteogenic differentiation. The impact of WNT3A and WNT4 silencing on hPDLSCs' osteogenic differentiation was evaluated based on ALP staining (A) (magnification × 50), ALP activity (B), alizarin red staining (C) (magnification × 50), absorbance of alizarin red staining (D), and the mRNA expression of osteogenic-specific genes (E and F).
To investigate the impact of WNT3A and WNT4 on the osteogenic differentiation of hPDLSCs, we utilized lentiviral vectors carrying shRNA targeting WNT3A and WNT4 to inhibit their expression at both the mRNA and protein levels (Fig. 3). Subsequently, we assessed the effect of WNT3A and WNT4 silencing on hPDLSCs osteogenic differentiation. The staining of alkaline phosphatase (ALP) in the silenced groups of WNT3A and WNT4 exhibited significant attenuation (Fig. 4A), which corresponded to a reduction in ALP activity (Fig. 4B). Alizarin red staining after 14 days of hPDLSCs osteogenesis induction revealed a notable decrease in the formation of insoluble red calcium salt deposits in the WNT3A and WNT4 silenced groups compared to the control group (Fig. 4C). Moreover, the absorbance values measured after dissolving the alizarin red-stained calcium deposits using cetylpyridinium chloride were significantly lower in the silenced groups (Fig. 3D). The decreased expression of osteogenic-specific genes (ALP, OPN, OCN, COL1) further supported the inhibitory effect resulting from the silencing of both WNT3A (Fig. 4E) and WNT4 (Fig. 4F) on the osteogenic differentiation of hPDLSCs.
The conditioned medium of WNT3A facilitates early osteogenic differentiation of hPDLSCs, whereas WNT4 appeared to promote their late osteogenesis (Fig. 5 )
Effects of WNT3A/WNT4 conditioned medium on hPDLSCs' proliferation and osteogenic differentiation. The effects of WNT3A/WNT4 conditioned medium on hPDLSCs' proliferation and osteogenic differentiation were assessed through Western blotting (A), CCK-8 assay (B), mRNA expression of osteogenic-specific genes (C), ALP staining (magnification × 50) and its activity (D), and alizarin red staining (magnification × 50) along with its absorbance value (E).
Green fluorescence was observed in the HEK293T cells under fluorescence microscopy (Supplementary Fig. 3), and significant upregulation in the conditioned medium of WNT3A and WNT4 expression was confirmed by western blotting of the affinity tag Flag (Fig. 5A), indicating successful transfection of the WNT3A and WNT4 plasmids into HEK293T cells and the presence of WNT3A and WNT4 proteins in the collected conditioned medium.
The CCK-8 assay results showed that the experimental group treated with WNT3A/WNT4 conditioned medium had no significant effect on the proliferation of hPDLSCs (Fig. 5B). To evaluate its impact on osteogenic differentiation, we examined the formation of calcified nodules, expression of osteogenesis-related genes, and ALP activity. Our findings revealed that WNT3A conditioned medium promoted early osteogenesis of the ALP activity (Fig. 5D) and the expression of osteogenesis-related genes (Fig. 5C); however, there was no significant difference in the production of late mineralized nodules based on the results of alizarin red staining (Fig. 5E). Interestingly, WNT4 conditioned medium appeared to exhibit a slightly promotion effect on late osteogenesis in hPDLSCs (Fig. 5E).
Discussion
Jaw bone defects or loss pose significant challenges in oral disease treatment. Despite numerous studies focusing on bone regeneration and osteogenesis promotion using various approaches, more efficient strategies for osteogenesis remain to be investigated.
Wnt signaling ligands, crucial components of the Wnt signaling pathway, have been extensively studied for their role in osteogenic or osteolytic differentiation and their impact on bone mass or volume [30,31,32,33]. Different Wnt ligands play distinct roles in bone diseases. For instance, mutations in WNT1 have been associated with osteogenesis imperfecta (OI) type XV and early-onset osteoporosis [34, 35]. Additionally, WNT3, WNT5A, WNT6, WNT7A, WNT10B, and others have been implicated in limb development and skeletal bone disorders [28, 32, 36,37,38]. In this study, we examined the changes in 19 Wnt ligands during the osteogenic differentiation of hPDLSCs using real-time qPCR and specifically investigated the functions of WNT3A and WNT4.
The WNT3A gene, located on human chromosome 1q42, activates the classical Wnt signaling pathway by inducing the accumulation of β-linked proteins. It plays important roles in various cell types, such as inhibiting apoptosis in mouse embryonic liver stem cells to promote their survival and upregulating genes associated with melanocyte differentiation [38]. On the other hand, WNT4 operates through a non-classical signaling pathway and has been shown to promote bone regeneration in craniofacial defective MSCs [39]. Preliminary experiments indicated that WNT3A and WNT4 might be closely related to the osteogenesis of hPDLSCs. Consequently, we further suppressed the expression of WNT3A and WNT4 in hPDLSCs to investigate their effects on osteogenic differentiation.
Previous studies have demonstrated that WNT3A, one of the most extensively studied classical signaling pathway ligands, has effects on both osteoblasts and osteoclasts. In an experimental periodontitis study, researchers examined the expression of WNT3A and Dkk1 in rat periodontal tissues and found a time-dependent decrease in WNT3A expression and an increase in the expression of its inhibitor, Dkk1[40]. This suggests that WNT3A and Dkk1 may play crucial roles in alveolar bone loss. While some studies have shown that WNT3A activates classical signaling pathways to promote osteogenic differentiation or inhibit osteoclasts, others argue against it, proposing that WNT3A inhibits osteogenesis. Boland et al. [41] demonstrated that WNT3A promoted the proliferation of human bone marrow stem cells while inhibiting their differentiation into osteoblasts. In our study, we observed a reduction in hPDLSCs osteogenic differentiation upon silencing WNT3A and the overexpression of WNT3A conditional medium promoted the early osteogenic differentiation of hPDLSCs.
Wnt4 operates through a non-classical signaling pathway and its ectopic expression has been associated with renal dysplasia. Genome-wide association studies have also revealed a close association between WNT4 and osteogenesis [42]. WNT4 has been shown to promote the osteogenic differentiation of craniofacial defect mesenchymal stem cells through activation of p38 MAPK via a non-classical signaling pathway, thereby accelerating the healing of craniofacial defects [39]. Previous studies have demonstrated that Wnt4 overexpression enhances the proliferation and mineralization of dental pulp stem cells and prevents bone aging and inflammation by inhibiting NF-κB [43]. However, there is currently a lack of conclusive evidence regarding the effect of WNT4 on the osteogenic differentiation of hPDLSCs. Building upon our previous findings, this study employed lentiviral infection to suppress WNT4 expression and investigate its specific effects on the osteogenic differentiation of hPDLSCs. Our results revealed a diminished capacity for osteogenic differentiation. Interestingly, the conditioned medium containing WNT4 had no significant effect on the proliferation, but promote the late osteogenic differentiation of hPDLSCs in some degree, suggesting the need for further investigation into the underlying mechanism.
In summary, our study highlights the importance of Wnt ligands in the osteogenic differentiation of hPDLSCs. We observed that the inhibition of WNT3A and WNT4 expression impairs the osteogenic capacity of hPDLSCs, while WNT3A conditioned medium promotes early osteogenesis in hPDLSCs. Surprisingly, the conditioned medium containing WNT4 did not affect the proliferation but seems to have an effect on late osteogenic differentiation of hPDLSCs. The underlying mechanisms of these observations require further elucidation. Future studies should aim to validate these findings in vivo and explore the underlying mechanisms in more detail.
Conclusion
In conclusion, our study investigated the expression of 19 different Wnt ligands during the osteogenic differentiation of hPDLSCs. Silencing WNT3A and WNT4 resulted in a reduction in hPDLSCs' osteogenic differentiation, highlighting them as promising new targets for the treatment of alveolar bone destruction.
Data availability
The data in our study are available and can be obtained from corresponding author for suitable reasons.
Abbreviations
- hPDLSCs :
-
Human periodontal ligament stem cells
- Wnt :
-
Wingless and int-1
- real-time qPCR :
-
Real-time quantitative polymerase chain reaction
- ALP :
-
Alkaline phosphatase
- GSK-3β :
-
Glycogen synthase kinase 3β
- MOI :
-
Multiplicity of infection
- OCN :
-
Osteocalcin
- OPN :
-
Osteopontin
- COL1 :
-
Collagen I
- OD :
-
Optical density
- ANOVA :
-
One-way analysis of variance
References
Li Y, Ling J, Jiang Q (2021) Inflammasomes in Alveolar Bone Loss. Front Immunol 12:691013. https://doi.org/10.3389/fimmu.2021.691013.Published2021Jun9
Ketharanathan V, Torgersen GR, Petrovski BÉ, Preus HR (2019) Radiographic alveolar bone level and levels of serum 25-OH-Vitamin D3 in ethnic Norwegian and Tamil periodontitis patients and their periodontally healthy controls. BMC Oral Health 19(1):83. https://doi.org/10.1186/s12903-019-0769-6.Published2019May14
Lee Y, Lee JE, Lee AR, Choi EY, Choi IS, Kim SJ (2023) Nifedipine attenuates alveolar bone destruction and improves trabecular microarchitectures in mice with experimental periodontitis. Naunyn Schmiedebergs Arch Pharmacol 396(12):3627–3633. https://doi.org/10.1007/s00210-023-02557-8
Zhao J, Zhou YH, Zhao YQ et al (2023) Oral cavity-derived stem cells and preclinical models of jaw-bone defects for bone tissue engineering. Stem Cell Res Ther 14(1):39. https://doi.org/10.1186/s13287-023-03265-zPublished2023Mar16
Seo BM, Miura M, Gronthos S et al (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364(9429):149–155. https://doi.org/10.1016/S0140-6736(04)16627-0
Kuberan S, Ravi MS, Manjappa AB, Rao S, Basavarajappa MK, Shetty V (2022) Assessment of proliferation, clonogenic assay, and osteogenic differentiation of human periodontal ligament stem cells following application of orthodontic forces. Indian J Dent Res 33(4):382–387. https://doi.org/10.4103/ijdr.ijdr_554_22
Gao W, Liang Y, Wu D, Deng S, Qiu R (2023) Graphene quantum dots enhance the osteogenic differentiation of PDLSCs in the inflammatory microenvironment. BMC Oral Health 23(1):331. https://doi.org/10.1186/s12903-023-03026-7Published2023May27
Purwaningrum M, Giachelli CM, Osathanon T, Rattanapuchpong S, Sawangmake C (2023) Dissecting specific Wnt components governing osteogenic differentiation potential by human periodontal ligament stem cells through interleukin-6. Sci Rep 13(1):9055. https://doi.org/10.1038/s41598-023-35569-8. (Published 2023 Jun 3)
Manescu A, Giuliani A, Mohammadi S et al (2016) Osteogenic potential of dualblocks cultured with human periodontal ligament stem cells: in vitro and synchrotron microtomography study. J Periodontal Res 51(1):112–124. https://doi.org/10.1111/jre.12289
Zhao Z, Sun Y, Qiao Q et al (2021) Human Periodontal Ligament Stem Cell and Umbilical Vein Endothelial Cell Co-Culture to Prevascularize Scaffolds for Angiogenic and Osteogenic Tissue Engineering. Int J Mol Sci 22(22):12363. https://doi.org/10.3390/ijms222212363Published2021Nov16
Zhang Y, Luo W, Zheng L et al (2022) Efficient bone regeneration of BMP9-stimulated human periodontal ligament stem cells (hPDLSCs) in decellularized bone matrix (DBM) constructs to model maxillofacial intrabony defect repair. Stem Cell Res Ther 13(1):535. https://doi.org/10.1186/s13287-022-03221-3Published2022Dec27
Zhu Y, Wang W, Chen Q et al (2023) Bioprinted PDLSCs with high-concentration GelMA hydrogels exhibit enhanced osteogenic differentiation in vitro and promote bone regeneration in vivo. Clin Oral Investig 27(9):5153–5170. https://doi.org/10.1007/s00784-023-05135-7
Zhao Z, Liu J, Weir MD et al (2022) Periodontal ligament stem cell-based bioactive constructs for bone tissue engineering. Front Bioeng Biotechnol 10:1071472. https://doi.org/10.3389/fbioe.2022.1071472
Behm C, Blufstein A, Gahn J et al (2020) Cytokines Differently Define the Immunomodulation of Mesenchymal Stem Cells from the Periodontal Ligament. Cells 9(5):1222. https://doi.org/10.3390/cells9051222Published2020May14
Tomokiyo A, Wada N, Maeda H (2019) Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev 28(15):974–985. https://doi.org/10.1089/scd.2019.0031
Zhu W, Liang M (2015) Periodontal ligament stem cells: current status, concerns, and future prospects. Stem Cells Int 2015:972313. https://doi.org/10.1155/2015/972313
Shim NY, Ryu JI, Heo JS (2022) Osteoinductive function of fucoidan on periodontal ligament stem cells: Role of PI3K/Akt and Wnt/β-catenin signaling pathways. Oral Dis 28(6):1628–1639. https://doi.org/10.1111/odi.13829
Fitri AR, Pavasant P, Chamni S, Sumrejkanchanakij P (2018) Asiaticoside induces osteogenic differentiation of human periodontal ligament cells through the Wnt pathway. J Periodontol 89(5):596–605. https://doi.org/10.1002/JPER.17-0471
Chen LJ, Hu BB, Shi XL et al (2017) Baicalein enhances the osteogenic differentiation of human periodontal ligament cells by activating the Wnt/β-catenin signaling pathway. Arch Oral Biol 78:100–108. https://doi.org/10.1016/j.archoralbio.2017.01.019
Quan H, Dai X, Liu M, Wu C, Wang D (2019) Luteolin supports osteogenic differentiation of human periodontal ligament cells. BMC Oral Health. 19(1):229. https://doi.org/10.1186/s12903-019-0926-y. (Published 2019 Oct 26)
Kim SY, Yoo JY, Ohe JY et al (2014) Differential expression of osteo-modulatory molecules in periodontal ligament stem cells in response to modified titanium surfaces. Biomed Res Int 2014:452175. https://doi.org/10.1155/2014/452175
Han P, Ivanovski S, Crawford R, Xiao Y (2015) Activation of the Canonical Wnt Signaling Pathway Induces Cementum Regeneration. J Bone Miner Res 30(7):1160–1174. https://doi.org/10.1002/jbmr.2445
Zhang LN, Wang XX, Wang Z, Li KY, Xu BH, Zhang J (2019) Berberine improves advanced glycation end products-induced osteogenic differentiation responses in human periodontal ligament stem cells through the canonical Wnt/β-catenin pathway. Mol Med Rep 19(6):5440–5452. https://doi.org/10.3892/mmr.2019.10193
Liu N, Shi S, Deng M et al (2011) High levels of β-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. J Bone Miner Res 26(9):2082–2095. https://doi.org/10.1002/jbmr.440
Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10(7):468–477. https://doi.org/10.1038/nrm2717
Jähn K, Lara-Castillo N, Brotto L et al (2012) Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of β-catenin. Eur Cell Mater 24:197–210. https://doi.org/10.22203/ecm.v024a14
Javaheri B, Stern AR, Lara N et al (2014) Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res 29(3):705–715. https://doi.org/10.1002/jbmr.2064
Witte F, Dokas J, Neuendorf F, Mundlos S, Stricker S (2009) Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns 9(4):215–223. https://doi.org/10.1016/j.gep.2008.12.009
Sun X, Ping Y, Li X et al (2023) Activation of PGC-1α-dependent mitochondrial biogenesis supports therapeutic effects of silibinin against type I diabetic periodontitis. J Clin Periodontol 50(7):964–979. https://doi.org/10.1111/jcpe.13811
Kornsuthisopon C, Chansaenroj A, Manokawinchoke J, Tompkins KA, Pirarat N, Osathanon T (2022) Non-canonical Wnt signaling participates in Jagged1-induced osteo/odontogenic differentiation in human dental pulp stem cells. Sci Rep 12(1):7583. https://doi.org/10.1038/s41598-022-11596-9. (Published 2022 May 9)
Baron R, Gori F (2018) Targeting WNT signaling in the treatment of osteoporosis. Curr Opin Pharmacol 40:134–141. https://doi.org/10.1016/j.coph.2018.04.011
Marini F, Giusti F, Palmini G, Brandi ML (2023) Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders. Osteoporos Int 34(2):213–238. https://doi.org/10.1007/s00198-022-06523-7
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19(2):179–192. https://doi.org/10.1038/nm.3074
Zhu J, Liu K, He S et al (2023) Type XV osteogenesis imperfecta: A novel mutation in the WNT1 gene, c.620G >A (p.R207H), is associated with an inner ear deformity. Intractable Rare Dis Res. 12(1):58–61
Hu J, Lin X, Gao P et al (2023) Genotypic and Phenotypic Spectrum and Pathogenesis of WNT1 Variants in a Large Cohort of Patients With OI/Osteoporosis. J Clin Endocrinol Metab 108(7):1776–1786. https://doi.org/10.1210/clinem/dgac752
Ming WH, Luan ZL, Yao Y, et al (2023) Pregnane X receptor activation alleviates renal fibrosis in mice via interacting with p53 and inhibiting the Wnt7a/β-catenin signaling. Acta Pharmacol Sin 44(10):2075–2090. https://doi.org/10.1038/s41401-023-01113-7
Sukarawan W, Rattanawarawipa P, Yaemkleebbua K et al (2023) Wnt3a promotes odonto/osteogenic differentiation in vitro and tertiary dentin formation in a rat model. Int Endod J 56(4):514–529. https://doi.org/10.1111/iej.13888
Chien AJ, Moore EC, Lonsdorf AS et al (2009) Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A 106(4):1193–1198. https://doi.org/10.1073/pnas.0811902106
Chang J, Sonoyama W, Wang Z et al (2007) Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem 282(42):30938–30948. https://doi.org/10.1074/jbc.M702391200
Liu J, Ren X, Zhang M, Lei Y, Chen Y, He H (2017) Roles of Wnt3a and Dkk1 in experimental periodontitis. J Dent Sci 12(3):220–225. https://doi.org/10.1016/j.jds.2016.11.006
Boland GM, Perkins G, Hall DJ, Tuan RS (2004) Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem 93(6):1210–1230. https://doi.org/10.1002/jcb.20284
Hsu YH, Kiel DP (2012) Clinical review: Genome-wide association studies of skeletal phenotypes: what we have learned and where we are headed. J Clin Endocrinol Metab 97(10):E1958–E1977. https://doi.org/10.1210/jc.2012-1890
Liu Z, Jiang T, Wang Y, Wang X (2013) Fluocinolone acetonide promotes the proliferation and mineralization of dental pulp cells. J Endod 39(2):217–222. https://doi.org/10.1016/j.joen.2012.09.012
Funding
This work was funded by Fujian Provincial Health Technology Project (grant number: 2020CXB030).
Author information
Authors and Affiliations
Contributions
The study protocol was meticulously designed by Xiao Zhang, Hanrui Lin, Dali Zheng, You-guang Lu, Yuchun Zou, and Bohua Su collectively. Yuchun Zou and Xiao Zhang took the lead in drafting the manuscript. Xiao Zhang and Hanrui Lin were primarily responsible for conducting the experiments and overseeing the procedures. Xiao Zhang, Hanrui Lin, and Dali Zheng collectively conducted the data analysis. The manuscript was revised under the guidance and contributions of Dali Zheng, You-guang Lu, and Bohua Su. All authors actively participated in reviewing and providing feedback on the manuscript, ultimately approving the final version. The final version of the manuscript was read and approved by all authors to ensure its accuracy and scientific integrity.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval and Consent to Participate
Our research was granted approval by the Medical Ethics Committee of the Hospital of Stomatology, Fujian Medical University under reference number 2018-IRB-28.
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Lin, H., Zheng, Dl. et al. Exploring the Role of Wnt Ligands in Osteogenic Differentiation of Human Periodontal Ligament Stem Cells. Clin Oral Invest 28, 64 (2024). https://doi.org/10.1007/s00784-023-05449-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-023-05449-6