Abstract
Chondrogenesis is a developmental process that is controlled and coordinated by many growth and differentiation factors, in addition to environmental factors that initiate or suppress cellular signaling pathways and the transcription of specific genes in a temporal-spatial manner. As key signaling molecules in regulating cell proliferation, homeostasis and development, both mitogen-activated protein kinases (MAPK) and the Wnt family participate in morphogenesis and tissue patterning, playing important roles in skeletal development, especially chondrogenesis. Recent findings suggest that both signals are also actively involved in arthritis and related diseases. Despite the implication that crosstalk between MAPK and Wnt signaling has a significant function in cancer, few studies have summarized this interaction and its regulation of chondrogenesis. In this review, we focus on MAPK and Wnt signaling, referencing their relationships in various types of cells and particularly to their influence on chondrogenesis and cartilage development. We also discuss the interactions between MAPK and Wnt signaling with respect to cartilage-related diseases such as osteoarthritis and explore potential therapeutic targets for disease treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chondrogenesis is a morphogenetic event that includes proliferation, condensation and differentiation of mesenchymal cells into chondrocytes with the production of a cartilage-specific extracellular matrix (ECM) rich in type II collagen and sulfated proteoglycans (Cancedda et al. 2000). Mitogen-activated protein kinase (MAPK) is one of the conserved signal transduction systems in cartilage and plays a crucial role in chondrogenic differentiation. The MAPK cascades constituting three sequentially activated kinase complexes, which include p38 MAPK, c-Jun N-terminal kinase (JNK) and extracellular regulated kinase (ERK), are substrates for phosphorylation by MAPK kinases (MKKs; Fig. 1). The MKKs are, in turn, phosphorylated by MAPK kinase kinases (MEKKs). With regard to chondrogenesis and chondrocyte differentiation, ERK and p38 MAPK have central roles in mediating chondrocyte proliferation and related gene expression (Krens et al. 2006), whereas JNK has a minor role in chondrogenesis, as JNK phosphorylation is not affected during the process (Nakamura et al. 1999; Stanton et al. 2003). p38 MAPK is usually phosphorylated during chondrogenesis and is generally accepted as a positive regulator in chondrogenesis and chondrocyte differentiation (Oh et al. 2000; Stanton et al. 2003; Watanabe et al. 2001); however, the role of the ERK MAPK pathway (also known as the MEK-ERK kinase cascade) is still controversial. Murakami et al. (2000) reported that ERK is a positive regulator in chondrogenesis, as the increase in SRY (sex-determining region Y)-box 9 (SOX9) levels induced by basic fibroblast growth factor (FGF2) is inhibited by a specific ERK kinase (MEK) inhibitor (U0126) in primary chondrocytes. Co-expression of a constitutively active mutant of MEK1 increases the activity of the Sox9-dependent enhancer in primary chondrocytes and C3H10T1/2 cells (Murakami et al.2000). However, the authors of some studies interpreted MEK-ERK as a negative factor for chondrogenesis. For example, ERK1/2 activities have been observed to decrease as chondrogenesis proceeds and the inhibition of ERK1/2 with PD98059 enhances chondrogenesis (Oh et al. 2000); other studies have also shown similar results (Bobick and Kulyk 2006; Chang et al. 1998).
The best characterized mitogen-activated protein kinase (MAPK) modules are the extracellular regulated kinase (ERK) pathway, the stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK) pathway, and the p38 MAPK pathway. The MAPK cascades consist of an MAPK kinase kinase (MEKK), an ERK kinase (MEK), and an MAPK. MEKKs are activated through a large variety of extracellular signals such as growth factors, cytokine factors, and stress. The activated MEKKs can phosphorylate and activate one or several MEKs, which, in turn, phosphorylate and activate a specific MAPK. Activated MAPK phosphorylates and activates various substrates in the cytoplasm and the nucleus of the cell, including transcription factors. These downstream targets control cellular responses (e.g., apoptosis, proliferation, and differentiation)
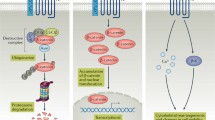
The Wnt family of secreted glycoproteins are signaling molecules that play important roles in controlling a wide range of developmental processes, including tissue patterning, cell proliferation and cell fate, through two distinct canonical and non-canonical Wnt pathways (Fig. 2). In the canonical Wnt signaling pathway, the binding of secreted Wnts to the Frizzled family of cell-surface receptors inactivates glycogen synthase kinase 3beta (GSK3β), resulting in stabilization and nuclear translocation of β-catenin and activation of Wnt target genes. The non-canonical pathways also signal through the Frizzled receptors; the planar cell polarity (PCP) pathway activates the Rho family of GTPases and JNK and modifies cytoskeletal organization and epithelial cell polarization. The Wnt/Ca2+ pathway stimulates the intracellular increase of Ca2+ through the activation of protein kinase C (PKC) and calmodulin-dependent kinase II (CaMKII; Akiyama et al. 2004). During embryonic skeletogenesis, Wnt components act as both positive and negative regulators of key events, including chondroblast differentiation, chondrocyte maturation and joint formation (Church and Francis-West 2002).
Three Wnt-dependent pathways have been categorized: canonical Wnt/β-catenin and non-canonical Wnt/planar cell polarity (PCP) and Wnt/Ca2+ pathways. Canonical Wnt/β-catenin pathway: In cells, with an inactive state of canonical Wnt signaling, cytosolic β-catenin is targeted to proteolytic degradation through phosphorylation by the adenomatous polyposis coli (APC)–Axin–glycogen synthase kinase 3beta (GSK3β) complex and further ubiquitination through the action of the beta-transducin repeat containing protein (βTrCP)-dependent E3 ubiquitin ligase complex. On stimulation by Wnt ligands, through binding to Frizzled (Fzd) receptors and its co-receptor LDL-related protein (LRP), Fzd recruits and phosphorates Dishevelled (Dsh) and inhibits APC–Axin–GSK3β complex formation by the recruitment and inhibition of GSK3β. Consequently, β-catenin can accumulate in the cytoplasm and enter the nucleus, activating the transcription of target genes through an association with the lymphoid enhancer-binding factor-1 (LEF)/T-cell factor (TCF) transcription factor family. Non-canonical Wnt/Ca2+ pathway: Interaction of Wnt ligands with Fzd receptors can lead to an increase in the intracellular calcium level, possibly through the activation of phospholipase C (PLC). Intracellular calcium will subsequently activate Ca2+/calmodulin-dependent protein kinase II (CAMKII) and protein kinase C (PKC) in cells, and the transcription factor called nuclear factor of activated T cells (NFAT). This pathway is particularly important for convergent-extension movements during gastrulation. Additionally, Fzd receptors can also activate JNK, promoting the expression of specific genes through the activation of activator protein-1 (AP-1). Non-canonical Wnt/PCP pathway: This pathway is characterized by an asymmetric distribution of Fzd and related receptors, resulting in the polarization of the cell. Moreover, Wnt-signaling activates Cdc42, RhoA, and Rac1 leading to cytoskeleton rearrangement. Rac1 can also activate JNK, activating specific gene transcription through the modulation of the AP-1 protein complex (dsDNA double-stranded DNA)
In embryos, low levels of Wnt/β-catenin signaling stimulate chondrogenic differentiation of stem cells, whereas high levels of Wnt/β-catenin signaling inhibit that process (Hartmann 2006; Johnson and Rajamannan 2006; Westendorf et al. 2004). Wnts have also been shown to both inhibit and stimulate chondrogenic differentiation of adult progenitor cells (Day et al. 2005; Hill et al. 2005; Hu et al. 2005). Removal of β-catenin early in mesenchymal progenitor cells promotes chondrocyte differentiation, whereas ectopic expression of an activated form of β-catenin in early differentiating chondrocytes induces ectopic joint formation both morphologically and molecularly (Guo et al. 2004). In adult progenitor cells, osteoblast precursors lacking β-catenin are blocked in their differentiation and develop into chondrocytes instead. Detailed in vivo and in vitro loss- and gain-of-function analyses have revealed that β-catenin activity is necessary and sufficient to repress the differentiation of mesenchymal cells into runt-related transcription factor 2 (RUNX2)- and SOX9-positive skeletal precursors (Hill et al. 2005), suggesting that Wnt/β-catenin signaling controls osteoblast and chondrocyte formation when they differentiate from mesenchymal progenitors.
The MAPK pathway has also been reported to regulate Wnt/β-catenin signaling. Wnt/β-catenin signaling is activated by LIT1 and MOM4, which separately encode a homolog of the MAPK-related Nemo-like Kinase (NLK) and a homolog of transforming growth factor beta (TGFβ)-activated kinase (TAK-1; Meneghini et al. 1999). TAK-1, which is a MEKK activated by TGFβ, by bone morphogenetic protein (BMP) and by other MAPK signaling components, plays a critical role in chondrogenesis. Deletion of TAK1 in chondrocytes results in novel embryonic developmental cartilage defects including decreased chondrocyte proliferation, reduced proliferating chondrocyte survival, delayed onset of hypertrophy and reduced matrix metalloproteinase-13 (MMP13) expression (Gunnell et al. 2010). Since both MAPK and Wnt signaling pathways have crucial regulatory functions in the development of cartilage and bone formation, studies of their interactions and crosstalk are of extreme importance for the elucidation of the complex signaling networks in chondrogenesis and for the exploration of potential therapeutic targets for related diseases such as osteoarthritis (OA).
Influence of canonical Wnt signals on MAPK pathway
In totipotent mouse F9 teratocarcinoma cells, the canonical Wnt-β-catenin-JNK signaling pathway has been found to be activated by G-proteins, which can propagate the signals downstream through Dishevelled isoforms. Suppression of Dishevelled-1 or Dishevelled-3 abolished the Wnt3a activation of JNK (Bikkavilli et al. 2008a). Wnt3a treatment enhances the mRNA and protein expression of c-Jun and stimulates the phosphorylation of c-Jun and JNK. Furthermore, Wnt3a activation of activator protein-1 (AP-1) is blocked by the inhibition of JNK with SP600125 and by the inhibition of AP-1 with N-acetyl-L-cysteine and nordihydroguaiaretic acid (Hwang et al. 2005). AP-1 is also activated by ERK1/2 in C3H10T1/2 cells (Seghatoleslami et al. 2003). In NIH3T3 fibroblast cells, ERK pathway activation by Wnt signaling can occur at multiple levels, including β-catenin-independent direct signaling resulting from a Wnt3a (Wnt3a-Raf-1-MEK-ERK) and a β-catenin-/Tcf-4-dependent post gene transcription event (Yun et al. 2005). In addition to JNK and ERK, p38 MAPK is strongly activated by Wnt3a in mouse F9 teratocarcinoma cells and the activated p38 MAPK regulates canonical Wnt-β-catenin signaling through the regulation of GSK3β. Chemical inhibitors of p38 MAPK (SB203580) and the expression of a dominant-negative version of p38 MAPK attenuates the Wnt3a-induced accumulation of β-catenin, lymphoid enhancer-binding factor-1/T-cell factor (Lef/Tcf)-sensitive gene activation and primitive endoderm formation (Bikkavilli et al. 2008b). The above evidence indicates the influence of canonical Wnt signals on the MAPK pathway (Fig. 3).
Influence of canonical Wnt signals on the MAPK pathway. Wnt3a treatment activates the Raf-1-MEK-ERK cascade (Yun et al. 2005) and the JNK pathways (Bikkavilli et al. 2008a). Wnt3a activation of activator protein-1 (AP-1) is blocked by the inhibition of JNK with SP600125 and by the inhibition of AP-1 with N-acetyl-L-cysteine and nordihydroguaiaretic acid (Hwang et al. 2005). In C3H10T1/2 cells, AP-1 is activated by ERK1/2 (Seghatoleslami et al. 2003). In totipotent mouse F9 teratocarcinoma cells, canonical Wnt-β-catenin-JNK signaling is activated by G-proteins, which propagate the signals downstream through Dishevelled (Dsh) isoforms; suppression of Dsh-1 or Dsh-3 abolishes Wnt3a activation of JNK (Bikkavilli et al. 2008a). In addition to JNK and ERK, p38 MAPK is strongly activated by Wnt3a, and the activated p38 MAPK regulates canonical Wnt-β-catenin signaling through the regulation of GSK3β. Chemical inhibitors of p38 MAPK (SB203580) and expression of a dominant-negative version of p38 MAPK attenuates Wnt3a-induced accumulation of β-catenin, Lef/Tcf-sensitive gene activation, and primitive endoderm formation (Bikkavilli et al. 2008b)
The reduced expression of adhesion molecules is known to be associated with the formation and differentiation of cartilage nodules; this is supported by the finding that N-cadherin is expressed in prechondrogenic mesenchymes during cell condensation but not in differentiated chondrocytes (Oberlender and Tuan 1994; Tavella et al. 1994). One study has indicated that the inhibition of p38 MAPK results in the sustained expression of N-cadherin and eventually inhibits chondrogenic differentiation in chick limb mesenchymal micromass cultures (Oh et al. 2000). Wnt regulation of limb mesenchymal chondrogenesis is also involved in the modulation of N-cadherin. Wnt7a signaling has been shown to inhibit the chondrogenic differentiation of limb mesenchymal cells in vitro by modulating the expression of N-cadherin and the turnover of N-cadherin-dependent cell-cell adhesion complexes (Tufan and Tuan 2001). The combination of Wnt7a misexpression and ERK inhibition partially recovers the Wnt7a inhibition of chondrogenic differentiation, whereas the combination of Wnt7a misexpression and p38 inhibition acts in a synergistic chondro-inhibitory fashion (Tufan et al. 2002).
Wnt3a can also induce a rapid and transient activation of p38 MAPK, which in turn regulates alkaline phosphatase activity and mineralization of nodules, directing the differentiation of mesenchymal cells into osteoprogenitors. Dickkopf1, a selective antagonist of Wnt proteins, does not influence the activation of p38 MAPK and ERK induced by Wnt3a (Caverzasio and Manen 2007), implying that non-canonical Wnt pathways participate in the regulatory process of mesenchymal cell differentiation into osteogenic cells.
Influence of non-canonical Wnt signals on MAPK pathway
As a non-canonical Wnt signal, Wnt5a specifically promotes entry into the prehypertrophic phase, whereas it conversely blocks chondrocyte hypertrophy, acting in a stage-specific context (Kawakami et al. 1999; Yang et al. 2003). This finding was confirmed by a study showing that Wnt5a misexpression delays the maturation of chondrocytes and the onset of bone collar formation (Hartmann and Tabin 2000). Wnt5a increases chondrocyte differentiation at an early stage through the CaMK/calcineurin (CaN)/nuclear factor of activated T cells (NFAT)-dependent induction of Sox9, while repressing chondrocyte hypertrophy via the IкB kinase (IKK)/nuclear factor-кB (NF-κB)-dependent inhibition of Runx2 expression (Bradley and Drissi 2010). In mouse F9 embryonal teratocarcinoma cells, strong activation of p38 MAPK has been observed in response to Wnt5a; treatment with SB203580 effectively abolishes the stimulatory effects of Wnt5a (Ma and Wang 2007). Both exogenous TGFβ3 and the overexpression of Wnt5a stimulates PKCα and p38 MAPK activation early in culture, resulting in cellular condensation and chondrogenesis. Comparatively, the inhibition of PKCα or p38 MAPK activity abolishes the promotion of chondrogenic differentiation by overexpressing Wnt5a or exogenous TGFβ3. On the other hand, the partial reduction of endogenous WNT5A by small interfering RNA diminishes TGFβ3-stimulated chondrogenesis through the inhibition of PKCα and p38 MAPK activity (Jin et al. 2006a). Wnt5a has also been found to promote ERK1/2 phosphorylation in endothelial cells (Masckauchán et al. 2006); the expression of Wnt5a blocks canonical Wnt signaling in endothelial cells and other cell types (Topol et al. 2003) (Fig. 4).
Influence of non-canonical Wnt signals on the MAPK pathway. In mouse F9 teratocarcinoma embryonal cells, p38 MAPK is strongly activated in response to Wnt5a, and treatment with SB203580 effectively abolishes the stimulatory effects of Wnt5a (Ma and Wang 2007). Wnt5a also promotes ERK1/2 phosphorylation, enhancing endothelial cell survival and proliferation (Masckauchán et al. 2006), and the expression of Wnt5a blocks canonical Wnt signaling in endothelial cells (Masckauchán et al. 2006) and other cell types (Topol et al. 2003). However, non-canonical Wnt signaling more commonly functions through the Wnt-JNK pathway. Activation of Wnt5a signaling by interleukin-1β (IL-1β) induces the expression of matrix metalloproteinase (MMP) via the JNK pathway in rabbit temporomandibular joint condylar chondrocytes, whereas blockage of JNK signaling impairs the Wnt5a-induced up-regulation of MMPs (Ge et al. 2009). Wnt5a increases chondrocyte differentiation at an early stage through the CaMK/NFAT-dependent induction of Sox9, while repressing chondrocyte hypertrophy via the nuclear factor-кB (NF-κB)-dependent inhibition of Runx2 expression (Bradley and Drissi 2010). Wnt5b activates JNK, a component of the PCP pathway and contributes to an increase in cellular migration but Wnt5b also decreases cell-cell adhesion through the activation of Src and subsequent cadherin receptor turnover (Bradley and Drissi 2011)
However, non-canonical Wnt signaling more commonly functions through the Wnt-JNK pathways (Logan and Nusse 2004). Activation of Wnt5a signaling by interleukin 1beta (IL-1β) induces the expression of MMPs via the JNK pathways in rabbit temporomandibular joint (TMJ) condylar chondrocytes, whereas blockage of JNK signaling impairs the Wnt5a-induced up-regulation of MMPs (Ge et al. 2009). The highly homologous non-canonical Wnt signals, Wnt5a and Wnt5b, have differential effects on cartilage development with regard to cell proliferation and the expression of type II collagen. Unlike Wnt5a, Wnt5b represses chondrocyte differentiation in both the initial stages of cartilage condensation and the late hypertrophic stage (Yang et al. 2003). Wnt5b activates JNK, a component of the PCP pathway, thereby contributing to an increase in cellular migration and Wnt5b-mediated decreases in cell-cell adhesion through the activation of Src and subsequent cadherin receptor turnover (Bradley and Drissi 2011; Fig. 4).
Wnt5a also plays an important role in osteoblast differentiation. The MAPK pathway is altered in Wnt5a-deficient mouse calvarial cells, suggesting that Wnt5a signaling influences the MAPK/JNK pathway (Guo et al. 2008). Other studies provide evidence for crosstalk between Wnt5a and MAPK. Ishitani et al. (2003) found that the overexpression of Wnt5a in HEK293 cells activates NLK MAPK through TAK-1; furthermore, the overexpression of Wnt5a antagonizes the canonical Wnt/β-catenin pathway (Ishitani et al. 2003). Through CaMKII-TAK1-TAB2-NLK, non-canonical Wnt signaling transcriptionally represses the transactivation of peroxisome proliferator-activated receptor gamma (PPARG) and induces RUNX2 expression, promoting osteoblastogenesis in preference to adipogenesis in bone marrow mesenchymal progenitors (Takada et al. 2007). Wnt4, conventionally regarded as a non-canonical Wnt class (Wong et al. 1994), has been found potently to enhance the osteogenic differentiation of mesenchymal stem cells (MSCs) isolated from human adult craniofacial tissue in vitro and bone formation in vivo, through the activation of p38 MAPK, which is known positively to regulate the osteogenic differentiation induced by BMPs and other growth factors (Gallea et al. 2001; Guicheux et al. 2003). The inhibition of p38 MAPK abolishes the osteogenic differentiation of MSCs promoted by Wnt4.
Wnt11 belongs to the Wnt5a subclass that exerts diverse effects through the activation of the non-canonical Wnt signaling pathway (Du et al. 1995). Recently, Rye and Chun (2006) demonstrated that Wnt11 stimulates the accumulation of type II collagen in articular chondrocytes. In three-dimensional alginate gels, WNT11 expression peaks at the late stage of chondrogenic differentiation of human MSCs (Xu et al. 2008). In Xenopus laevis and mouse P19 cells, signaling cascades activated by Wnt11 are crucial for the initiation of cardiogenesis; furthermore, Wnt11 not only inhibits β-catenin signaling but also activates JNK, suggesting crosstalk between Wnt11 and MAPK signals (Pandur et al. 2002). In human hepatocellular carcinoma (HCC) cell lines, the overexpression of Wnt11 activates PKC signaling, which antagonizes canonical Wnt signaling through the phosphorylation of β-catenin and the reduction of T-cell factor (TCF)-mediated transcriptional activity (Toyama et al. 2010). However, few studies have been reported about the interplay between Wnt11 and MAPK signaling in the regulation of chondrogenesis.
Influence of MAPK signals on Wnt pathway
Many studies have shown that MAPKs participate in the regulation of Wnt pathway activities (Fig. 5). Expression of constitutively active MKK6, an upstream activator of p38 MAPK, in 293T cells is sufficient to increase the expression of β-catenin proteins through the direct phosphorylation of GSK3β protein, both in vitro and in vivo; this phosphorylation is blocked by SB203580 or the knock-out of MKK3 and MKK6 (Ding et al. 2005; Thornton et al. 2008). Since MAPK signals are required for the phosphorylation of PPPS/TP motifs of endogenous LDL-related protein 6 (LRP6), Wnt3a-induced phosphorylation of endogenous LRP6 is significantly attenuated by the knock-down of JNK1 and p38β. These results are further confirmed by pharmacological inhibition of p38 MAPK by SB203580 and that of JNK by SP600125 (Červenka et al. 2011). Rac1 can activate JNK2 to phosphorylate β-catenin, which is responsible for controlling limb outgrowth in mouse embryos (Wu et al. 2008). In Xenopus embryos, the activation of JNK antagonizes the canonical Wnt pathway through the activation of the nuclear export of β-catenin rather than its cytoplasmic stability (Liao et al. 2006). Many receptor tyrosine kinase (RTK) systems facilitate Wnt/β-catenin signaling by the phosphatidylinositol 3-kinase (PI3K)/AKT (or alternatively protein kinase B, PKB) pathway through the inhibition of GSK3 activity (Dailey et al. 2005). Interestingly, RTKs have also been found to utilize ERK/LRP6 pathways for the direct phosphorylation of β-catenin to activate WNT/β-catenin signaling (Krejci et al. 2012).
Influence of MAPK signals on the Wnt pathway. Expression of constitutively active MKK6, an upstream activator of p38 MAPK, in 293 T cells increases the expression of β-catenin proteins through the direct phosphorylation of GSK3β protein, both in vitro and in vivo; this phosphorylation is blocked by SB203580 or the knock-out of MKK3 and MKK6 (Thornton et al. 2008). Members of MAPKs such as ERK1/2, p38 MAPK, and JNK contribute to the phosphorylation of PPPS/TP clusters of endogenous LDL-related protein 6 (LRP6) phosphorylation, stimulating Wnt/β-catenin expression. Rac1, a small signaling G protein, can activate JNK2 to phosphorylate β-catenin (Wu et al. 2008). In Xenopus embryos, activation of JNK antagonizes the canonical Wnt pathway through activating the nuclear export of β-catenin instead of maintaining its cytoplasmic stability (Liao et al. 2006). Receptor tyrosine kinase (RTK) systems facilitate Wnt/β-catenin signaling by the phosphatidylinositol 3-kinase (PI3K)/AKT pathway through the inhibition of GSK3 activities (Dailey et al. 2005); RTKs can also phosphorylate β-catenin through involving ERK/LRP6 pathways to activate Wnt/β-catenin signaling (Krejci et al. 2012)
Some critical transcriptional factor activities such as Sox9 or Runx2 are regulated by changing key signals of the Wnt pathways and eventually determine the differentiation fate of cells. BMP2, for instance, promotes chondrogenesis by activating p38 MAPK, which in turn down-regulates Wnt7a/β-catenin signaling. Inhibition of p38 MAPK by using a dominant-negative mutant leads to a sustained Wnt7a increase and decreased Sox9 expression, with the consequent inhibition of pre-cartilage condensation and chondrogenic differentiation (Jin et al. 2006b). Similarly, TGFβ-1-mediated MAPK activation, which controls WNT7A gene expression and Wnt-mediated signaling through the intracellular β-catenin-TCF pathway, probably regulates N-cadherin expression and subsequent N-cadherin-mediated cell-adhesion complexes during the early steps of mesenchymal progenitor cell chondrogenesis (Tuli et al. 2003).
Environmental factors such as mechanical stress and cytokines might also activate the MAPK pathway. Static compressive loading of cartilage activates the MAPK pathway, which is also known as the stress-activated protein kinase (SAPK) pathway (Fanning et al. 2003; Tibbles and Woodgett 1999). Wnt/β-catenin signaling not only is involved in the bone response to mechanical loading (Robinson et al. 2006; Sawakami et al. 2006) but is also associated with the response to mechanical damage to cartilage, which results in an increase in Wnt16 expression (Dell'accio et al. 2008). In MC3T3-E1 osteoblastic cells, the activation of the pathway by treatment with a GSK3β inhibitor results in an anabolic bone formation response, whereas the application of an inhibitor combined with mechanical loading produces a synergistic effect on the expression of Wnt/β-catenin pathway target genes (Robinson et al. 2006). These results indicate that mechanical loading activates the Wnt/β-catenin signaling pathway, at least in part, through the MAPK signaling pathway (Thornton et al. 2008).
Crosstalk of MAPK and Wnt signals in cartilage inflammation and regeneration
OA is a common disease clinically manifested by joint pain, swelling and impairment of joint function and leads to disability and the need for joint replacement. Levels of β-catenin and cyclooxygenase 2 (COX2) are increased in osteoarthritic and rheumatoid arthritic cartilage, suggesting that the accumulation of β-catenin contributes to the inflammatory responses of cartilage by inducing COX2 expression in the chondrocytes of arthritis-affected cartilage (Kim et al. 2002). Activation of β-catenin in mature chondrocytes stimulates hypertrophy and matrix mineralization, as evidenced by the expression of MMP13 and vascular endothelial growth factor (VEGF; Day et al. 2005; Tamamura et al. 2005). Overexpression of β-catenin in chondrocytes markedly increases the expression of matrix degradation enzymes such as MMP-2, MMP-3, MMP-7, MMP-9, membrane-type 3 MMP (MT3-MMP) and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5; Tamamura et al. 2005). Animals and in vitro models exhibiting the knock-out of FRZB, which encodes a secreted Frizzled-related protein that can bind Wnt proteins, are more prone to lose proteoglycans from the articular cartilage in the knee (Lories et al. 2007). Through β-catenin stabilization and its nuclear translocation, Wnt signaling is associated with the negative regulation of early chondrogenesis and the stimulation of chondrocyte hypertrophy during development. Overexpression of Frzb1 lowers the expression of β-catenin (Enomoto-Iwamoto et al. 2002). FRZB −/− mice can experience induced OA formation through the up-regulation and stabilization of β-catenin in the canonical Wnt pathway. Interestingly, Frzb deficiency also results in thicker cortical bone, with increased stiffness and higher cortical appositional bone formation after loading; this seems to support the hypothesized inverse relationship between OA and osteoporosis (Dequeker et al. 2003). In addition, canonical Wnt signaling is influenced by local factors, including alterations in glycosaminoglycan sulfation, cartilage matrix content, TGFβ and vitamin D. Notably, the MMPs and ADAMTSs boosted by the experimental activation of the Wnt/β-catenin pathway are similar to those triggered by treatment with IL-1β or tumor necrosis factor alpha (TNFα) in chondrocytes (Burrage et al. 2006). However, the role of β-catenin in the homeostasis of cartilage is still controversial; as suggested by Zhu et al. (2008), the inhibition of β-catenin signaling in articular chondrocytes causes increased cell apoptosis and articular cartilage destruction in COL2A1-ICAT-transgenic mice. The Wnt/β-catenin pathway is assumed to be part of integrated signal transduction mechanisms through which chondrocytes respond to deranging and catabolic cues, activate the expression of MMP and ADAMTS genes and corresponding proteolytic activity and undermine their phenotypic status and ultimate tissue function (Yuasa et al. 2008).
Accumulating evidence supports a central regulatory role of MAPK in mediating inflammatory and matrix-degrading processes that contribute to joint tissue destruction in OA. Both OA and normal chondrocytes express p38 MAPK; however, OA chondrocytes show a much higher phosphorylated p38 MAPK level compared with normal chondrocytes (Fan et al. 2007; Takebe et al. 2011). Activated JNK has been detected in the cytoplasm of OA chondrocytes but not in healthy controls (Clancy et al. 2001). In a dog model of surgically induced OA, p38 MAPK, JNK and ERK1/2 are all activated to a greater degree compared with those in normal tissue (Boileau et al. 2006). Among the possible MAPK therapeutic targets for OA or rheumatoid arthritis (RA), p38 MAPK is generally considered to be the most promising, as p38 MAPK isoforms have been implicated in the regulation of processes (such as the migration and accumulation of leukocytes and the production of cytokines and pro-inflammatory mediators and angiogenesis) that promote disease pathogenesis (Korb et al. 2006; Schett et al. 2000). p38 MAPK inhibitors have been proven effective in reducing clinical severity, paw swelling, inflammation, cartilage breakdown and bone erosion in a rat streptococcal cell wall arthritis model (Mbalaviele et al. 2006; Mclay et al. 2001), a collagen-induced-arthritis (CIA) model in mice (Medicherla et al. 2006) and adjuvant and CIA models in rats (Badger et al. 2000; Nishikawa et al. 2003). JNK appears to be a critical MAPK pathway for IL-1-induced collagenase gene expression in synoviocytes and joint arthritis (Han et al. 1999, 2001). The JNK inhibitor SP600125 completely blocks not only IL-1-induced accumulation of phosphorylated Jun and induction of c-Jun transcription in synoviocytes but also AP-1 binding and collagenase mRNA accumulation (Han et al. 2001). ERK is known to be involved in the regulation of IL-6, IL-12, IL-23 and TNF-α synthesis, suggesting a possible involvement of ERK in joint damage associated with pro-inflammatory cytokine production by macrophages (Feng et al. 1999; Goodridge et al. 2003). ERK inhibitors have been found to be successful in reducing inflammation in an experimental OA model in rabbits (Pelletier et al. 2003); therapeutic intervention with the goal of MEK1/2 inhibition might have interesting potential for the development of agents for the treatment of OA. Because of their important roles in transducing inflammation and joint destruction, MAPK signals are key molecular targets for therapeutic intervention in inflammatory diseases such as OA and RA. However, inhibitors targeting the ablation or reduction of MAPK activity are likely to have serious side effects (Thalhamer et al. 2008).
Recently, several non-canonical Wnt isoforms such as Wnt5a and Wnt11 were reported to be involved in the IL-1β-induced dedifferentiation of articular chondrocytes (Ryu and Chun 2006). Wnt5a is detectably expressed in OA and RA and is involved in the IL-1β-induced up-regulation of MMP-1, MMP-3, MMP-9 and MMP-13 in primary TMJ condylar chondrocyte via the JNK pathway, suggesting the role of Wnt5a in arthritic pathology and the regulation of cartilage destruction. Furthermore, the blockage of JNK signaling impairs the Wnt5a-induced up-regulation of MMPs (Ge et al. 2009). This finding indicates that crosstalk between Wnt5a and JNK contributes to the pathogenesis of OA; meanwhile, the disturbance or intervention of the interaction between these signals might provide new targets for OA treatment. Several studies have indicated that signaling pathways involving MAPKs mediate the catabolic response of chondrocytes to these inflammatory cytokines; specific inhibitors to these pathways can counteract cytokine effects on matrix protease gene expression (Geng et al. 1996; Hwang et al. 2005; Kumar et al. 2001; Liacini et al. 2002; Ryu et al. 2002).
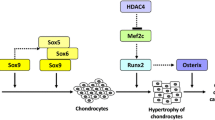
In addition to the activation of inflammatory cytokines such as IL-1, the occurrence of OA can also take the route whereby articular chondrocytes lose their differentiated phenotype and exhibit a behavior with similarities to that of terminal differentiating chondrocytes (hypertrophy-like), as can be found in the growth plate of growing individuals (Dreier 2010; von der Mark et al. 1992). Chondrocytes in OA cartilage show an aberrant phenotype and actively produce cartilage-degrading enzymes, such as MMP-13 and aggrecanases (Moldovan et al. 1997; Shlopov et al. 2000; Song et al. 2007). The higher expression of hypertrophic chondrocyte markers, type X collagen and MMP-13 (Kirsch and von der Mark 1992; Nurminskaya and Linsenmayer 1996) in OA suggests a correlation between hypertrophy and OA. Some studies have demonstrated that Wnt signaling promotes chick chondrocyte hypertrophy through the induction of the bone and cartilage-related transcription factor Runx2. Dong et al. (2006) reported that β-catenin is able to induce RUNX2 and COL10A1 transcription as the molecular mechanism through which Wnt signaling regulates chondrocyte hypertrophy. Protein levels of β-catenin, which accumulates in OA chondrocytes, are extremely low in differentiated articular chondrocytes; however, low levels of β-catenin are up-regulated during phenotypic loss after a serial monolayer culture. Ectopic expression or inhibition of β-catenin degradation causes the cessation of cartilage-specific ECM molecule synthesis via the activation of β-catenin-Tcf/Lef transcriptional activity (Ryu et al. 2002). The activation of Wnt/β-catenin signaling is usually accompanied by a shift in chondrocyte cytoarchitecture. This event might result from a reduction of proteoglycan pericellular matrix or interactions between chondrocyte surface and substrate or fibrillar components such as collagen or fibronectin, which could change intracellular signaling and up-regulate cell adhesion pathways such as that of MAPK (Gemba et al. 2002). Because the loss of a differentiated phenotype of chondrocytes is associated with cartilage destruction during arthritis (Sandell and Aigner 2001), the canonical Wnt pathway-mediated cell phenotype change via crosstalk with MAPK signals is another pathway through which OA forms. For the non-canonical Wnt signal pathway, Wnt5a has been reported as a key parameter influencing the phenotypic stability of chondrocytes (Benya and Shaffer 1982; Yuasa et al. 2008). Wnt5a inhibits type II collagen expression in rabbit TMJ condylar chondrocytes (Ge et al. 2009), suggesting that Wnt5a signaling regulates pathologic cartilage degeneration by inducing chondrocyte dedifferentiation (Ryu and Chun 2006). Increased p38 activity is accompanied by type X collagen staining in osteochondrocytes and marginal synovial cells in a mouse OA model (Seto et al. 2004). During monolayer culture, p38 MAPK is responsible for the loss of chondrocyte phenotypes including type II collagen and Sox9, whereas the blockade of p38 MAPK enhances chondrocyte phenotypes, which suggests a blockade of dedifferentiation (Rosenzweig et al. 2013). Inhibition of p38 signaling in chondrocytes results in decreased expression of the COL10A1 gene (Beier and LuValle 1999; Stanton et al. 2003; Zhen et al. 2001).
Concluding remarks and future directions
Although awareness is increasing with regard to the importance of MAPK and Wnt signaling pathways in regulating cell activities and in relevant diseases such as cancer, their interaction networks and potential roles in disease are still not fully appreciated, especially in the cartilage regeneration area (Table 1). Cartilage differentiation and the maintenance of homeostasis are finely tuned by a complex network of signaling molecules; interplay of these signaling pathways leads to changes in cell activities and eventually influences their differentiation fates. Over the past two decades, extensive studies on the Wnt and MAPK regulation of chondrogenesis and cartilage development have shown that Wnt and MAPK signals have both positive and negative regulatory effects on cartilage development. Accumulating evidence indicates the involvement of Wnt and MAPK signals in the regulation of differentiated chondrocyte functions and cartilage disease.
Recent evidence from both animal experiments and clinical samples has demonstrated the role of both Wnt and MAPK signaling in OA pathology, making these pathways attractive targets for therapy. Some chemicals and drugs targeting MAPK or Wnt have been designed and applied clinically; whereas some are effective in the treatment of OA, their side effects have caused concern. Direct targeting to Wnt or MAPK has been reported to be too risky, because of the crucial role of these signals in the maintenance of articular chondrocyte stability. A promising method would be to identify the mis-regulated genes in the Wnt or MAPK pathways and to try to determine balanced therapeutic targets. The ideal therapeutic goal would be treatment with few or even no side effects in patients. In order to achieve the goal of clinical application, comprehensive appreciation and meticulous evaluation of the interactions of these signaling pathways are essential. Future research should focus on the elucidation of the network between MAPK and Wnt signaling, their interplay and the exploration of clinical application in cartilage regeneration by intervention in specific signaling pathways.
References
Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, Crombrugghe B de (2004) Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev 18:1072–1087
Badger AM, Griswold DE, Kapadia R, Blake S, Swift BA, Hoffman SJ, Stroup GB, Webb E, Rieman DJ, Gowen M, Boehm JC, Adams JL, Lee JC (2000) Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum 43:175–183
Beier F, LuValle P (1999) Serum induction of the collagen X promoter requires the Raf/MEK/ERK and p38 pathways. Biochem Biophys Res Commun 262:50–54
Benya PD, Shaffer JD (1982) Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30:215–224
Bikkavilli RK, Feigin ME, Malbon CC (2008a) G alpha o mediates WNT-JNK signaling through dishevelled 1 and 3, RhoA family members, and MEKK 1 and 4 in mammalian cells. J Cell Sci 121:234–245
Bikkavilli RK, Feigin ME, Malbon CC (2008b) p38 mitogen-activated protein kinase regulates canonical Wnt-beta-catenin signaling by inactivation of GSK3beta. J Cell Sci 121:3598–3607
Bobick BE, Kulyk WM (2006) MEK-ERK signaling plays diverse roles in the regulation of facial chondrogenesis. Exp Cell Res 312:1079–1192
Boileau C, Martel-Pelletier J, Brunet J, Schrier D, Flory C, Boily M, Pelletier JP (2006) PD-0200347, an alpha2delta ligand of the voltage gated calcium channel, inhibits in vivo activation of the Erk1/2 pathway in osteoarthritic chondrocytes: a PKC alpha dependent effect. Ann Rheum Dis 65:573–580
Bradley EW, Drissi MH (2010) WNT5A regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-kappaB pathways. Mol Endocrinol 24:1581–1593
Bradley EW, Drissi MH (2011) Wnt5b regulates mesenchymal cell aggregation and chondrocyte differentiation through the planar cell polarity pathway. J Cell Physiol 226:1683–1693
Burrage PS, Mix KS, Brinckerhoff CE (2006) Matrix metalloproteinases: role in arthritis. Front Biosci 11:529–543
Cancedda R, Castagnola P, Cancedda FD, Dozin B, Quarto R (2000) Developmental control of chondrogenesis and osteogenesis. Int J Dev Biol 44:707–714
Caverzasio J, Manen D (2007) Essential role of Wnt3a-mediated activation of mitogen-activated protein kinase p38 for the stimulation of alkaline phosphatase activity and matrix mineralization in C3H10T1/2 mesenchymal cells. Endocrinology 148:5323–5330
Červenka I, Wolf J, Mašek J, Krejci P, Wilcox WR, Kozubík A, Schulte G, Gutkind JS, Bryja V (2011) Mitogen-activated protein kinases promote WNT/beta-catenin signaling via phosphorylation of LRP6. Mol Cell Biol 31:179–189
Chang SH, Oh CD, Yang MS, Kang SS, Lee YS, Sonn JK, Chun JS (1998) Protein kinase C regulates chondrogenesis of mesenchymes via mitogen-activated protein kinase signaling. J Biol Chem 273:19213–19219
Church VL, Francis-West P (2002) Wnt signalling during limb development. Int J Dev Biol 46:927–936
Clancy R, Rediske J, Koehne C, Stoyanovsky D, Amin A, Attur M, Iyama K, Abramson SB (2001) Activation of stress-activated protein kinase in osteoarthritic cartilage: evidence for nitric oxide dependence. Osteoarthritis Cartilage 9:294–299
Dailey L, Ambrosetti D, Mansukhani A, Basilico C (2005) Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 16:233–247
Day TF, Guo X, Garrett-Beal L, Yang Y (2005) Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8:739–750
Dell'accio F, De Bari C, Eltawil NM, Vanhummelen P, Pitzalis C (2008) Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum 58:1410–1421
Dequeker J, Aerssens J, Luyten FP (2003) Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res 15:426–439
Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC (2005) Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell 19:159–170
Dong YF, Soung do Y, Schwarz EM, O'Keefe RJ, Drissi H (2006) Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol 208:77–86
Dreier R (2010) Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther 12:216
Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT (1995) Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol 15:2625–2634
Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, Koike T, Okada H, Komori T, Yoneda T, Church V, Francis-West PH, Kurisu K, Nohno T, Pacifici M, Iwamoto M (2002) The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol 251:142–156
Fan Z, Söder S, Oehler S, Fundel K, Aigner T (2007) Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am J Pathol 171:938–946
Fanning PJ, Emkey G, Smith RJ, Grodzinsky AJ, Szasz N, Trippel SB (2003) Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. J Biol Chem 278:50940–50948
Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY (1999) Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol 163:6403–6412
Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S (2001) Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone 28:491–498
Ge X, Ma X, Meng J, Zhang C, Ma K, Zhou C (2009) Role of Wnt-5A in interleukin-1beta-induced matrix metalloproteinase expression in rabbit temporomandibular joint condylar chondrocytes. Arthritis Rheum 60:2714–2722
Gemba T, Valbracht J, Alsalameh S, Lotz M (2002) Focal adhesion kinase and mitogen-activated protein kinases are involved in chondrocyte activation by the 29-kDa amino-terminal fibronectin fragment. J Biol Chem 277:907–911
Geng Y, Valbracht J, Lotz M (1996) Selective activation of the mitogen activated protein kinase subgroups c-Jun NH2 terminal kinase and p38 by IL-1 and TNF in human articular chondrocytes. J Clin Invest 98:2425–2430
Goodridge HS, Harnett W, Liew FY, Harnett MM (2003) Differential regulation of interleukin-12 p40 and p35 induction via Erk mitogen-activated protein kinase-dependent and -independent mechanisms and the implications for bioactive IL-12 and IL-23 responses. Immunology 109:415–425
Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J (2003) Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res 18:2060–2068
Gunnell LM, Jonason JH, Loiselle AE, Kohn A, Schwarz EM, Hilton MJ, O'Keefe RJ (2010) TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. J Bone Miner Res 25:1784–1797
Guo J, Jin J, Cooper LF (2008) Dissection of sets of genes that control the character of wnt5a-deficient mouse calvarial cells. Bone 43:961–971
Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y (2004) Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev 18:2404–2417
Han Z, Boyle DL, Aupperle KR, Bennett B, Manning AM, Firestein GS (1999) Jun N-terminal kinase in rheumatoid arthritis. J Pharmacol Exp Ther 291:124–130
Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS (2001) c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 108:73–81
Hartmann C (2006) A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol 16:151–158
Hartmann C, Tabin CJ (2000) Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development 127:3141–3159
Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C (2005) Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8:727–738
Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F (2005) Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 132:49–60
Hwang SG, Yu SS, Lee SW, Chun JS (2005) Wnt-3a regulates chondrocyte differentiation via c-Jun/AP-1 pathway. FEBS Lett 579:4837–4842
Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K (2003) The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol 23:131–139
Jin EJ, Lee SY, Choi YA, Jung JC, Bang OS, Kang SS (2006a) BMP-2-enhanced chondrogenesis involves p38 MAPK-mediated down-regulation of Wnt-7a pathway. Mol Cells 22:353–359
Jin EJ, Park JH, Lee SY, Chun JS, Bang OS, Kang SS (2006b) Wnt-5a is involved in TGF-beta3-stimulated chondrogenic differentiation of chick wing bud mesenchymal cells. Int J Biochem Cell Biol 38:183–195
Johnson ML, Rajamannan N (2006) Diseases of Wnt signaling. Rev Endocr Metab Disord 7:41–49
Kawakami Y, Wada N, Nishimatsu SI, Ishikawa T, Noji S, Nohno T (1999) Involvement of Wnt-5a in chondrogenic pattern formation in the chick limb bud. Develop Growth Differ 41:29–40
Kim SJ, Im DS, Kim SH, Ryu JH, Hwang SG, Seong JK, Chun CH, Chun JS (2002) Beta-catenin regulates expression of cyclooxygenase-2 in articular chondrocytes. Biochem Biophys Res Commun 296:221–226
Kirsch T, Mark K von der (1992a) Remodelling of collagen types I, II and X and calcification of human fetal cartilage. Bone Miner 18:107–117
Korb A, Tohidast-Akrad M, Cetin E, Axmann R, Smolen J, Schett G (2006) Differential tissue expression and activation of p38 MAPK alpha, beta, gamma, and delta isoforms in rheumatoid arthritis. Arthritis Rheum 54:2745–2756
Krejci P, Aklian A, Kaucka M, Sevcikova E, Prochazkova J, Masek JK, Mikolka P, Pospisilova T, Spoustova T, Weis M, Paznekas WA, Wolf JH, Gutkind JS, Wilcox WR, Kozubik A, Jabs EW, Bryja V, Salazar L, Vesela I, Balek L (2012) Receptor tyrosine kinases activate canonical WNT/β-catenin signaling via MAP kinase/LRP6 pathway and direct β-catenin phosphorylation. PLoS One 7:e35826
Krens SF, Spaink HP, Snaar-Jagalska BE (2006) Functions of the MAPK family in vertebrate-development. FEBS Lett 580:4984–4990
Kumar S, Votta BJ, Rieman DJ, Badger AM, Gowen M, Lee JC (2001) IL-1- and TNF-induced bone resorption is mediated by p38 mitogen activated protein kinase. J Cell Physiol 87:294–303
Liacini A, Sylvester J, Li WQ, Zafarullah M (2002) Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors downregulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol 21:251–262
Liao G, Tao Q, Kofron M, Chen JS, Schloemer A, Davis RJ, Hsieh JC, Wylie C, Heasman J, Kuan CY (2006) Jun NH2-terminal kinase (JNK) prevents nuclear beta-catenin accumulation and regulates axis formation in Xenopus embryos. Proc Natl Acad Sci U S A 103:16313–16318
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, Thomas JT, Luyten FP (2007) Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum 56:4095–4103
Ma L, Wang HY (2007) Mitogen-activated protein kinase p38 regulates the Wnt/cyclic GMP/Ca2+ non-canonical pathway. J Biol Chem 282:28980–28990
Mark K von der, Kirsch T, Nerlich A, Kuss A, Weseloh G, Glückert K, Stöss H (1992) Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum 35:806–811
Masckauchán TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, Khoo A, Tycko B, Brown AM, Kitajewski J (2006) Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell 17:5163–5172
Mbalaviele G, Anderson G, Jones A, De Ciechi P, Settle S, Mnich S, Thiede M, Abu-Amer Y, Portanova J, Monahan J (2006) Inhibition of p38 mitogen-activated protein kinase prevents inflammatory bone destruction. J Pharmacol Exp Ther 317:1044–1053
Mclay LM, Halley F, Souness JE, McKenna J, Benning V, Birrell M, Burton B, Belvisi M, Collis A, Constan A, Foster M, Hele D, Jayyosi Z, Kelley M, Maslen C, Miller G, Ouldelhkim MC, Page K, Phipps S, Pollock K, Porter B, Ratcliffe AJ, Redford EJ, Webber S, Slater B, Thybaud V, Wilsher N (2001) The discovery of RPR 200765A, a p38 MAP kinase inhibitor displaying a good oral anti-arthritic efficacy. Bioorg Med Chem 9:537–554
Medicherla S, Ma JY, Mangadu R, Jiang Y, Zhao JJ, Almirez R, Kerr I, Stebbins EG, O'Young G, Kapoun AM, Luedtke G, Chakravarty S, Dugar S, Genant HK, Protter AA (2006) A selective p38 alpha mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J Pharmacol Exp Ther 318:132–141
Meneghini MD, Ishitani T, Carter JC, Hisamoto N, Ninomiya-Tsuji J, Thorpe CJ, Hamill DR, Matsumoto K, Bowerman B (1999) MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature 399:793–797
Moldovan F, Pelletier JP, Hambor J, Cloutier JM, Martel-Pelletier J (1997) Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: in vitro mimicking effect by transforming growth factor beta. Arthritis Rheum 40:1653–1661
Murakami S, Kan M, McKeehan WL, Crombrugghe B de (2000) Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A 97:1113–1118
Nakamura K, Shirai T, Morishita S, Uchida S, Saeki-Miura K, Makishima F (1999) p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Exp Cell Res 250:351–363
Nishikawa M, Myoui A, Tomita T, Takahi K, Nampei A, Yoshikawa H (2003) Prevention of the onset and progression of collagen-induced arthritis in rats by the potent p38 mitogen-activated protein kinase inhibitor FR167653. Arthritis Rheum 48:2670–2681
Nurminskaya M, Linsenmayer TF (1996) Identification and characterization of up-regulated genes during chondrocyte hypertrophy. Dev Dyn 206:260–271
Oberlender SA, Tuan RS (1994) Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development 120:177–187
Oh CD, Chang SH, Yoon YM, Lee SJ, Lee YS, Kang SS, Chun JS (2000) Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem 275:5613–5619
Pandur P, Läsche M, Eisenberg LM, Kühl M (2002) Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418:636–641
Pelletier JP, Fernandes JC, Brunet J, Moldovan F, Schrier D, Flory C, Martel-Pelletier J (2003) In vivo selective inhibition of mitogen-activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthritis Rheum 48:1582–1593
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ (2006) Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281:31720–31728
Rosenzweig DH, Ou SJ, Quinn TM (2013) P38 mitogen-activated protein kinase promotes dedifferentiation of primary articular chondrocytes in monolayer culture. J Cell Mol Med 17:508–517
Ryu JH, Chun JS (2006) Opposing roles of WNT-5A and WNT-11 in interleukin-1beta regulation of type II collagen expression in articular chondrocytes. J Biol Chem 281:22039–22047
Ryu JH, Kim SJ, Kim SH, Oh CD, Hwang SG, Chun CH, Oh SH, Seong JK, Huh TL, Chun JS (2002) Regulation of the chondrocyte phenotype by beta-catenin. Development 129:5541–5550
Sandell LJ, Aigner T (2001) Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res 3:107–113
Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH (2006) The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 281:23698–23711
Schett G, Tohidast-Akrad M, Smolen JS, Schmid BJ, Steiner CW, Bitzan P, Zenz P, Redlich K, Xu Q, Steiner G (2000) Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum 43:2501–2512
Seghatoleslami MR, Roman-Blas JA, Rainville AM, Modaressi R, Danielson KG, Tuan RS (2003) Progression of chondrogenesis in C3H10T1/2 cells is associated with prolonged and tight regulation of ERK1/2. J Cell Biochem 88:1129–1144
Seto H, Kamekura S, Miura T, Yamamoto A, Chikuda H, Ogata T, Hiraoka H, Oda H, Nakamura K, Kurosawa H, Chug UI, Kawaguchi H, Tanaka S (2004) Distinct roles of Smad pathways and p38 pathways in cartilage-specific gene expression in synovial fibroblasts. J Clin Invest 113:718–726
Shlopov BV, Gumanovskaya ML, Hasty KA (2000) Autocrine regulation of collagenase 3 (matrix metalloproteinase 13) during osteoarthritis. Arthritis Rheum 43:195–205
Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW (2007) Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum 56:575–585
Stanton LA, Underhill TM, Beier F (2003) MAP kinases in chondrocyte differentiation. Dev Biol 263:165–175
Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S (2007) A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol 9:1273–1285
Takebe K, Nishiyama T, Hayashi S, Hashimoto S, Fujishiro T, Kanzaki N, Kawakita K, Iwasa K, Kuroda R, Kurosaka M (2011) Regulation of p38 MAPK phosphorylation inhibits chondrocyte apoptosis in response to heat stress or mechanical stress. Int J Mol Med 27:329–335
Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M (2005) Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem 280:19185–19195
Tavella S, Raffo P, Tacchetti C, Cancedda C, Castagnola P (1994) N-CAM and N-cadherin expression during in vitro chondrogenesis. Exp Cell Res 215:354–362
Thalhamer T, McGrath MA, Harnett MM (2008) MAPKs and their relevance to arthritis and inflammation. Rheumatology 47:409–414
Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M (2008) Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science 320:667–670
Tibbles LA, Woodgett JR (1999) The stress-activated protein kinase pathways. Cell Mol Life Sci 55:1230–1254
Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y (2003) Wnt-5a inhibits the canonical wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol 162:899–908
Toyama T, Lee HC, Koga H, Wands JR, Kim M (2010) Noncanonical Wnt11 inhibits hepatocellular carcinoma cell proliferation and migration. Mol Cancer Res 8:254–265
Tufan AC, Tuan RS (2001) Wnt regulation of Limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. FASEB J 15:1436–1438
Tufan AC, Daumer KM, DeLise AM, Tuan RS (2002) AP-1 transcription factor complex is a target of signals from both WnT-7a and N-cadherin-dependent cell-cell adhesion complex during the regulation of limb mesenchymal chondrogenesis. Exp Cell Res 273:197–203
Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS (2003) Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem 278:41227–41236
Watanabe H, Caestecker MP de, Yamada Y (2001) Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem 276:14466–14473
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39
Wong GT, Gavin BJ, McMahon AP (1994) Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol 14:6278–6286
Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F (2008) Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 133:340–353
Xu J, Wang W, Ludeman M, Cheng K, Hayami T, Lotz JC, Kapila S (2008) Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng A 14:667–680
Yang Y, Topol L, Lee H, Wu J (2003) Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 130:1003–1015
Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M (2008) Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest 88:264–274
Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY (2005) Both ERK and Wnt/beta-catenin pathways are involved in Wnt3a-induced proliferation. J Cell Sci 118:313–322
Zhen X, Wei L, Wu Q, Zhang Y, Chen Q (2001) Mitogen-activated protein kinase p38 mediates regulation of chondrocyte differentiation by parathyroid hormone. J Biol Chem 276:4879–4885
Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, O'Keefe RJ, Chen D (2008) Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum 58:2053–2064
Acknowledgments
The authors thank Suzanne Danley for help in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was partially supported by the AO Foundation (S-12-19P) and the National Institutes of Health (NIH) (1 R03 AR062763-01A1).
The authors declare no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhang, Y., Pizzute, T. & Pei, M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res 358, 633–649 (2014). https://doi.org/10.1007/s00441-014-2010-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-2010-x