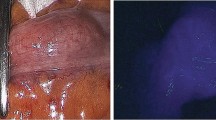
Abstract
Background
Indocyanine green fluorescence angiography (ICG-FA) has been used in colorectal surgery to assess anastomotic perfusion and reduce the risks of anastomotic leaks. The main objective of this paper is to review the data on the transanal application of ICG-FA for the intraluminal assessment of colorectal anastomosis.
Methods
A literature search was conducted for articles published between 2011 and 2021 using PubMed and Cochrane databases, related to the application of ICG for the intraluminal assessment of colorectal anastomosis. Original scientific manuscripts, review articles, meta-analyses, and case reports were considered eligible.
Results
A total of 305 studies have been identified. After abstract screening for duplicates, 285 articles remained. Of those, 271 were not related to the topic of interest, 4 were written in a language other than English, and 4 had incomplete data. Six articles remained for the final analysis. The intraluminal assessment of colorectal anastomosis with ICG-FA is feasible, safe, and may reduce the incidence of leaks.
Conclusion
The intraluminal assessment of anastomotic perfusion via ICG-FA may be a promising novel application of ICG technology. More data is needed to support this application further to reduce leak rates after colorectal surgery, and future randomized clinical trials are awaited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anastomotic leak (AL) is one of the most devastating complications in colorectal surgery, with a reported incidence ranging from 5 to 24%, despite progressive advances in surgical technique.1,2,3,4,5 Besides the impairment of functional outcomes and the detrimental effects on the quality of life of an AL occurring after colorectal surgery, an AL following cancer resection can have even more devastating consequences, as it can delay the start of adjuvant therapy with a subsequent increased risk of local recurrence and reduced survival.6,7,8 Several risk factors for its occurrence have been identified, including patient comorbidities and technical aspects. Among them are the surgical procedure type, operative time, anastomotic configuration, number of linear stapler firings, height, tension, and poor perfusion of the anastomosis,9,10,11,12,13,14 and the exposure to neoadjuvant chemo-radiotherapy.15 Different techniques to test the integrity and perfusion of colorectal anastomosis have been developed over time, although their adoption among surgeons differs among institutions. One of the most promising tools to reduce AL after colorectal surgery is indocyanine green fluorescence angiography (ICG-FA) for the intraoperative evaluation of bowel perfusion. ICG is administered intravenously, and real-time vascular perfusion is evaluated from the serosal coloring detected with visible high-definition endoscopic fluorescence or near-infrared (NIR) spectroscopic laparoscopic cameras. Some authors, however, have questioned the use of ICG-FA due to inherent limits of the technique. For example, the observation from outside the lumen cannot assess the whole circumference of the anastomotic site, perfusion evaluation before the anastomosis is constructed cannot predict the final changes in perfusion after the anastomosis is completed, and the distal side of the anastomosis is often hard to evaluate. The intraluminal assessment of ICG uptake can overcome all these limitations. The main objective of this paper is to review the data on the transanal application of ICG-FA for the intraluminal assessment of colorectal anastomosis.
Methods
This review focuses on two key questions: how effective is the intervention in reducing postoperative complications after colorectal surgery, and what are its effects on patient outcomes? The PRISMA 2020 (preferred reporting items for systematic reviews and meta-analyses) guidelines have been followed.16 The PRISMA diagram is shown in Fig. 1. A literature search was conducted using PubMed and Cochrane databases for eligible articles from 2011 to 2021. A combination of keywords was searched: “indocyanine green fluorescence angiography,” intraluminal indocyanine green,” “colorectal surgery,” “endoscopic colorectal anastomoses assessment,” “colonic perfusion assessment,” and “anastomotic leak.” Inclusion criteria were original scientific manuscripts, review articles, meta-analyses, case reports, English language, and articles regarding the intraluminal assessment of colorectal anastomosis with ICG-FA. No limitation was applied to study type, sample size, and patient age. Exclusion criteria were articles with incomplete data, incorrect or repeated citations, and unrelated to the review topic. Studies were also excluded whether, after construction of the anastomosis and injection of ICG bolus, the endoscopic assessment of the proximal and distal mucosal perfusion was not performed. Additionally, studies that did not report the number of patients, without a clear definition of intraluminal viability or incomplete postoperative follow-up were not deemed eligible for the analysis. SL and DP double screened titles and abstracts and checked full texts against eligibility criteria. Disagreements were resolved by discussion or, if unresolved, through arbitration with a third reviewer (PS). For each study, the following data were extracted: number of patients, study design, median age, benign or malignant indication, level of anastomosis from the anal verge (cm), postoperative complications, and anastomotic leakage. The Joanna Briggs Institute (JBI) Critical Appraisal checklist tools were used to assess the methodological quality of the studies. The risk of bias in the included studies was assessed using the Risk Of Bias In Non-Randomized Studies—of Interventions (ROBINS-I) tool.17
Results
A total of 305 articles have been identified, and 285 remained after removing duplicates (Fig. 1). After the abstract screening, 271 studies were excluded because they were not related to the topic of interest, and the other 8 articles were for the following reasons: non-English language (n = 4), incomplete article or abstract (n = 2), and incomplete or lacking postoperative information (n = 2). Six articles remained for the final analysis (Table 1).
The JBI checklists evaluation is reported in Tables 2 and 3. Overall quality criteria were fulfilled.
The use of intraluminal perfusion assessment of colorectal anastomoses was first described by Sherwinter DA et al.18 using the PINPOINT® Endoscopic Fluorescence Imaging System (Novadaq, ON, Canada) on a cohort of 7 patients undergoing low anterior resection (LAR) for both benign and malignant disease. The study was later prolonged with the inclusion of 13 more patients.19 All patients received mechanical bowel preparation and perioperative antibiotics. All operations were performed laparoscopically using a standardized double-stapled technique. After completion of the anastomosis, a single bolus of 2.5 mg of ICG was administered intravenously, providing fluorescent imaging of mucosal and anastomotic blood flow. Out of the 20 patients in this study, 4 exhibited flow abnormalities on transanal ICG evaluation. In 2 cases, hypofluorescence coincided with visual signs of hypoperfusion in white light. Thus, the surgical team decided to perform a diverting loop ileostomy. In the other 2 cases, blood flow abnormalities were apparent on ICG, and there were no changes to the operative strategy. Both patients developed a peri-anastomotic collection and were successfully treated conservatively with antibiotics. The remaining 16 patients were discharged home without complications with a mean hospital stay of 4.75 (± 1.0) days.
Jafari et al.20 provided the results of a multicenter prospective, open-label clinical trial of 139 patients undergoing LAR for rectal cancer (25%) or left colectomy for diverticulitis (44%) and colon cancer (21%). The mean level of anastomosis was 10 ± 4 cm from the anal verge. Splenic flexure mobilization was performed in 81% and high ligation of the inferior mesenteric artery in 61.9% of patients. The use of ICG-FA (single bolus of 3.75 to 7.5 mg ICG intravenously) changed surgical plans in 11 patients (8%) without the occurrence of leaks. The changes included the revision of the point of proximal colon transection in 9 cases (6.5%), the takedown and revision of the anastomosis after transanal perfusion assessment in 1 patient, and confirmation of the viability of anastomosis in 1 case with concerns of malperfusion under white light. In the latter case, the transanal ICG-FA altered the intraoperative plan for diversion to no diversion. The overall complication rate reported in the study was 17%. Anastomotic leaks occurred in 2 patients (1.4%) that were successfully treated conservatively. This study presents a moderate risk of bias after the ROBIN-I assessment.
The study from Amagai H. et al.21 was conducted prospectively on 71 patients undergoing left-sided colectomy or rectal surgery. All patients received standard bowel preparation and antibiotic prophylaxis the day before the operation. ICG was administered intraoperatively at a dosage of 0.2 mg/kg. Side-to-side anastomoses were performed in 16 cases, side-to-end in 40 cases, and end-to-end in 13 cases. One case of side-to-end anastomosis and three cases of end-to-end anastomosis were hand-sewn. Anastomotic leaks occurred in nine patients (13%); two required reoperation, and the remaining were treated with antibiotics and drainage. The group of Amagai was one of the first to attempt to correlate the quantification of ICG fluorescence with the risk of dehiscence. Fluorescence was measured using the ImageJ software (National Institutes of Health, Maryland, USA), and a time-intensity fluorescence curve was created. The authors then localized the areas of the anastomosis with the highest and lowest fluorescence intensity and the contrast pattern of the mucosa. Significant differences in the time from ICG injection to maximum fluorescence (p = 0.015) and in time from the initial coloring to maximum fluorescence (p = 0.04) were evident in cases where a leak occurred. However, the described ICG quantification method was deemed impractical by the same authors for real-time intraoperative use. Therefore, they visually analyzed the contrast pattern of the colonic mucosa. The areas showing the lowest fluorescence location in the proximal and distal intestine that lacked visible vessels after ICG injection had a significantly higher incidence of leaks than those in which vessels were depicted (p = 0.031 and p = 0.030, respectively). Some of the areas in which vessels were not characterized by ICG fluorescence observation from the luminal side corresponded to the leakage points. This study presents a moderate risk of bias after the ROBIN-I assessment.
Mizrahi I et al.22 evaluated the impact of ICG-FA on any change in proximal resection margin and/or anastomotic leak following transanal total mesorectal excision (taTME) for rectal cancer in a retrospective dual center cohort study. All patients had preoperative mechanical and antibiotic bowel preparation. In the application of bowel perfusion, ICG was used in the range of 0.1–0.3 mg/kg. All patients followed an enhanced recovery after surgery protocol. Fifty-four patients (31 males, 23 females; mean age 63 ± 12 years) were included; 30 (55%) received neoadjuvant chemoradiation therapy. The average anastomotic height was 3.6 cm from the anal verge, 8 (14.5%) patients required intersphincteric dissection, and 46 (85%) had loop ileostomy.
The study did not restrict the type of anastomotic technique; that was left to the surgeon’s judgment. In 44.5% of the cases, the anastomosis was hand-sewn and stapled in the remaining 55.5%.
A straight anastomosis was created in 26 patients (48%), a side-to-end in 20 (37%), and a colonic J pouch in 8 (15%). The use of endoluminal ICG-FA led to a change in the proximal resection margin in 10 patients (18.5%), one of whom had an anastomotic leak, although ICG-FA showed adequate blood supply to the anastomosis. This patient had a straight anastomosis, requiring reintervention with the creation of a loop ileostomy. A second patient undergoing an intersphincteric dissection with the construction of a hand-sewn colonic J pouch-anal anastomosis 2 cm from the anal verge and a diverting loop ileostomy without a change in the proximal resection margin after ICG-FA also had an anastomotic leak. In this case, the patient did not require reintervention and was treated conservatively. The overall leak rate of the study was 3.7%. The splenic flexure was not mobilized, and the specimen was extracted transanally in both patients who suffered a leak. This study presents a moderate risk of bias after the ROBIN-I assessment.
The largest study on the assessment of ICG-FA for the evaluation of the mucosa by transanal visualization was conducted by Otero-Piñeiro AM et al.23 on 284 rectal cancer patients undergoing taTME. The patients were divided into two groups: no ICG-FA assessment (204 patients) and ICG-FA assessment (80 patients). The study’s primary aim was to compare anastomotic leak rates among groups. The use of ICG-FA altered the surgical plan in 23 patients (28.7%), changing the proximal colonic transection in 22 cases. In the remaining case, the transanal assessment showed insufficient anastomotic perfusion that led to splenic flexure mobilization with an improvement of tissue perfusion. Anastomotic leaks occurred in 23 cases (11.3%) in the non-ICGA group vs. two (2.5%) cases in the ICGA group (p = 0.020). Univariate analysis identified three variables to be associated with the risk of developing anastomotic leak: transabdominal specimen extraction (OR 2.551; 95% CI 1.031–6.317, p = 0.043), diverting stoma (OR 0.265; 95% CI 0.114–0.612, p = 0.002), and ICG-FA (OR 0.204; 95 CI 0.047–0.888, p = 0.034). This study presents a moderate risk of bias after the ROBIN-I assessment.
Discussion
AL remains the most feared complication after colorectal resections. Prevention and management of AL are challenging, with treatment strategies ranging from conservative management to radiological, endoscopic, or surgical interventions.
Because of the multitude of factors that contribute to its occurrence, a reliable predictor of anastomotic leakage is not available. Some studies support the concept that altered microperfusion is the predominant factor resulting in AL in cases where a negative air leak test and complete proximal and distant doughnuts were achieved at the surgery.14,24 In a prospective study by Vignali et al., a linear correlation was found between reduced blood flow measured intraoperatively by a laser-Doppler probe at the proximal or rectal stump and the occurrence of AL.24 They found that a blood flow reduction in microperfusion of 16% occurred in patients who ended up developing a leak vs. 6.1% observed in patients without AL.
Several factors can impact perfusion, including anatomic variations in the blood supply to the anastomotic conduit, low perfusion pressure probably associated with atherosclerotic or microvascular disease, injury to the vasa recta from excessive dissection, twisting of the mesentery, and undue tension on the anastomosis. Hence, strategies to assess colonic vascular perfusion intraoperatively are of significant interest and may help identify occult bowel ischemia and help decrease the rate of AL.
The use of ICG-FA has been reported in colorectal surgery for both benign and malignant diseases applied to different surgical approaches (laparoscopic, robotic, and transanal).19,25,26 The use of such technology has increased considerably in recent years as a complementary tool for surgeons to assess intestinal serosal microcirculation in real time. Therefore, ICG-FA has become a precious and objective method for surgeons to obtain well-perfused anastomosis.
Revision rates of colonic resection margins following ICG-FA and colorectal leak rates are variably reported in current literature.20,27,28 According to the meta-analysis conducted by Blanco-Collino et al. that included 4 retrospective non-randomized studies and one prospective cohort study, the intraoperative use of ICG fluorescence following elective rectal surgery resulted in a lower AL rate compared to standard of care (1.1% vs. 6.1%, respectively).29 The recent meta-analysis29 reported pooled outcomes for 1302 patients from 5 non-randomized studies that compared the intraoperative use of ICG-FA in 555 patients with the standard of care in 747 patients. A change in the proximal colonic resection margins occurred in 7.4% of patients in the ICG group. However, no significant differences in AL rates were observed between groups (p = 0.10). The meta-analysis was limited by the retrospective nature of most of the studies included in the pooled analysis (4/5) and by randomized studies, which presented a moderate risk of bias when the quality of studies was assessed.
In a multicenter prospective phase II trial involving 504 patients undergoing elective colorectal surgery for benign or malignant indications, the use of ICG-NIR resulted in a change of proximal colonic resection margins in 29 patients (5.8%) with no subsequent AL.30 The results observed in the ICG group were compared to data collected from 1173 patients undergoing colorectal surgery in the same study centers before or during the study period without the use of ICG-NIR. The overall leak rate for colorectal resections where ICG-NIR was not used was 5.8% compared with 2.6% (12 of 453) in the cohort where NIR-ICG imaging was used (p = 0⋅009). The retrospective case-matched study by Kin et al. reported questionable results when ICG-FA was performed to assess vascular bowel perfusion for minimizing the risk of AL.31 Six hundred nine patients underwent colorectal resections for benign and malignant indications during a 7-year study period; intraoperative ICG-FA was used in 224 patients with outcomes compared with 384 patients in whom ICG-FA was not used. Although no significant difference in AL rate was observed between groups (7.5% vs. 6.4%, p = 0.67), the use of ICG-FA altered the proximal resection point in 8 patients. However, only the perfusion of the proximal bowel was assessed; the perfusion to the rectal stump was not confirmed.
More recently, in a multicenter randomized trial involving 240 patients undergoing laparoscopic colorectal resections, 118 were enrolled in the ICG-FA group, while 122 were in the control group without ICG. A change in the planned proximal colonic resection margins was observed in 13% of patients in the ICG-FA group. However, the AL rate was found not statistically different between the groups (5% vs. 9%, p > 0.05): this divergent result cast doubts about the role of ICG-FA in preventing postoperative anastomotic dehiscence.32
Overall, the data published so far supports the use of ICG perfusion in colorectal surgery to test anastomotic vascular perfusion at two critical points: just before bowel resection and after completion of the anastomosis. Benefits of ICG-FA include confirmation of adequate or inadequate perfusion, especially in high-risk anastomoses and in scenarios where the adequacy of bowel perfusion is questionable.
Regardless of the surgical approach, tumors in the middle and lower third of the rectum pose a significant challenge to the surgeon, especially in patients with a deep and narrow pelvis, males, obese, following neoadjuvant chemoradiation, and those with a bulky tumor. The distal transection in the deep and narrow pelvis using the currently available laparoscopic or robotic staplers can be difficult and may require multiple linear stapler firings, which may be associated with increased rates of AL.
The introduction of total mesorectal excision (TME) as the gold standard for the surgical management of rectal cancer has increased the rate of sphincter-preserving surgery with low anastomoses, resulting in an overall increase in AL rates.33 Surgery for rectal cancer has progressed greatly, and the development of minimally invasive approaches to TME has garnered considerable interest in the last few decades. Since its introduction, transanal total mesorectal excision (taTME) has been adopted by colorectal surgeons worldwide. It is advantageous in patients with a narrow pelvis that would otherwise make a transabdominal approach difficult.34 Transanal total mesorectal excision (taTME) utilizes a “bottom-up” approach to perform rectal and mesorectal dissection. It is mainly used for distal rectal tumors to ensure a complete TME specimen and negative margins in low-lying tumors that might otherwise require an abdominoperineal resection. Considering the higher reported incidence of AL on the international registry during taTME performed with low and ultralow coloanal anastomoses (7.8% vs. 5.4% among the initial 720 registry cases), there has been a growing interest in incorporating strategies, such as intraoperative perfusion assessment of high-risk anastomoses during taTME to mitigate the risk of AL.35
Endoscopic intraluminal assessment of mucosal perfusion in high-risk anastomoses provides a 360-degree view of the bowel, which is otherwise not easily achievable. Transanal ICG-FA provides additional confirmation of anastomotic integrity by assessing vascular perfusion of the entire circumference of stapled or hand-sewn anastomosis. To date, just a few studies have reported the use of ICG-FA for endoscopic assessment of vascular perfusion of the anastomosis.18,23 Sherwinter DA et al. were the first to describe the rectal mucosal flow transanally on a small cohort of patients undergoing LAR.18,19 A qualitative evaluation of the intraluminal intensity of ICG uptake was performed. The subjective nature of these data clearly limits any conclusion regarding the positive predictive value of an abnormal ICG angiogram.
Jafari et al. provided the results of the largest published prospective case series.20 The study reported an AL rate of 1.4% (n = 139), with no leakages in 11 patients (7.9%) who had a change in the surgical plan after ICG-FA. The takedown and revision of the anastomosis after transanal perfusion assessment occurred in 1 patient while serosal signal was present. The work of Amagai H et al.21 was one of the first to conduct a quantitative analysis of colon perfusion using ICG-FA. The work of Mizhrai I et al. was the first to report outcomes of FA exclusively during taTME.22 No discrepancy of FA in the extra or intraluminal assessment of the adequacy of vascular perfusion during taTME was demonstrated; hence, no surgical revision of the anastomoses was required. The decision to perform a protective stoma, the splenic flexure mobilization, and the anastomotic configuration were left to the surgeon’s judgment. These variations added an important selection bias, especially when focusing on AL, and the small sample size limited the strength of their conclusions. In the study reported by Otero-Piñeiro AM et al., the transanal assessment showed insufficient anastomotic perfusion in one patient while serosal signal was present.23 This led to splenic flexure mobilization, and tissue perfusion improved without further measures. A key limitation was the retrospective nature and the small size of the study.
The use of intraluminal NIR-ICG has several advantages. First, the mucosa is more susceptible than the serosa to alterations in perfusion, and adequate serosal perfusion may not imply adequate mucosal perfusion. During laparoscopic LAR and when combined with taTME, it might be challenging to assess perfusion of the rectal stump from a transabdominal approach, especially during very low dissection. On the other hand, endoscopic intraluminal assessment of the anastomosis provides a 360-degree evaluation of the bowel above and below the anastomosis, including low and ultralow coloanal anastomoses that would otherwise be inaccessible in the extraluminal assessment using a transabdominal approach. Intraluminal perfusion assessment of low colorectal anastomoses using this ICG-FA platform is quick and can easily be performed by a single operator at any time during the case. Since the camera is connected to the fluorescence imaging system, the entire surgical team can visualize the transanal images on the monitor. In addition, intraoperative proctoscopy allows for assessing the integrity of the anastomotic line and provides an air leak test at the same time. Both perfusion and structural defects of the anastomosis can be identified and corrected with this single diagnostic modality.
Even though ICG-FA has shown to be promising in the extra or intraluminal assessment of the adequacy of vascular perfusion of high-risk colorectal anastomoses, there are still some limitations in surgical practice that need to be overcome.
Following intravenous injection of ICG, assessment of the adequacy of colonic perfusion is subject to variable interpretation. Despite some recent exceptions, most NIR imaging systems cannot quantify tissue perfusion; thus, the determination of adequate perfusion is determined by subjective measures with no clear cut‐off to guide the surgeon on the adequacy or inadequacy of anastomotic perfusion. The work by Amagai et al. highlights the need to better quantify anastomotic perfusion with ICG-FA.21 Further studies focusing on quantitative rather than qualitative fluorescence assessment at the anastomosis level are awaited.
Additionally, due to the costs associated with the devices, many facilities still do not have the technology to perform endoluminal perfusion assessment, and although several devices from many different companies are commercially available, to date, no study comparing platforms and systems has been conducted. Further studies incorporating other NIR imaging systems will be necessary to demonstrate the impact of endoscopic anastomotic perfusion assessment in preventing AL.
Higher-quality studies are needed to evaluate better the dual role of extra and intraluminal ICG-FA perfusion assessment in preventing AL rates, ideally large prospective randomized controlled trials (RTCs). The low level of evidence supporting the routine use of ICG-FA in colorectal surgery stems from poorly designed observational, retrospective case–control studies and prospective non-randomized trials. As a result, there is a lack of consensus on the optimal technique for ICG-FA (dose, timeline for perfusion assessment) and the clinical benefits of routine use in colorectal surgery.
IntAct is a prospective, unblinded, multicenter randomized controlled trial that will compare AL rates at 90 days postoperatively among 880 patients undergoing laparoscopic or robotic low anterior resection for rectal cancer with or without intraoperative FA.36 Following ICG administration, intestinal vascular perfusion will be assessed in two critical steps: before bowel resection and after anastomosis construction, either intracorporeally or transanally. Clinical and radiological AL, changes in the planned resection margins, postoperative complications, quality of life, and a health economic evaluation will be examined as secondary outcomes.
Conclusion
Anastomotic perfusion assessment using ICG-FA during colorectal resections has shown promise in predicting and possibly preventing AL. Reliable identification of the demarcation line for ischemic tissue allows surgeons to revise the proximal transection margin to create a well-perfused anastomosis, thereby minimizing the risk of postoperative AL. More recently, transanal intraluminal assessment of anastomotic perfusion using ICG-NIR imaging is safe and feasible to evaluate anastomotic viability in real time. A dual extra and intraluminal approach for anastomotic perfusion assessment might particularly benefit high-risk anastomoses. Routine use of intraluminal ICG-NIR may become a standard tool in evaluating high-risk colorectal and coloanal anastomoses.
However, to date, large-scale prospective RCTs are lacking. Additionally, intraluminal perfusion assessment is only feasible for coloanal and colorectal anastomoses, but it is still not demonstrated for left-sided, transverse, or right-sided colonic anastomoses. New technologies incorporating fluorescence assessment in standard endoscopic equipment will be necessary.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its appendix.
References
McDermott FD, Heeney A, Kelly ME, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015; 102:462–479
Pommergaard HC, Gessler B, Burcharth J et al. Pre‐operative risk factors for anastomotic leakage after resection for colorectal cancer: a systematic review and meta‐analysis. Colorectal Dis. 2014; 16: 662–7
Trencheva K, Morrissey KP, Wells M, et al. Identifying important predictors for anastomotic leak after colon and rectal resection: prospective study on 616 patients. Ann Surg. 2013; 257:108–113
Bertelsen CA, Andreasen H, Jørgensen T et al. Anastomotic Leakage After Anterior Resection for Rectal Cancer: Risk Factors; Colorectal Dis. 2010; 12:37-43
Kanellos I, Vasiliadis K, Angelopoulos S et al. Anastomotic leakage following anterior resection for rectal cancer. Tech Coloproctol. 2004; 8: s79–81
Goto S, Hasegawa K, Hida K, et al. Multicenter analysis of impact of anastomotic leakage on long-term oncologic outcomes after curative resection of colon cancer. Surgery. 2017; 162: 317–324
Ramphal W, Boeding JRE, Gobardhan PD, et al; Oncologic Outcome and Recurrence Rate Following Anastomotic Leakage After Curative Resection for Colorectal Cancer; Surg Oncol. 2018; 27:730-736
Mongin C, Maggiori L, Agostini J, et al. Does anastomotic leakage impair functional results and quality of life after laparoscopic sphincter-saving total mesorectal excision for rectal cancer? A case-matched study. Int J Colorectal Dis. 2014; 29:459e467
Vallance A, Wexner S, Berho M et al. A Collaborative Review of the Current Concepts and Challenges of Anastomotic Leaks in Colorectal Surgery. Colorectal Dis. 2017; 19:01-012
2015 European Society of Coloproctology collaborating group. The relationship between method of anastomosis and anastomotic failure after right hemicolectomy and ileo-caecal resection: An international snapshot audit. Colorectal Dis. 2017. https://doi.org/10.1111/codi.13646
Shiomi A, Ito M, Maeda K, et al. Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1014 consecutive patients. J Am Coll Surg. 2015; 220:186–194
Qu H, Liu Y, Bi DS. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc. 2015; 29:3608–3617
Kang CY, Halabi WJ, Chaudhry OO, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013; 148:65–71
Meyer J, Naiken S, Christou N, et al. Reducing anastomotic leak in colorectal surgery: The old dogmas and the new challenges. World J Gastroenterol. 2019; 25: 5017-5025
Choi HK, Law WL, Ho JW. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors. Dis Colon Rectum. 2006; 49:1719-1725
Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ2021;372:n160. https://doi.org/10.1136/bmj.n160 pmid:33781993
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ2016;355:i4919. https://doi.org/10.1136/bmj.i4919 pmid:27733354
Sherwinter DA. Transanal Near-Infrared Imaging of Colorectal Anastomotic Perfusion. Surg Laparosc Endosc Percutan Tech. 2012; 22:433-6
Sherwinter DA, Gallagher J, Donkar T. Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Colorectal Dis. 2013 Jan;15(1):91-6. https://doi.org/10.1111/j.1463-1318.2012.03101.x. PMID: 22632448
Jafari MD, Wexner SD, Martz JE, et al. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 2015; 220:82–92
Amagai H, Miyauchi H, Muto Y, et al. Clinical utility of transanal indocyanine green near‐infrared fluorescence imaging for evaluation of colorectal anastomotic perfusion. Surg Endosc. 2019. https://doi.org/10.1007/s00464-019-07315-7
Mizrahi I, De Lacy FB, Abu Gazala M et al. Transanal Total Mesorectal Excision for Rectal Cancer With Indocyanine Green Fluorescence Angiography; Tech Coloproctol. 2018; 22:785-791
Otero-Piñeiro AM, de Lacy F B, Van Laarhoven JJ et al. The Impact of Fluorescence Angiography on Anastomotic Leak Rate Following Transanal Total Mesorectal Excision for Rectal Cancer: A Comparative Study. Surg Endosc. 2020. https://doi.org/10.1007/s00464-020-07442-6
Vignali A, Giannotti L, Braga M, et al. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum. 2000 ; 43 :76-82
Hellan M, Spinoglio G, Pigazzi A, et al. The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery. Surg Endosc. 2014; 28:1695–1702
Ris F, Hompes R, Cunningham C et al. Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surg Endosc. 2014; 28:2221–2226
Jafari MD, Lee KH, Halabi WJ, et al. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc. 2013; 27:3003-3008
Kim JC, Lee JL, Yoon YS, et al. Utility of indocyanine-green fluorescent imaging during robot-assisted sphincter-saving surgery on rectal cancer patients. Int J Med Robot. 2016; 12:710–717
Blanco-Collino R, Espin-Basany E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta‐analysis; Tech Coloproctol. 2018; 22:15-23
Ris F, Liot E, Buchs NC et al. Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. BR J Surg. 2018 ; 105 :1359-1367
Kin C, Vo H, Welton L, et al. Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis Colon Rectum. 2015; 58:582–587
De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti E et al. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc. 2020; 34:53-60
Peeters KCMJ, Tollenaar RAEM, Marijnen CAM, et al. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg. 2005; 92:211–216
Sylla P, Rattner DW, Delgado S, et al. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010; 24:1205–1210
Penna M, Hompes R, Arnold S, et al Incidence and Risk Factors for Anastomotic Failure in 1594 Patients Treated by Transanal Total Mesorectal Excision: Results From the International TaTME Registry. Ann Surg. 2019; 269:700-711
Armstrong G, Croft J, Corrigan N, et al. IntAct: intra‐operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: a randomized controlled trial. Colorectal Dis. 2018; 20: O226-O234
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
No ethical approval is required for this study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lauricella, S., Peyser, D., Carrano, F.M. et al. Intraluminal Anastomotic Assessment Using Indocyanine Green Near-Infrared Imaging for Left-Sided Colonic and Rectal Resections: a Systematic Review. J Gastrointest Surg 27, 615–625 (2023). https://doi.org/10.1007/s11605-022-05564-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05564-x