Abstract
Background and Purpose
Submucosal tunneling endoscopic resection (STER) was initially used to remove submucosal tumors (SMTs) located at the esophagus and cardia; only few researchers have reported the feasibility of STER for gastric SMTs beyond cardia due to the technical difficulty, and little is known about the comparison of STER for cardia and non-cardia gastric SMTs. The purpose was to compare the feasibility and efficacy of STER for cardia and non-cardia gastric SMTs, as well as to explore the risk factors for failure of en bloc resection.
Methods
We retrospectively collected the clinical data about patients with gastric SMTs who received STER at our hospital from June 2012 to June 2018. Demographics, tumor size, procedure-related parameters, complications, hospital stay, and follow-up data were compared between cardia and non-cardia SMTs. And multivariate analyses were conducted to look for the risk factors for failure of en bloc resection.
Results
A total of 46 SMTs were removed, and 25 of them were located at cardia, while the other 21 at non-cardia position. There was no significant difference between the two groups in terms of gender, age, tumor size, en bloc resection rate, operation time, complications, and hospital stay (p > 0.05). No recurrence was noticed in all the cases. Multivariate analyses revealed that irregular shape was an independent risk factor for failure of en bloc resection.
Conclusion
STER is feasible for both cardia and non-cardia gastric SMTs, and the efficacy between cardia and non-cardia location is comparable. Irregular shape was an independent risk factor for failure of en bloc resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the widespread use of esophagogastroduodenoscopy (EGD) and endoscopic ultrasonography (EUS), the diagnostic rate of gastric submucosal tumors (SMTs) has increased obviously.1 Periodical surveillance by EGD and/or EUS remains one of the recommended strategies; however, it involves issues related to the patient’s compliance and stress, cost-effectiveness, and the risk associated with repeated endoscopic procedures and delayed diagnosis of malignancy.2,3 Moreover, a majority of the gastric SMTs are gastrointestinal stromal tumors (GISTs), which have malignant potential, especially when they are large in diameter.1 Therefore, it is necessary to remove these SMTs.
Current methods to remove gastric SMTs include surgical and endoscopic resection.1,3,4 Endoscopic resection has several advantages over surgical approaches, such as minimally invasive, a shorter hospital stay, a lower cost, etc.1,3,4 And current endoscopic modalities include endoscopic band ligation (EBL), endoscopic submucosal dissection (ESD), endoscopic submucosal excavation (ESE), endoscopic full-thickness resection (EFTR), and submucosal tunneling endoscopic resection (STER).1,3,4 STER is a novel endoscopic technique and has several advantages over other endoscopic methods, such as mucosa integrity, easy to close the mucosal defect, etc.5,6,7,8 STER was initially used for treatment of SMTs located at esophagus and gastric cardia,9,10,11 and gastric SMTs beyond cardia were not recommended for STER due to technical difficulty. Until now, only several studies have reported the feasibility of STER for gastric SMTs in non-cardia location12,13,14,15; however, little is known about the comparison of the efficacy of STER for gastric cardia and non-cardia SMTs. In this retrospective study, we aimed to compare the feasibility and efficacy of STER on SMTs located at the above two sites, as well as to explore the risk factors for failure of en bloc resection.
Materials and Methods
Patients
This is a single-center, retrospective study and was approved by the ethics committee of the Second Xiangya Hospital of Central South University, and all the patients signed informed consent. The inclusion criteria of the study were as follows: (a) presence of gastric SMTs originating from the muscularis propria (MP) layer confirmed by EGD, EUS, and/or computerized tomography (CT), and the greatest diameter of SMT per EUS and/or CT is ≤ 5 cm; (b) EUS shows no high-risk features of malignancy, such as internal heterogeneity, heterogeneous enhancement; (c) no signs of metastasis or invasion outside the gastrointestinal tract during CT examination; and (d) patient consent to undergo an STER procedure at our hospital. Those patients who could not tolerate anesthesia, those with severe cardiopulmonary disease or blood coagulation disorders (international normalized ratio > 2.0, platelet count < 100,000/mm3), and those with multiple gastric SMTs or gastric SMTs with co-existed esophageal SMT were excluded from the study. Forty-six consecutive patients were retrospectively included in the study between June 2012 and June 2018. Their demographics (age, gender), tumor size, procedure-related parameters, complications, hospital stay, and follow-up data were retrospectively collected and recorded.
STER Procedure
STER was performed under general anesthesia through tracheal intubation. A carbon dioxide insufflator (UCR; Olympus) was used in all the procedures. Other equipment and accessories included a high-frequency generator (VIO 200D; ERBE, Tübingen, Germany), an argon plasma coagulation unit (APC300; ERBE), a hybrid knife (ERBE), a dual knife (KD-650Q; Olympus), an insulation-tip knife (KD611L, IT2; Olympus), an injection needle (NM-4L-1; Olympus), and hemostatic clips (HX-600-90; Olympus).
STER was performed as follows: (a) identification of the SMT: for SMTs located at gastric fundus, submucosal injection with methylene-blue was used to help locate the tumor; (b) for cardia SMTs, submucosal injection was made at about 3~5 cm proximal to the SMT; submucosal injection at cardia or lower esophagus for gastric fundus SMTs, while 2–3 cm proximal to the SMT for gastric antrum or corpus SMTs; (c) a longitudinal mucosal incision was made to create the tunnel entry; (d) create the submucosal tunnel; (e) dissect the tumor; and (f) close the mucosal entry. Figure 1 and Fig. 2 depict the procedure of STER for cardia and antrum SMT, respectively.
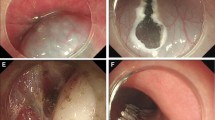
Case illustration of STER for cardia submucosal tumor. a Esophagogastroduodenoscopy revealed a submucosal tumor at cardia. b We could see the tumor in the submucosal tunnel. c Dissected the tumor in the submucosal tunnel. d Wound surface after the tumor was removed. e Close the mucosal entry with clips. f The resected tumor
Case illustration of STER for antrum submucosal tumor. a Esophagogastroduodenoscopy revealed a submucosal tumor at gastric antrum. b Create the submucosal tunnel. c Dissected the tumor in the submucosal tunnel. d Wound surface after the tumor was removed. e Close the mucosal entry with clips. f The resected tumor
Postoperative Management
All the patients were kept nil per os (NPO) for 48 h, a liquid diet for 3 days, and returned gradually to a normal diet within 2 weeks. Intravenous proton pump inhibitor (PPI) and antibiotics were used for 3 days. A thoracoabdominal radiography or sometimes a CT was performed for suspicious patients on postoperative day 2 to check for the occurrence of emphysema, pneumothorax, pneumoperitoneum, etc.
Pathological Evaluation
The specimens were fixed, embedded with paraffin, and then sectioned. Hematoxylin and eosin and immunohistochemical staining (SMA, Ki67, CD34, CD117, S-100, desmin, Dog-1, etc) were carried out to determine the characteristic of the SMTs. The greatest diameter measured after removal of the SMT was recorded as tumor size. A tumor with lobulated outer margin was defined as a tumor with irregular shape. En bloc resection was defined as the intact fibrous capsule of the resected tumor and the absence of any remnant of tumor observed on endoscopy.
Follow-up
Surveillance EUS was performed at 3, 6, and 12 months and annually thereafter to observe healing of the wound and to check for any residual tumor. And CT scanning was recommended if any recurrence was noticed during EUS examination.
Statistical Analysis
SPSS 21.0 software was applied for data analysis. Continuous variables were presented as mean ± standard deviation and analyzed using Student’s t test. And qualitative data were presented as frequencies and calculated using the Chi-square test or Fisher exact test. Univariate and multivariate analyses were conducted to look for the risk factors for failure of en bloc resection. A two-tailed P value < 0.05 was considered as statistically significant in all cases.
Results
General Clinical Characteristics
A total of 46 cases were retrospectively included during June 2012 to June 2018, among whom 26 were females and 20 were males, and the mean age was 46.9 ± 12.6 years. All the patients underwent STER successfully, and the mean operation time was 76.1 ± 29.5 min. A total of 46 SMTs were removed with a mean diameter of 2.3 ± 1.3 cm, and 25 of them were located at the cardia, while the other 21 cases non-cardia (17 at gastric fundus, 2 at gastric antrum, 1 at gastric corpus, and 1 at gastric antrum-corpus junction). Histologically, 25 cases were GISTs, 19 cases were leiomyoma, 1 ectopic pancreas, and 1 myofibroblastic tumor. Among the 25 GISTs, 14 were very low risk and the other 11 were low risk on the basis of the National Comprehensive Cancer Network guidelines.16 Three patients (6.5%) encountered complications, namely 1 mucosal laceration, 1 pneumoperitoneum, and 1 pleural effusion and pneumoperitoneum. The mucosal laceration was closed with two clips without subsequent leakage, the one with pneumoperitoneum was resolved by abdominal puncture deflation, and the one with both pleural effusion and pneumoperitoneum was managed by conservative treatment with NPO and antibiotics. No recurrence was noticed during a mean follow-up of 27.9 months (range, 1–72 months).
Comparison Between Cardia and Non-cardia SMTs
Comparison results are shown in Table 1; there was no significant difference between cardia and non-cardia SMTs in regarding to gender, age, tumor size, tumor growth way, operator’s experience, tumor shape, operation time, rate of complications,and en bloc resection, length of hospital stay, and follow-up time; SMTs located in non-cardia are more likely to be GISTs, while leiomyomas are the main pathological type for cardia SMTs. The results suggested that the efficacy of STER for cardia and non-cardia SMTs was comparable.
Risk Factors for Failure of En Bloc Resection
Univariate analysis revealed that large tumor size, irregular shape, and long operation time were risk factors for failure of en bloc resection, and multivariate analyses revealed that irregular shape was an independent risk factor for failure of en bloc resection (Tables 2 and 3).
Discussion
In the present study, we found for the first time that the efficacy of STER for gastric cardia and non-cardia SMTs was comparable.
STER is a relatively new technique, and several studies have shown that STER is a feasible, safe, and effective for treatment of gastric SMTs.7,8,12,13,14,17,18 Theoretically, STER has several advantages over other endoscopic methods in the following aspects. Firstly, STER could maintain the gastrointestinal mucosal integrity and the submucosal tunnel has a good “leak-proofing” effect because of the distance between the site of mucosal incision and tumor dissection, which would promote early wound healing, reduce the duration of NPO, the necessity of gastrointestinal decompression, and the potential risk of gastrointestinal tract leakage and secondary infection.19,20 Secondly, the site of resection and clip closure is not the same in the STER technique, and only a longitudinal mucosal incision was performed during STER, while circumferencial mucosal incision with a larger mucosal defects is usually performed during other endoscopic treatments (such as ESD, ESE, EFTR) which would reduce the tension for suturing as the MP and serosal layer immediately underlying the mucosal incision is intact. Thirdly, the submucosal tunnel in the STER technique helps maintain the visual field and facilitates precise hemostasis. Several studies have compared the safety and efficacy of STER with other endoscopic methods for gastric SMTs.5,6,7,8 We found in our previous study that the treatment efficacy between STER and EFTR was comparable, but STER takes advantages over EFTR in a shorter suture time and less clips to close the gastric wall defect, which means that it is easy to close the defect when using the STER technique.5,6 However, Du et al.8 found that ESE (n = 40) was superior to STER (n = 47) with reduced operation time and less clips required, without any compromise in treatment safety and efficacy for cardial SMTs. Zhang et al.7 conducted a comparison between STER and non-tunneling methods (ESD, EFR) accompanying with a systematic review and found that STER has no distinct advantages over endoscopic nontunneling methods, but has a longer procedure time.
However, due to the specific anatomical and physiological features of the stomach such as a large lumen, high flexibility, unfixed position, and relative thick mucosa, creating a submucosal tunnel may be more difficult than that of in the esophagus, STER was only recommended for gastric SMTs located at the cardia with the submucosal tunnel created from the esophagus. Only few researchers have reported the feasibility of STER for gastric SMTs beyond cardia, and technique modification is necessary. Lu et al.14 successfully treated 18 patients with gastric fundus SMTs with transcardiac tunneling technique, 19 SMTs were removed, and en bloc resection was achieved in all the patients without severe complications. The same group reported a higher success rate of STER for gastric SMTs; 43 of 45 cases were successfully treated with 47 SMTs removed, of whom 18 cases located at gastric fundus.13 For SMTs located at gastric fundus, transcardiac tunneling technique was used, while a submucosal tunnel was created 3 cm proximal to the SMTs for other sites. Subsequently, Li et al.12 and Zhang et al.7 both demonstrated the feasibility of STER for gastric SMTs. For gastric SMTs located at non-cardia location, several technical pointers may be helpful to successfully remove the SMTs: (1) for SMTs at gastric fundus, sometimes submucosal injection with methylene-blue was used to help locate the tumor, and transcardiac tunnel is recommended; for SMTs difficult to retrieve, a double-opening method may be tried.17 (2) For SMTs located at gastric corpus or antrum, a short submucosal tunnel is recommended (usually 2–3 cm proximal to the SMTs) to reduce technical difficulty. Zhang and Li et al. reported a novel technique-endoscopic mucosa-sparing lateral dissection (EMSLD) for gastric SMTs.21,22,23 The procedure of EMSLD was as follows: mark the border of the SMT, submucosal injection, semicircular mucosal cut along the marks, separate the submucosal layer to expose the SMT, dissect the SMT, replace the retained mucosa to cover the wound, and close with clips. This method, to some extent, is a modified STER with extremely short submucosal tunnel, which is very helpful to overcome the difficulty to create submucosal tunnel in the stomach. Although the above studies have demonstrated the feasibility, safety, and efficacy of STER for non-cardia SMTs, little is known concerning the comparison of STER between cardia and non-cardia location. Herein, we found that STER is feasible for both cardia and non-cardia gastric SMTs and found for the first time that the efficacy of STER for gastric cardia and non-cardia SMTs was comparable, which suggested that STER could be applied to endoscopic resection of gastric SMTs regardless of the tumor location in experienced hands.
Although piecemeal resection does not affect the long-term outcome of leiomyoma,24 its influence on GISTs remains unknown. What is more, a majority of gastric SMTs are GISTs, and piecemeal resection does affect postoperative pathological evaluation, en bloc resection should be achieved as possible as we can. Although Du et al.8 reported a relatively low en bloc rate (70.2%, 33/47) for cardia SMTs, no significant difference was seen in the present study in regarding to en bloc resection rate between cardia and non-cardia SMTs (84.0% vs 85.7%). Currently, three studies have explored the risk factors for failure of en bloc resection,8,24,25 and reported risk factors included irregular shape, large tumor diameter. We found that irregular shape was an independent risk factor for piecemeal resection during STER treatment for gastric SMTs. To the best of our knowledge, this is the first time to demonstrate the risk factors of failure of en bloc resection during STER for gastric SMTs.
The present study has several limitations. Firstly, this was a single center, retrospective study. Secondly, the sample size was relatively small with only 46 cases enrolled, thus warranting large-scale studies. Thirdly, although the mean follow-up duration was 27.9 months, 12 patients had a follow-up with less than 12 months with the minimum follow-up of only 1 month; however, the follow-up time between cardia and non-cardia location is comparable; thus, it has little effect on the study conclusion. Continuous follow-up is ongoing to observe the long-term outcomes.
Conclusion
In conclusion, our study shows that STER is feasible for both cardia and non-cardia gastric SMTs, and the efficacy between them was comparable, and irregular shape was an independent risk factor for failure of en bloc resection. Randomized, large-scale studies are warranted for a more confirmed conclusion.
References
Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc 2013;25:479–489.
Kim GH. Endoscopic resection of subepithelial tumors. Clin Endosc 2012;45: 240–244.
Kim SY, Kim KO. Management of gastric subepithelial tumors: The role of endoscopy. World J Gastrointest Endosc 2016;8:418–424.
Tan Y, Tan L, Lu J, Huo J, Liu D. Endoscopic resection of gastric gastrointestinal stromal tumors. Transl Gastroenterol Hepatol 2017;2:115.
Tan Y, Tang X, Guo T, Peng D, Tang Y, Duan T, Wang X, Lv L, Huo J, Liu D. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc 2017;31:3376–3382.
Duan TY, Tan YY, Wang XH, Lv L, Liu DL. A comparison of submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric fundus submucosal tumors. Rev Esp Enferm Dig 2018;110:160–165.
Zhang Q, Wang F, Wei G, Cai JQ, Zhi FC, Bai Y. Endoscopic resection of gastric submucosal tumors: A comparison of endoscopic nontunneling with tunneling resection and a systematic review. Saudi J Gastroenterol 2017;23: 52–59.
Du C, Chai N, Linghu E, Gao Y, Li Z, Li L, Zhai Y, Lu Z, Meng J, Tang P. Treatment of cardial submucosal tumors originating from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation. Surg Endosc 2018; doi: https://doi.org/10.1007/s00464-018-6206-0.
Wang H, Tan Y, Zhou Y, Wang Y, Li C, Zhou J, Duan T, Zhang J, Liu D. Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol 2015;27:776–780.
Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy 2012;44:225–230.
Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, Yao LQ. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 2012;75:195–199.
Li QL, Chen WF, Zhang C, Hu JW, Zhou PH, Zhang YQ, Zhong YS, Yao LQ, Xu MD. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc 2015;29:3640–3646.
Lu J, Jiao T, Li Y, Liu Y, Wang Y, Wang Y, Zheng M, Lu X. Heading toward the right direction--solution package for endoscopic submucosal tunneling resection in the stomach. PLoS One 2015;10:e0119870.
Lu J, Zheng M, Jiao T, Wang Y, Lu X. Transcardiac tunneling technique for endoscopic submucosal dissection of gastric fundus tumors arising from the muscularis propria. Endoscopy 2014;46:888–892.
Zhou H, Tan Y, Wang C, Yang J, Zhou Y, Liu D. Removal of an extraluminal gastric gastrointestinal stromal tumor: the role of submucosal tunneling endoscopic resection. Endoscopy 2017;49:E11-E13.
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Cancer Netw 2010; Suppl 2:S1–41; quiz S42–44
Zhang Q, Cai JQ, Xiang L, Wang Z, de Liu S, Bai Y. Modified submucosal tunneling endoscopic resection for submucosal tumors in the esophagus and gastric fundus near the cardia. Endoscopy 2017;49:784–791.
Mao XL, Ye LP, Zheng HH, Zhou XB, Zhu LH, Zhang Y. Submucosal tunneling endoscopic resection using methylene-blue guidance for cardial subepithelial tumors originating from the muscularis propria layer. Dis Esophagus 2017;30:1–7.
Tan Y, Huo J, Liu D. Current status of submucosal tunneling endoscopic resection for gastrointestinal submucosal tumors originating from the muscularis propria layer. Oncol Lett 2017;14:5085–5090.
Du C, Linghu E. Submucosal Tunneling Endoscopic Resection for the Treatment of Gastrointestinal Submucosal Tumors Originating from the Muscularis Propria Layer. J Gastrointest Surg 2017;21(12):2100–2109.
Zhang Q, Li Y, Meng Y, Bai Y, Cai JQ, Han ZL, Wang Z, Zhi FC, Liu SD. Should the Integrity of Mucosa Be Considered in Endoscopic Resection of Gastric Submucosal Tumors? Gastroenterology 2016;150:822–824 e829.
Li Y, Zhang Q, Zhu C, Luo Y, Han Z, Qing H, Cai J, Li L, Huang Y, Liu S. Endoscopic mucosa-sparing lateral dissection for treatment of gastric submucosal tumors: a prospective cohort study. Endoscopy 2018;50:886–890.
Zhang Q, Li Y, Lian ZY, Wang Z, Wang LH, Bai Y, Liu SD. A modified endoscopic method for resection of gastric submucosal tumor. Surg Endosc 2018;32:536–543.
Chen T, Zhou PH, Chu Y, Zhang YQ, Chen WF, Ji Y, Yao LQ, Xu MD. Long-term Outcomes of Submucosal Tunneling Endoscopic Resection for Upper Gastrointestinal Submucosal Tumors. Ann Surg 2017;265:363–369.
Li Z, Gao Y, Chai N, Xiong Y, Ma L, Zhang W, Du C, Linghu E. Effect of submucosal tunneling endoscopic resection for submucosal tumors at esophagogastric junction and risk factors for failure of en bloc resection. Surg Endosc 2018;32:1326–1335.
Grant Support
This study was funded by Development and Reform Commission of Hunan Province (XFGTZ2014713).
Author information
Authors and Affiliations
Contributions
Conception of the work: YT and DL; acquisition, analysis, and interpretation of the data: YT, BZ, SZ, FD, RL, and SG; drafting the manuscript: YT; critically revise for important intellectual content: JH and DL; final approve of the manuscript: all authors.
Corresponding author
Ethics declarations
This is a single-center, retrospective study and was approved by the ethics committee of the Second Xiangya Hospital of Central South University, and all the patients signed informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, Y., Zhou, B., Zhang, S. et al. Submucosal Tunneling Endoscopic Resection for Gastric Submucosal Tumors: a Comparison Between Cardia and Non-cardia Location. J Gastrointest Surg 23, 2129–2135 (2019). https://doi.org/10.1007/s11605-019-04182-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04182-4