Abstract
Previous studies examining short- and long-term outcomes of pancreaticoduodenectomy with vascular resection for pancreatic adenocarcinoma have not graded perioperative complication severity. These studies may provide incomplete assessments of the efficacy of vascular resection. In the current study, we evaluated 36 patients who had pancreaticoduodenectomy with major vascular resection. These were matched 1:3 by tumor stage and age to patients who had pancreaticoduodenectomy without vascular resection. Charts were reviewed to identify all complications and 90-day readmissions. Complications were graded as either severe or minor adverse postoperative outcomes, taking into account the total length of stay. There were no statistical differences in patient demographics, comorbidities, or symptoms between the groups. Patients who had vascular resection had significantly increased rates of severe adverse postoperative outcomes, readmissions, lengths of hospital stay, as well as higher hospital costs. Hypoalbuminemia and major vascular resection were independent predictors of severe adverse postoperative outcomes. On multivariate Cox-regression survival analysis, patients who had vascular resection had decreased recurrence-free (12 vs. 17 months) and overall (17 vs. 29 months) survival. Major vascular resection was a predictor of mortality, may be an independent prognostic factor for survival, and may warrant incorporation into future staging systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular resection for pancreatic adenocarcinoma with porto-venous involvement is being utilized with increasing frequency.1 Most of the evidence evaluating the safety and efficacy of vascular resection in these cases comes from single institutional retrospective studies. These studies have shown mixed results in terms of both complications and survival. Some have demonstrated increased perioperative complication rates2 and decreased survival3 , 4 with major vascular resection, while others have reported similar short- and long-term outcomes with and without vascular resection.5 – 7
There have been two recent large retrospective evaluations of the approach. A multicenter trial from the UK demonstrated that pancreaticoduodenectomy with vascular resection was comparable to pancreaticoduodenectomy alone in terms of perioperative complications and that both operations had superior long-term outcomes than palliative bypass alone.8 In contrast, pancreaticoduodenectomy with vascular resection was found to have increased in-hospital perioperative morbidity and mortality in a National Surgical Quality Improvement Program (NSQIP) study. No long-term outcomes were available in this study.9
None of the prior reports evaluating outcomes following major vascular resection with pancreaticoduodenectomy have graded perioperative complications or taken into account total hospital stay including 90-day readmission data. Given this, these studies may provide an incomplete assessment of the morbidity associated with the approach. In the current study, we used a case-matched cohort analysis to compare a group of patients who had pancreaticoduodenectomy with vascular resection to a similar control group without vascular resection with regard to the grade of their perioperative complications to more accurately assess the short-and long-term outcomes associated with pancreaticoduodenectomy requiring vascular resection.
Materials and Methods
Data Source
From May 2007 to December 2014, 345 patients who had pancreaticoduodenectomy were identified from our IRB-approved, prospectively collected pancreatic surgery database. Of these, 36 had pancreaticoduodenectomy with major vascular resection (PDVR) for a confirmed diagnosis of pancreatic adenocarcinoma. Major vascular resection was defined as a partial or circumferential portal or mesenteric vein resection reconstructed with primary closure, end-to-end anastomosis, vein patch, or interposition graft. Propensity score matching for age and pathologic stage was used to match this cohort 1:3 to a total of 108 similar patients who underwent pancreaticoduodenectomy (PD) without vascular resection for pancreatic adenocarcinoma in the same time period. Patients with metastatic disease, a diagnosis other than pancreatic adenocarcinoma, or vascular resection due to injury unrelated to tumor infiltration were excluded. Prospectively collected data in our database included demographic information such as age, race, and gender; patient comorbidities; smoking and alcohol use; symptoms including jaundice, pruritis, pain, weight loss, anorexia, early satiety, nausea, abdominal distention, and change in bowel habits; and preoperative lab values including serum bilirubin, albumin, CA 19-9, and hemoglobin. The data also included treatment information such as preoperative biliary stent placement, use of neoadjuvant therapy, operative time and estimated blood loss, type of procedure performed, final pathology including TNM staging, tumor grade, and resection margins. Follow-up data including recurrence, death, and last follow-up were also available. Retrospective chart review was used to identify all complications and deviations from care pathways, 90-day readmission events, and interventions required to manage postoperative complications. For purposes of this paper, delayed gastric emptying was defined as decreased motility requiring promotility agents, supplemental enteral nutrition, or parenteral nutrition. Intra-abdominal abscesses and pancreatic fistulas were grouped together as one type of complication. Retrospective chart review was used to classify patients as resectable or borderline resectable by the MD Anderson criteria.10 Hospital cost data was available though an electronic data warehouse which links clinical information from the electronic medical record to all inpatient and outpatient hospital charges and reimbursements.
Complication Grading
Complications were first classified by the Clavien-Dindo system11 and then reclassified as severe or minor adverse postoperative outcomes using a grading system previously described and published by our research group.12 Severe adverse postoperative outcomes (SAPO) were defined as any Clavien-Dindo grade IIIb, IV, or V complication or a Clavien-Dindo grade IIIa complication requiring more than one intervention (endoscopic or interventional). In addition, any Clavien-Dindo grade I, II, or IIIa complication that resulted in a total length of stay (including readmissions) greater than 3 standard deviations beyond the mean for patients without complications were also graded as SAPO. In a previous review of our institution’s pancreaticoduodenectomy experience, this was defined as 17 days. All other complications were considered minor adverse postoperative outcomes (MAPO). Patients who had more than one complication during their recovery (e.g., pneumonia and pancreatic fistula) were given one grade based on the most severe complication experienced.
Statistical Analysis
Propensity score matching was done using SAS software (SAS version 9.3; Cary, NC). All other analyses utilized SPSS software (Version 19; Chicago, IL). Chi-square and ANOVA analysis were used to compare categorical variables. Independent t-tests were used to compare continuous variables. Univariate and multivariate logistic regressions were utilized to identify predictors of severe adverse postoperative outcomes, with an odds ratio (OR) >1 representing increased odds of SAPO. Multivariate Cox-regression survival analysis was used to estimate cumulative survival and generate plots of recurrence-free and overall survival, with recurrence-free survival being measured from the time of surgery and overall survival being measured from the time of diagnosis. Both univariate and multivariate Cox-regression analyses were used to determine predictors of poorer overall survival. Hazard ratio (HR) >1 represented an increased hazard of mortality. Variables examined as potential predictors of SAPO and survival were those felt to be determinants of outcome by the operating surgeons or identified as significant risk factors in prior studies of outcome. Statistical significance was set at a p value of 0.05. Two-sided p values were reported for all variables. All confidence intervals (CI) are reported at a 95 % significance level.
Results
Cohort Characteristics
We identified 36 patients who underwent PDVR for pancreatic adenocarcinoma at our institution between May 2007 and December 2014. A propensity score cohort matching of 1:3 by pathologic stage and age was utilized to further identify 108 patients who underwent PD during the same time frame for a total cohort of 144 patients. Of the 36 patients who underwent vascular resection, 35 (97 %) had superior mesenteric vein or portal vein confluence involvement (SMV/PV) and 1 had both SMV/PV and hepatic artery involvement. A total of 18 patients (50 %) had vascular repair with primary transverse closure, 11 (31 %) required end-to-end anastomosis, 3 (8 %) had a vein patch repair, and 4 (11 %) required an interposition graft–1 with a vein graft and 3 with a PTFE graft.
The mean age of patients in our cohort was 67 ± 10 years old. There were no significant differences in the demographics between the PDVR and PD groups. About 50 % of the patients were male in both groups, 28 % were age 75 or older, and over 80 % were Caucasian (Table 1). There were no significant differences in any patient comorbidities, including chronic obstructive lung disease (COPD), asthma, preoperative coronary artery disease (CAD), diabetes mellitus (DM), renal failure, pancreatitis, bleeding or clotting disorders, cirrhosis, congestive heart failure, hypertension, hepatitis, history of previous malignancy, stroke, smoking history, and alcohol history (p > 0.05). There were no significant differences in patient symptoms at presentation between the groups, with similar rates of jaundice, weight loss, anorexia, abdominal pain, early satiety, and change in bowel habits (p > 0.05). There was also no significant difference in the rates of preoperative biliary stenting between the PDVR and PD groups, with rates of 76 and 79 % respectively. Neoadjuvant therapy was significantly more common in the PDVR group (56 % compared to 13 %, p < 0.01). This was related to the high proportion of patients with borderline resectable pancreatic cancer in the PDVR category–18 of the 36 patients were initially classified as borderline resectable on presentation, and 94 % (17) of them received neoadjuvant therapy. Only 8 of 108 patients were classified as borderline resectable in the PD group, all of whom received neoadjuvant therapy. The type of pancreaticoduodenectomy performed was also different between the two groups, with only 19 % of PDVR patients having a pylorus-preserving pancreaticoduodenectomy compared to 63 % of the PD patients (p < 0.01). Pathologic stage and grade was equivalent between groups, as the patients were matched by stage (p > 0.05).
Perioperative Outcomes
Estimated operative blood loss (EBL) was significantly higher in the vascular resection group (1.2 ± 1.0 L vs. 0.5 ± 0.3 L, p < 0.01). The reoperation rate was also higher in the PDVR group (14 % vs. 4 %, p = 0.04). There was no difference in 90-day mortality between the two groups (3 % vs. 1 %, p > 0.05). Rates of severe adverse postoperative outcomes (56 % vs. 29 %), readmissions (42 % vs. 19 %), and total length of stay including readmissions (21 ± 14 days vs. 14 ± 8 days) were significantly higher in the PDVR group compared to the PD group (all p values ≤0.01). Hospital charges were also significantly higher in the PDVR group ($190,000 ± 120,000 vs. $120,000 ± 78,000, p < 0.01) (Table 2).
Oncologic Outcomes
The margin negative (R0) resection rates were equivalent between the PDVR (81 %) and PD (82 %) groups (p = 0.80). The lymph node ratio of positive to examined lymph nodes was 0.08 ± 0.11 in the PDVR group and 0.13 ± 0.16 in the PD group (p = 0.02). The overall recurrence rate was similar between the groups (56 % vs. 48 %, p = 0.56), as was the local recurrence rate (32 % vs. 29 %, p = 0.78). However, at a mean follow-up of 18 months, the median time to recurrence was significantly shorter in the PDVR group at 12 months compared to the PD group at 17 months (p = 0.02) (Table 3).
Complications and Severe Adverse Postoperative Outcomes
The most common postoperative complications were wound infection (50 %), anemia (41 %), and delayed gastric emptying (35 %). Malnutrition, anemia, intra-abdominal hemorrhage, and respiratory failure were more common in the PDVR group (all p values <0.05). When classified using the Clavien-Dindo grading scale, there was no statistically significant difference in the complications between the PDVR and PD groups; however, when using our modified grading scale of SAPO and MAPO, patients undergoing PDVR were nearly twice as likely to have had a SAPO as patients who had PD alone (56 % vs. 29 %, p < 0.01) (Table 4).
We performed logistic regression to identify independent predictors of severe adverse postoperative outcomes. On univariate analysis, neoadjuvant therapy (OR 2.21, CI 1.01–4.84), intraoperative blood transfusion (OR 3.11, 95 % CI 1.43–6.76), and vascular resection (OR 3.11, CI 1.43–6.76) were significant predictors of SAPO. American Society of Anesthesiologists (ASA) class, hypoalbuminemia, age, type of pancreaticoduodenectomy performed, and tumor size were not significant. On adjusted multivariate analysis, the only significant independent predictors of SAPO were albumin ≤3 g/dL, with an odds ratio of 2.73 (95 % CI 1.11–6.69) and vascular resection, with an odds ratio of 2.68 (95 % CI 1.05–6.85) (Table 5). Due to its high correlation with PDVR (56 % of PDVR vs. 15 % of PD, p < 0.01), intraoperative blood transfusion was not included as a covariate in the multivariate analysis.
Survival Analysis
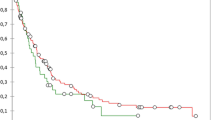
The mean length of follow-up from the time of diagnosis was 20 months. Adjusted multivariate Cox-regression survival analysis was used to examine recurrence-free and overall survival trends between the PDVR and PD groups. After adjusting for age, comorbidities, chemotherapy, complications, vascular resection, and pathologic stage, there was a significantly shorter recurrence-free survival in the PDVR group, with a median recurrence-free survival of 12 months compared to 17 months in the PD group, p = 0.02. A similar difference was noted in our analysis of overall survival, with a median overall survival of 17 months in the PDVR group compared to 29 months in the PD group, p = 0.04 (Fig. 1). Cox-regression modeling was also used to identify determinants of overall survival. On univariate analysis, vascular resection (OR 2.01, CI 1.21–3.36), higher pathologic stage (OR 2.99, CI 1.09–8.20), and severe adverse postoperative outcome (OR 1.76, CI 1.10–2.81) were associated with worse overall survival. However, on multivariate modeling adjusted for age, comorbidities, chemotherapy, complications, vascular resection, and pathologic stage, the only independent predictors that remained significant were vascular resection (OR 1.79, CI 1.04–3.09) and higher pathologic stage (OR 2.97, CI 1.03–8.52) (Table 6).
A significant percentage of patients undergoing PDVR were treated with neoadjuvant chemotherapy. To evaluate the impact of the neoadjuvant approach, we performed adjusted Cox-survival analysis for the vascular resection cohort. In patients who had PDVR, the only significant independent predictor of overall survival was neoadjuvant chemotherapy, with a HR of 0.19 (95 % CI 0.04–0.94, p = 0.04) from the time of diagnosis (adjusting for age, ASA class, neoadjuvant chemotherapy, adjuvant chemotherapy, complications, and pathologic stage).
Discussion
In this study, we used a case-matched cohort analysis to compare patients undergoing pancreaticoduodenectomy with vascular resection for pancreatic adenocarcinoma to a pathologic stage and age-matched cohort of patients undergoing pancreaticoduodenectomy without vascular resection. We applied a system for grading postoperative complications that included all 90-day readmissions to fully evaluate perioperative morbidity in addition to short- and long-term recurrence and survival outcomes between groups. Our study demonstrated that major vascular resection was associated with an increased rate of severe adverse postoperative outcomes, readmissions, total length of stay, and hospital costs. Although the rates of R0 resection and overall recurrence were equivalent between groups, there was a significantly shorter time to recurrence in the PDVR group as well as a decreased overall survival. We found the need for major vascular resection to be the only independent predictor of mortality on multivariate Cox-regression survival analysis.
Our findings contrast some of the published series examining PDVR. The two largest series to date on pancreaticoduodenectomy with vascular resection are a multi-institutional retrospective British study of high-volume centers and a NSQIP study. The British study examined 230 vascular resections of T3 pancreatic adenocarcinoma. They found similar overall complication rates between compared groups, although there were significantly increased rates of delayed gastric emptying and postoperative blood transfusion in the PDVR groups in both studies when compared to patients who underwent PD. Median overall survival was equivalent between the PDVR and PD groups (approximately 18 months).8 The NSQIP study examined 281 vascular resections for pancreatic cancer. They found significantly increased perioperative mortality and increased overall 30-day morbidity rates for the patients who underwent PDVR compared to patients who underwent PD alone. No staging or long-term survival data was available in the study.9 Both studies found similar postoperative hospital lengths of stay for the two groups. Meta-analyses of smaller retrospective studies have shown increased operative time and EBL for PDVR compared to PD with similar overall morbidity rates and overall long-term survival for venous resections.13 , 14 Increased perioperative morbidity and mortality as well as decreased long-term overall survival have been reported for arterial resections.15 However, none of these studies have taken into account the 90-day readmission rates and subsequent complications or graded complications to reflect the overall length of hospital stay.
In our study, we used a modified version of the Clavien-Dindo system to grade complications in an effort to better reflect complication severity accounting for readmission events and all interventional procedures out to 90 days post-resection. Severe adverse postoperative outcomes included any Clavien-Dindo IIIb-V complication as well as IIIa complications requiring >1 intervention and I–IIIa complications for which total length of hospital stay was >3 standard deviations beyond the mean for patients without complications (17 days). When comparing our overall complication rates based on the Clavien-Dindo system alone, there was no difference in the complication rates between the PDVR and PD groups. There was also no difference in initial postoperative length of stay between groups. However, when readmissions were included in our modified grading system, we found a significantly increased rate of severe adverse postoperative outcomes as well as a significantly longer total length of stay in the PDVR group. This finding demonstrates a potential pitfall of reporting overall morbidity rates without taking into account the 90-day readmission data and indicates that such efforts provide incomplete assessments of the morbidity of PDVR.
The underlying etiology for the increased complication severity profile is not clear. The higher complication profile may be related to the cross-clamping of the portal vein which might be expected to result in transient intestinal ischemia and then lead to increased propensity for delayed gastric emptying, ileus, and malnutrition. All of these complications were seen in increased frequency among PDVR patients in our series and have been seen at higher rates in vascular resection patients in other series as well.8 , 16 Regardless of the underlying mechanism, the morbidity is not inconsequential. These complications contribute to the prolonged hospitalization and admission. Longer length of hospital stay has been shown to be a major adverse contributor to the quality of life and patient debilitation and is a known risk factor for delay to adjuvant chemotherapy.17 , 18
The decreased recurrence-free and overall survival demonstrated in our series is likely multifactorial. First, pathologic tumor invasion of the resected vein has been shown to be associated with decreased long-term overall survival.13 , 19 , 20 Although R1 resection has been associated with decreased survival,21 – 23 rates of R1 were equivalent in our cohort groups and thus not likely to contribute to the survival difference. In addition, many of our patients in the PDVR group presented with borderline resectable tumors and received neoadjuvant therapy prior to resection. There was likely a pathologic down-staging in some of these patients. We used pathologic stage to cohort match our patients and we may be comparing non-equivalent groups as more of the PDVR patients may have started with more advanced tumors but have been down-staged by neoadjuvant treatment. Finally, uncinate process and pancreatic neck tumors may simply be diagnosed at a later point in the course of their disease due to their anatomic location. These tumors are less likely to narrow the bile duct and cause jaundice. They may be stage II tumors pathologically but may more likely harbor subclinical microscopic metastatic disease at the time of resection than tumors closer to the ampulla that cause symptoms earlier in the course of disease. This may result in a degree of lead-time bias that contributes to decreased survival in these patients.
Limitations of our study include the retrospective nature of the data and the small sample size. The extent of vascular resection was variable, with some patients requiring relatively long interposition graft reconstruction and others primary repair. The operations in the vascular group were heterogeneous, and the effect of vascular resection on outcome may be muted by inclusion of short segment and partial diameter vein reconstruction. All borderline resectable patients were treated with neoadjuvant therapy;24 however, because of the 7-year time span of our study, there was heterogeneity in the type of neoadjuvant chemotherapy ranging from gemcitabine-based therapy25 , 26 in the earlier years of the study to FOLFIRINOX therapy currently.27 , 28 Also, there may be a pathologic bias in the cohort matching. There were a greater proportion of borderline resectable patients in the vascular cohort. Patients in the vascular resection group may have had higher stage tumors at the time of treatment onset and been down-staged by neoadjuvant therapy. This may impact their overall survival and contribute to the finding that these patients have earlier recurrence and die of their disease sooner than the patients in the PD alone cohort. Finally, overall survival was calculated from the time of diagnosis. The retrospective nature of the data and the fact that the analysis includes only patients who underwent resection almost certainly introduce selection bias as patients who had cancer progression while on neoadjuvant therapy and did not undergo resection and ultimately died early were excluded from the study population.
In spite of these limitations, our investigation has substantive value. We demonstrate that vascular resection performed in the context of pancreaticoduodenectomy is associated with an increased complication severity profile and increased costs. The overall rate of complications is not dramatically different than that for PD alone and the severity of complications may not prove to be prohibitive of pursuing resection for these patients; but our findings do suggest that at least great care in selecting patients for pancreaticoduodenectomy with vascular resection is warranted. These findings may also ultimately serve to drive a better discussion of risks and benefits on an individual case-by-case basis. Further, our observation that patients with neoadjuvant chemotherapy prior to vascular resection have improved overall survival relative to those not receiving neoadjuvant treatment supports the notion that the best therapeutic approach for these individuals may be upfront chemotherapy/chemoradiation prior to resection. Given the risk of severe complications and early recurrence and the associated increased costs, it may make sense to treat all patients with the potential need for vascular resection with neoadjuvant chemoradiation for a longer period than would be done otherwise and to restage prior to resection in an effort to rightly identify those with more favorable tumor biology and no subclinical metastatic disease burden. Recent work by the group at MD Anderson has suggested that all patients with pancreatic cancer would be better served by undergoing neoadjuvant chemotherapy prior to surgery. In their experience, this approach does in fact clarify the biologic nature of the tumor (more definitively, rules out chemo-insensitive disease, aggressive disease, and patients with subclinical metastatic disease at the time of diagnosis) and establishes the fitness of the patient for surgical resection and allow for patients to receive more complete courses of systemic therapy.29 Our results of decreased overall survival in patients who underwent vascular resection but did not receive neoadjuvant therapy would support this argument. Our results in general also suggest that uncinate and neck tumors requiring vascular resection have more aggressive tumor biology or are prone to later presentation than stage-matched ampullary lesions and may thus have decreased recurrence-free and overall survival. The need for vascular resection may prove to be an independent prognostic indicator of decreased survival that should be integrated into future pathologic staging systems for pancreatic adenocarcinoma.
Conclusions
Vascular resection is an aggressive surgical approach to borderline resectable pancreatic cancers that is oncologically effective and frequently achieves negative resection margins without prohibitive perioperative mortality. It is associated with an increase in the severity of perioperative complications and with early disease recurrence and decreased overall survival. Larger studies that incorporate a graded system of complications to fully assess perioperative morbidity are needed to further determine the true value and costs of these procedures.
References
Worni M, Castleberry AW, Clary BM, Gloor B, Carvalho E, Jacobs DO, Pietrobon R, Scarborough JE, White RR. Concomitant vascular reconstruction during pancreatectomy for malignant disease: a propensity score-adjusted, population-based trend analysis involving 10,206 patients. JAMA Surg 2013;148(4):331-8.
Banz VM, Croagh D, Coldham C, Taniere P, Buckels J, Mayer D, Muiesan P, Bramhall S, Mirza DF. Factors influencing outcome in patients undergoing portal vein resection for adenocarcinoma of the pancreas. Eur J Surg Oncol 2012;38(1):72-9.
Hwang JW, Kim SC, Song KB, Yoon JH, Nam JS, Lee JS, Park KM, Lee YJ. Significance of radiologic location and extent of portal venous involvement on prognosis after pancreatic adenocarcinoma. Pancreas 2015;44(4):665-71.
Jahromi AH, Jaferimehr E, Dabbous HM, Chu Q, D’Agostino H, Shi R, Wellman GP, Zibari GB, Shokouh-Amiri H. Curative resection of pancreatic adenocarcinoma with major venous resection/repair is safe procedure but will not improve survival. JOP 2014;15(5):433-41.
Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, Gomez HF, Sun CC, Crane CH, Wolff RA, Evans DB. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004;8(8):935-49.
Riediger H, Makowiec F, Fischer E, Adam U, Hopt UT. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg 2006;10(8):1106-15.
Menon VG, Puri VC, Annamalai AA, Tuli R, Nissen NN. Outcomes of vascular resection in pancreaticoduodenectomy: single-surgeon experience. Am Surg 2013;79(10):1064-7.
Ravikumar R, Sabin C, Abu Hilal M, Bramhall S, White S, Wigmore S, Imber CJ, UK Vascular Resection in Pancreatic Cancer Study Group. Portal vein resection in borderline resectable pancreatic cancer: A United Kingdom multicenter study. J Am Coll Surg 2014;218(3):401-11.
Castleberry AW, White RR, De La Fuente SG, Clary BM, Blazer DG, McCann RL, Pappas TN, Tyler DS, Scarborough JE. The impact of vascular resection on early postoperative outcomes after pancreaticoduodenectomy: an analysis of the American College of Surgeons National Surgical Quality Improvement Program Database. Ann Surg Oncol 2012;19(13):4068-77.
Varadhachary GR, Tamm EP, Crane C, Evans DB, Wolff RA. Borderline resectable pancreatic cancer. Curr Treat Options Gastroenterol 2005;8(5):377-84.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250(2):187-96.
Baker MS, Sherman KL, Stocker SJ, Hayman AV, Bentren DJ, Prinz RA, Talamonti MS. Using a modification of the Clavien-Dindo system accounting for readmissions and multiple interventions: Defining quality for pancreaticoduodenectomy. J Surg Oncol 2014;110(4):400-6.
Yu XZ, Li J, Fu DL, Di Y, Yang F, Hao SJ, Jin C. Benefit from synchronous portal-superior mesenteric vein resection during pancreaticoduodenectomy for cancer: a meta-analysis. Eur J Surg Oncol 2014;40(4):371-8.
Chua TC, Saxena A. Extended pancreaticoduodenectomy with vascular resection for pancreatic cancer: a systemic review. J Gastrointest Surg 2010;14(9):1442-52.
Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoegger Y, Buchler MW, Weitz J. Arterial resection during pancreatectomy for pancreatic cancer: A systemic review and meta-analysis. Ann Surg 2011;254(6):882-93.
Müller SA, Hartel M, Mehrabi A, Welsch T, Martin DJ, Hinz U, Schied BM, Büchler MW. Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg 2008;13(4):784-92.
Mbah N, Brown RE, St Hill CR, Bower MR, Ellis SF, Scoggins CR, McMasters KM, Martin RC. Impact of post-oeprative complications on quality of life after pancreatectomy. JOP 2012;13(4):387-93.
Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, Ko CY, Bentrem DJ. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260(2);372-7.
Wang J, Estrella JS, Peng L, Rashid A, Varadhachary GR, Wang H, Lee JE, Pisters PW, Vauthey JN, Katz MH, Gomez HF, Evans DB, Abbruzzese JL, Fleming JB, Wang H. Histologic tumor involvement of superior mesenteric vein/portal vein predicts poor prognosis in patients with stage II pancreatic adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012;118:3801-11.
Fukuda S, Oussoultzoglou E, Bachellier P, Rosso E, Nakano H, Audet M, Jaeck D. Significance of the depth of portal vein wall invasion after curative resection for pancreatic adenocarcinoma. Arch Surg 2007;142(2):172-9.
Mathur A, Ross SB, Luberice K, Kurian T, Vice M, Toomey P, Rosemurgy AS. Margin status impacts survival after pancreaticoduodenectomy but negative margins should not be pursued. Am Surg 2014;80(4):353-60.
Dusch N, Weiss C, Ströbel P, Kienle P, Post S, Niedergethmann M. Factors predicting long-term survival following pancreatic resection for ductal adenocarcinoma of the pancreas: 40 years of experience. J Gastrointest Surg 2014;18(4):674-81.
Merkow RP, Billimoria KY, Bentrem DJ, Pitt HA, Winchester DP, Posner MC, Ko CY, Pawlik TM. National assessment of margin status as a quality indicator after pancreatic cancer surgery. Ann Surg Oncol 2014;21(4):1067-74.
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma Version 1.2015. http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
Marti JL, Hochster HS, Hiotis SP, Donahue B, Ryan T, Newman E. Phase I/II trial of induction chemotherapy followed by concurrent chemoradiotherapy and surgery for locoregionally advanced pancreatic cancer. Ann Surg Oncol 2008;15(12):3521-31.
O’Rielly EM, Perelshteyn A, Jarnagin WR, Schattner M, Gerdes H, Capanu M, Tang LH, LaValle J, Winston C, DeMatteo RP, D’Angelica M, Kurtz RC, Abou-Alfa GK, Kimstra DS, Lowery MA, Brennan MF, Coit DG, Reidy DL, Kingham TP, Allen PJ. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg 2014;260(1):142-8.
Christians KK, Tsai S, Mahmoud A, Ritch P, Thomas JP, Wiebe L, Kelly T, Erickson B, Wang H, Evans DB, George B. Neoadjuvant FULFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist 2014;19(3)266-74.
Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnel EI, Sabbatino F, Santos DD, Allen JN, Blaszkowsky LS, Clark JW, Faris JE, Goyal L, Kwak EL, Murphy JE, Ting DT, Wo JY, Zhu AX, Warshaw AL, Lillemoe KD, Fernandex-del Castillo C. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261(1):12-7.
Tzeng CW, Fleming JB, Lee JE, Xiao L, Pisters PW, Vauthey JN, Abdalia EK, Wolff RA, Varadhachary GR, Fogelman DR, Crane CH, Balachandran A, Katz MH. Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy. Ann Surg Oncol 2012;19(6):2045-53.
Author information
Authors and Affiliations
Corresponding author
Additional information
Primary Discussant
Jennifer F. Tseng, M.D. MPH (Boston, MA)
Dr Kantor, Dr. Baker, congratulations on your talk on an important subject in pancreatic surgery. I have 2 questions.
1). 50 % of your 36 patients underwent primary transverse closure and 31 % underwent end-to-end anastomosis over the 7 years of the study. This 81 % rate of primary reconstruction without patch or graft is higher than that reported in many studies and may suggest intraoperative decision making rather than preoperative planning for venous resection necessitating reconstruction. Were these vascular reconstructions planned a priori based on imaging or intraoperative necessity due to unexpected tumor involvement and/or bleeding at the time of operation? This might influence the increased complications and worse outcomes from the VR group.
2). You had a 56 % rate of neoadjvuant therapy in the VR group versus 13 % in the matched control group. Was survival (time to recurrence) calculated from diagnosis, as would be appropriate in a study where some patients received neoadjuvant therapy, or from surgery, which would bias the survival against the VR group?
Thank you for the opportunity to review.
Closing Discussant
Dr. Kantor
Dr. Tseng, thank you for reviewing our manuscript and your thoughtful comments.
1). With regard to your first question, for this study we identified all patients that had vascular resections by a retrospective query of our institutional pancreatic database. We then reviewed operative notes and had additional separate discussion with the operating surgeons to clarify the indications/reasons for the vein resection. For our analysis, we excluded vein resections that were done for accidental vascular injuries as well as unplanned vascular resections. .
2). With regard to your second question, overall survival was calculated from time of surgery. We felt that either choice for the point of origin for the survival analysis (from date of surgery or date of diagnosis) would have the potential to introduce bias. We did have many patients that received neoadjuvant chemotherapy in our vascular resection group. All of these patients were selected retrospectively and thus were by nature patients who survived through neoadjuvant therapy to surgery. We felt that using the time of diagnosis would create bias in the sense that none of these patients would have had any possibility of mortality during the course of neoadjuvant chemotherapy. We also do not have data on how many patients would have dropped out (developed metastatic disease) during neoadjuvant treatment. For these reasons, in an analysis starting at time of diagnosis, patients in this cohort would have thus had a built in survival advantage. On the other hand, using date of surgery, “takes” time away from these individuals in the sense that it excludes the time in chemotherapy from the survival calculations. At our institution we typically give short courses of neoadjuvant therapy: approximately two months of treatment with a third month left for recovery prior to surgery. While using the starting point for the survival analysis as the date of surgery could certainly introduce a bias that would reflect negatively on the survival statistics of patients in the vascular resection cohort, the observed difference in overall survival between the groups was wide enough (9 months) that it would be expected to continue to be significant even if neoadjuvant treatment time was added back to all affected vascular resection patients. In the end, we felt the least problematic method was to use the date of surgery as the start point for our survival calculation.
Plenary Presentation at 2015 SSAT Annual Meeting on May 19, 2015 in Washington, DC
Rights and permissions
About this article
Cite this article
Kantor, O., Talamonti, M.S., Stocker, S.J. et al. A Graded Evaluation of Outcomes Following Pancreaticoduodenectomy with Major Vascular Resection in Pancreatic Cancer. J Gastrointest Surg 20, 284–292 (2016). https://doi.org/10.1007/s11605-015-2957-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2957-2