Abstract
A simple wet chemical method was used to coat a layer of LaF3 on the surface of LiNi0.5Co0.2Mn0.3O2(NCM523) to improve the electrochemical performance. Through the characterization of X-ray diffractometer (XRD), scanning electron microscope (SEM), X-ray energy dispersive spectrometer (EDS), and X-ray photoelectron spectroscopy (XPS), it can be seen that the LaF3 coating can be uniformly coated on the surface of the material and will not change the crystal structure and micro-morphology of the material. In this work, the amount of LaF3 coating on the materials is 0.0 wt%, 0.5 wt%, 1.0 wt%, and 2.0 wt%, respectively. Under the condition of the optimum coating amount of 1 wt%, the rate performance and cycle performance of the coated material can be improved obviously. After 100 cycles at a high cut-off voltage of 4.6 V, the capacity retention of the 1 wt% LaF3 coated material is 88.7%, which is higher than 80.4% of the bare material. And through EIS analysis, the coated material after 50 cycles still has excellent lithium ion diffusion kinetics. This may be due to the fact that an appropriate amount of LaF3 coating can effectively avoid direct contact between cathode materials and electrolytes, inhibit the generation of oxygen vacancies, and reduce unnecessary side effects. This could provide a new idea for improving the electrochemical performance of LiNi0.5Co0.2Mn0.3O2 at high cut-off voltage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, with the continuous development of new energy field, lithium-ion battery (LIBs) has been widely used in portable electronic devices, electric vehicles (EVs), medical fields, and other fields because of its excellent energy density and safety performance [1,2,3]. However, the earlier commercial cathode materials, such as LiMn2O4 and LiCoO2, have seriously limited the commercial application of lithium-ion batteries due to their poor high-voltage performance, unstable cycling performance, and low discharge specific capacity [4, 5].
Layered ternary cathode material LiNixCoyM1-x-yO2 (NCM) has stable electrochemical performance and high energy density, making it an ideal cathode material for power lithium-ion batteries [6, 7]. LiNi0.5Co0.2Mn0.3O2 (NCM523) has attracted more and more attention for its high initial discharge capacity, low cost, and high safety [8]. However, NCM523 has some defects in the commercial process, such as Li+/Ni2+ mixing, long-period capacity attenuation, thermal instability, and gas escape [9]. The main reasons are as follows: (1) ion radii of Ni2+ and Li+ are similar, which is easy to cause mixed discharge of cations. (2) Serious side reactions occur at the interface between the electrolyte and the positive electrode, which accumulates a large number of by-products on the surface of the positive electrode. (3) The surface of the cathode particles is unstable at high temperature, which leads to the rapid attenuation of the capacity during cycling. (4) The layered structure changes to electrochemically inert spinel or even salt facies at high cut-off voltage [10,11,12,13,14]. Therefore, some improvement measures need to be taken to improve the cyclic stability and rate performance. There are two common modification methods: the coating method and the doping method. Surface coating can effectively avoid direct contact between cathode materials and electrolytes to reduce side reactions. Typical coating materials include metal oxides TiO2 [15], V2O5 [16], CeO2 [17], fluorides CaF2 [18] and AlF3 [19], lithium ion conductor Li2TiO3 [20], Li3VO4 [21], phosphate AlPO4 [22], and conductive polymer [23]. Zhao et al. obtained the precise Al2O3 coating on the surface of NCM523 by atomic layer deposition (ALD), which successfully inhibited the dissolution of TM during the NCM cycle and reduced the internal stress variation of NCM secondary particles during the cycle [24]. As a kind of rare earth fluoride, LaF3 has good ionic conductivity, so it is often used as a modified material. In comparison, most oxides and phosphates react with electrolytes containing LiPF6 to form corresponding fluoride, resulting in serious corrosion of cathode materials [25]. Therefore, fluoride as a coating material can effectively reduce the corrosion of HF to cathode materials in electrolyte, reduce the occurrence of side reactions, and effectively improve the cycle performance of materials, and fluoride is more stable than oxide in the ambient air, can effectively protect the cathode material, and reduce LiOH/Li2CO3 and other harmful impurities on the surface.
In this work, we used a simple wet coating method to coat the surface of LiNi0.5Co0.2Mn0.3O2 uniformly with LaF3 and studied in detail the improvement of cycle performance and rate performance of LaF3 coating on LiNi0.5Co0.2Mn0.3O2 cathode material at high cut-off voltage (4.6 V).
Experiments
Material synthesis
LiNi0.5Co0.2Mn0.3O2 cathode materials were synthesized by the coprecipitation method and high-temperature solid-state method. The chemicals used in this study are all analytical-grade reagents and can be used directly without further purification. The metal salts NiSO4·6H2O, CoSO4·7H2O, and MnSO4·H2O were mixed into a solution (2.0 M) according to the molar ratio at 5:2:3, which was added into the reactor slowly and stirred continuously in a nitrogen atmosphere with the rotating speed of 300 rpm. At the same time, the NaOH (3.0 M) and ammonia (2.0 M) solutions, which are used as precipitators and complexing agents, were added into the reactor at a pH value of 11 (± 0.1) and kept the temperature at 55 °C. After the reaction, standing, washing, filtering, and drying at 120 °C for 24 h to obtain the precursor Ni0.5Co0.2Mn0.3(OH)2. The precursor and Li2CO3 were weighed and ground uniformly according to the molar ratio of 1:0.54, and then pre-sintered at 500 °C for 6 h, then calcined at 850 °C for 12 h, and finally the material LiNi0.5Co0.2Mn0.3O2 was obtained.
A certain amount of LiNi0.5Co0.2Mn0.3O2 was supersonic dispersed in ethanol and stirred by magnetic force at 30 °C for 1 h. The configured NH4F and La(NO3)3·H2O solution were added slowly into the reactor, and the mole ratio was strictly controlled at 3:1. Subsequently, the mixed solution was heated to 65 °C and stirred quickly until all the solution was volatilized. The prepared samples were dried in a vacuum at 120 °C for 12 h and then calcined in air at 400 °C for 5 h. In order to explore the optimal coating amount, the prepared LaF3 coating amount is 0.0 wt%, 0.5 wt%, 1.0 wt%, and 2.0 wt%, respectively, marked as LF0, LF0.5, LF1, and LF2.
Material characterization
The structure of the sample was analyzed by X-ray diffractometer (XRD, Advance D8). The sample was collected in the 2θ range of 10 ~ 90°, and the scanning rate was 5°/min. In order to analyze the surface morphology and element distribution of the sample, the morphology and microstructure of the material were observed by S4800 scanning electron microscope (SEM), and the coating of elements on the original sample surface was detected by X-ray energy dispersive spectrometer (EDS, Noran System Six, USA). The thickness of the coating was observed by JEOL JEM2100F transmission electron microscope (TEM). The oxidation state of elements in the material was examined by X-ray photoelectron spectroscopy (XPS, Thermo K-alpha).
Electrochemical test
The positive active material, acetylene black (Super P), and polyvinylidene fluoride (PVDF) were uniformly mixed and ground according to the mass ratio of 8:1:1, and the proper amount of organic solvent N-methyl pyrrolidone (NMP) was added. The obtained slurry was uniformly coated on the aluminum foil with a Doctor blade technique and dried in a vacuum at 80 °C for 12 h. The electrode with a diameter of 14 mm was prepared by pressing and punching. The button battery (CR2032) was assembled in a glove box filled with argon with an electrode piece as the positive electrode, a metal lithium piece as the negative electrode, Celgard2325 PP microporous diaphragm and 1 mol·L−1 LiPF6 [EC + DMC + EMC (v: v: v = 1:1:1)] solution as the electrolyte. The constant current charge–discharge cycle test was carried out by using the LAND battery test system (CT2001A, Wuhan in China) at 25 °C. Cyclic voltammetry test (voltage range 2.5 ~ 4.6 V, scanning rate 150 μV/S) and impedance test were carried out on electrochemical workstation (IM6E, Zahenr, Germany), with a frequency range of 100 kHz ~ 0.01 Hz and amplitude of 5 mV.
Results and discussion
Microstructural characterization
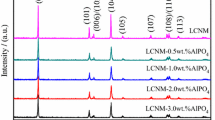
Figure 1a shows the XRD patterns of bare NCM523 and NCM523 with different LaF3 coating amounts. It can be seen that all the materials have two clear splitting peaks of (006)/(102) and (108)/(110), indicating that the layered structure of the four materials is sequential and showing a single hexagonal α-NaFeO2 structure and R-3 m space group [26, 27]. It can be seen from Fig. 1b that the (003) peak shifts obviously to the left after coating, which indicates that the lattice parameters increase. Tables 1, 2 lists the unit cell parameters after Rietveld refinement, and the c/a values of all samples are greater than 4.9, indicating that the layered structure of the materials is good. In addition, the ratio of I(003)/I(104) of LF1 is 1.36, which is higher than that of the bare sample (1.24), indicating that the LaF3 coating may reduce the mixing of cations and could improve the structural stability of the active materials [28]. There are no other impurity peaks in the XRD spectra of the coated samples, which indicate that the LaF3 coating will not change the crystal structure of the material.
In order to further study the effect of LaF3 coating on the morphology of NCM523, the SEM diagrams of LF0, LF0.5, LF1, and LF2 were compared. As shown in Fig. 2, it can be clearly observed that the samples are composed of submicron particles with an average particle size of about 12 μm, which is approximately spherical, and there is no obvious agglomeration. The surface of the sample LF0 is smooth, and there are no particles attached. However, the surface of the sample coated with LaF3 becomes rough, there are particles attached, and most of the angular features become blurred. And with the increase of the amount of coating, it is difficult to distinguish the gaps between the primary particles. Too many coating materials will hinder the deintercalation/intercalation of lithium ions, resulting in a decrease in capacity. Figure 3 shows the EDS pattern of the LF1 sample to test the uniformity of the elements on the sample surface. As shown in Fig. 3a–g, Mn, Ni, Co, O, La, and F elements are uniformly distributed on the surface of the particles, indicating that LaF3 is uniformly coated on the surface of NCM523, which will play an effective role in the cyclic stability.
Figure 4 shows the TEM image of LF1. It can be seen that the surface of NCM523 is coated with a layer of LaF3 with a thickness of about 5 nm. The uniform coating can effectively prevent the electrode material from direct contacting with the electrolyte and reduce unnecessary side reactions. Figure 4a is an enlarged view of the yellow area in Fig. 4b, and an obvious lattice stripe, about 0.461 nm, can be observed, corresponding to the (003) crystal plane of LiNO2, which indicates that the LaF3 coating will not change the microstructure of the sample and still maintains the layered structure [29].
In order to analyze the chemical valence of each element in the sample, LF1 was tested by XPS. In Fig. 5a, the whole spectrum shows obvious peaks of Mn, Co, Ni, F, and La, and no other impurities exist. Figure 5b–f shows the measured spectra of each element. The Mn-XPS spectrum has two main peaks including Mn 2p3/2(641.4 eV) and Mn 2p1/2(653.1 eV), which indicates that the chemical state of Mn in the sample is Mn4+ [30]. The peaks around 855.9 eV and 873.3 eV belong to Ni 2p3/2 and Ni 2p1/2 [31]. The peaks of 779.8 eV and 794.08 eV belong to Co 2p3/2 and Co 2p1/2, indicating that the main valence state of Co is + 3 [32]. Figure 5e shows that there is a main peak at 684.08 eV, which corresponds to F 1 s. Such value revealed that the F valence was − 1 [33]. In Fig. 5f, the peak at 834.34 eV corresponds to 3d5/2 of La3+ [34]. This indicates that the NCM523 sample was effectively coated by LaF3.
Electrochemical performance
In order to test the effect of LaF3 coating on the electrochemical performance of cathode materials, LF0, LF0.5, LF1, and LF2 were assembled into button cells for charge and discharge tests. Figure 6a shows the initial charge–discharge curve of cathode materials with different LaF3 coating amount at 0.1 C (1 C = 180 mAh·g−1), 3.0–4.6 V. The curves of all samples are similar and smooth, and the discharge platform is about 3.8 V. With the increase of coating amount, the discharge specific capacity of cathode materials decreases in varying degrees. The bare material has the highest discharge specific capacity of 190.7 mAh·g−1, while the discharge specific capacity of LF0.5, LF1, and LF2 is 188.9, 187.1, and 185.1 mAh·g−1, respectively, which are lower than that of LF0. This is because LaF3 is an inert material; no additional discharge specific capacity can be provided. However, LF1 has the highest coulombic efficiency (86.3%), which is significantly higher than that of bare samples (82.2%). This is due to the fact that an appropriate amount of coating can prevent direct contact between electrodes and electrolytes, thus improving coulombic efficiency [35]. However, the thicker coating of LF2 reduces the mass of active material and hinders the diffusion of lithium ion, which leads to the decrease of discharge specific capacity.
Figure 6b–d depicts the rate performance of LF0, LF0.5, LF1, LF2, and the discharge curves of LF0 and LF1 at different rates. All materials were cycled 5 times at 0.1, 0.2, 0.5, 1, 2, and 5 C, respectively, and then recovered to 0.1 C at 3.0–4.6 V. At low current density (0.1 C and 0.2 C), the original sample still has higher discharge specific capacity and smaller attenuation, which is slightly higher than that of the coated material. However, with the increase of current density, the capacity of the bare sample began to decrease rapidly, especially at 5 C; the capacity of the original sample was only 120.0 mAh·g−1, while the discharge capacities of LF0.5, LF1, and LF2 at 5 C were 133.1, 139.4, and 135.2 mAh·g−1, respectively, mainly due to the increase of polarization. In addition, when the current is restored to 0.1 C, LF1 can still maintain a discharge specific capacity of 91.5% (171.3 mAh·g−1), which shows that LF1 has good reversibility. The discharge capacity retention rates of LF0, LF0.5, and LF2 at 0.1 C were 85.6% (163.3 mAh·g−1), 88.7% (167.6 mAh·g−1), and 90.8% (168 mAh·g−1), respectively. The results show that an appropriate amount of LaF3 coating can improve the high-rate cycle performance.
In order to further explore the improvement of the electrochemical performance of NCM523 coated by LaF3, the cycle performance of LF0, LF0.5, LF1, and LF2 was tested at 25 °C. The cycle performance was tested at 0.5 C. As shown in Fig. 7a, after 100 cycles, the discharge specific capacities of LF0, LF0.5, LF1, and LF2 are 147.6, 154.2, 163.6, and 152.5 mAh·g−1, respectively, and the retention rates are 80.4%, 83.2%, 88.7%, and 84.4%, respectively. By comparison, it is obvious that the cycle performance of LF0 is the worst, which is mainly due to the corrosion of HF to the cathode material and the accumulation of a large number of by-products on the surface of the material. The main reactions are as follows:
And at a high cut-off voltage of 4.6 V, the reaction will be accelerated, which leads to the rapid attenuation of the discharge capacity. However, the cycle stability of LF1 is the best, which shows that an appropriate amount of LaF3 surface modification layer can effectively restrain the generation of oxygen vacancy and alleviate the corrosion of electrolyte to the material. In order to observe the redox reaction in detail during the cycle, the differential capacity curve (dQ/dV) curves of LF0 and LF1 at lap 1, 25, and 50 were drawn respectively. As shown in Fig. 7b, c, all materials show typical anodic peaks around 3.7–3.8 V. With the increase of the number of cycles, the oxidation peak of the sample shifts to the right and the reduction peak shifts to the left. It can be observed that the potential difference of LF1 is obviously smaller than that of LF0. This shows that the coated sample can better maintain the redox peak and eliminate the kinetic energy barrier of electrochemical redox reaction, thus reducing the capacity attenuation [36].
Cyclic voltammograms and electrochemical impedance spectroscopy
In order to determine the charge–discharge reaction process and analyze the mechanism of its improvement, LF0 and LF1 were tested by cyclic voltammetry. Figure 7d is the CV diagrams. It can be seen that the oxidation peaks of LF0 and LF1 are 3.954 V and 3.891 V, respectively, and the corresponding reduction peaks are 3.664 V and 3.643 V, respectively. The potential difference can reflect the polarization and cyclic reversibility of the electrode. The potential difference of LF0 is 0.29 V, which is significantly larger than the 0.248 V of LF1. This shows that LaF3-modified NCM523 has a lower degree of polarization, which is corresponding to its excellent rate performance and cyclic performance [22].
Electrochemical impedance spectroscopy (EIS) was performed to further analyze the electrochemical kinetic characteristics of LF0 and LF1 after 3 cycles and 50 cycles at 3.0–4.6 V, 0.5 C. As shown in Fig. 8a, each curve of the impedance spectrum consists of a semicircle and a straight line, and the frequency range is from 0.01 Hz to 100 kHz. The first intersection of the curve with the Zʹ represents the solution impedance (Rs), which includes solution resistance and diaphragm resistance. The semicircle in the intermediate frequency region represents the charge transfer resistance (Rct), which refers to the resistance encountered when Li+ passes through the double layer, and is related to the dynamic characteristics of lithium batteries. The diagonal part is related to the Warburg resistance and the diffusion impedance of lithium ion in the electrode. The impedance is fitted by Z-view software, and the calculated fitting value is shown in Table 3. It can be seen that the charge transfer resistance Rct of LF0 after 3 cycles is 38.98 Ω, which is larger than 19.75 Ω of LF1, and after 50 cycles, the Rct of LF0 increases rapidly to 223.3 Ω, while the Rct of LF1 is only 118.6 Ω, which is much smaller than that of bare samples. This is due to the dissolution of transition metal ions in the bare materials during the cycle, the transition of layered structure to spinel salt phase, and the increase of surface impedance, thus hindering the diffusion of Li+ [13, 37]. This shows that LaF3 coating can well protect the electrode material, reduce the occurrence of side reactions, and prevent the attack of HF. There is a slight change in Rs during the cycle, which indicates that the solution resistance is little affected [18].
The diffusion coefficient of lithium ion can also be calculated by impedance diagram, and the formula is as follows:
In the above formula, R is the gas constant, T is the absolute temperature, n is the number of transferred electrons, F is the Faraday constant, CLi is the molar concentration of lithium ion, Ac is the positive electrode area, and σ is the Warburg coefficient fitted by ω−0.5 and Zʹ. Figure 8b shows the equations fitted by ω−0.5 and Zʹ in circles 3 and 50, respectively. The lithium ion diffusion coefficients of LF0 in the third and 50th circles are calculated to be 1.08 × 10−12 and 1.79 × 10−14 cm2·s−1, respectively, while the lithium ion diffusion coefficients of LF1 in the third and 50th circles are 1.79 × 10–12 and 1.01 × 10–13 cm2·s−1, respectively, which are higher than those of the bare samples. This is due to the corrosion of the bare sample by HF, which shows that a large number of by-products are accumulated in the material, which seriously affects the diffusion of lithium ion, while an appropriate amount of LaF3 coating can effectively protect the cathode material from crystal collapse and still has excellent lithium ion diffusion kinetics.
Figure 9a–d show the SEM images of the LF0 and LF1 after 100 cycles at 3.0–4.6 V. Part of the spherical shape of the pristine particles was destroyed, and micro-cracks appeared on the surface, accompanied by a large number of scattered fragments. This is mainly due to the direct contact between the pristine particles and the electrolyte, which leads to the lattice corrosion, while LF1 is still mostly compact spherical secondary particles, with few scattered fragments. LaF3 coating can not only be used as a protective layer, but also shield HF in electrolyte. The results show that the structure and interface stability of NCM523 are greatly improved after LaF3 coating, which corresponds to its excellent cycle performance [24, 38].
Conclusion
The cathode material LiNi0.5Co0.2Mn0.3O2 was prepared by coprecipitation and high-temperature solid-state method, and a layer of LaF3 material was uniformly coated on the surface of the material by the wet chemical method. In this work, the amount of LaF3 coating on the materials is 0.0 wt%, 0.5 wt%, 1.0 wt%, and 2.0 wt%, respectively. Uniform LaF3 coating can effectively inhibit the formation of residual alkali on the cathode material and isolate the cathode material from the electrolyte to resist HF attack. At high rate, the capacity decay of uncoated samples is significantly faster than that of coated samples, and when returning to low rate, 1 wt% coated samples still have a capacity retention of 91.5%, while uncoated samples have only 85.6%. Through CV and EIS diagrams, it can be found that LaF3 coating can significantly reduce the electrochemical polarization and charge transfer resistance, which corresponds to its excellent rate performance and cycle performance. However, LaF3 coating can not provide an additional discharge capacitance, resulting in an initial discharge capacity slightly lower than that of the bare sample, which still needs further improvement.
References
Li M, Lu J, Chen Z, Amine K (2018) 30 years of lithium-ion batteries. Adv Mater 1800561:1–24
Zhou H, Xin F, Pei B, Whittingham MS (2019) What limits the capacity of layered oxide cathodes in lithium batteries? ACS Energy Lett 4:1902–1906
Zha Q, Hu N, Song C, Hou H, Liao S, Zha G (2021) Improving cycle stability of Ni-rich LiNi0.8Mn0.1Co0.1O2 cathode materials by Li4Ti5O12 coating. Ionics 28:1047–1054
Krishna KS, Ghosh S, Ghosal P, Martha SK (2017) Synergistic effect of 3D electrode architecture and fluorine doping of Li1.2Ni0.15Mn0.55Co0.1O2 for high energy density lithium-ion batteries. J Power Sources 356:115–123
Song L, Li X, Xiao Z, Li L, Cao Z, Zhu H (2018) Effect of Zr doping and Li2O-2B2O3 layer on the structural electrochemical properties of LiNi0.5Co0.2Mn0.3O2 cathode material: experiments and first-principle calculations. Ionics 25:2017–2026
Zhang Z, Qiu J, Yu M, Jin C, Yang B, Guo G (2020) Performance of Al-doped LiNi1/3Co1/3Mn1/3O2 synthesized from spent lithium ion batteries by sol-gel method. Vacuum 172
Choi NS, Han J-G, Ha SY, Park I, Back CK (2015) Recent advances in the electrolytes for interfacial stability of high-voltage cathodes in lithium-ion batteries. RSC Adv 5:2732–2748
Bak SM, Hu E, Zhou Y, Yu X, Senanayake SD, Cho SJ, Kim KB, Chung KY, Yang XQ, Nam KW (2014) Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl Mater Interfaces 6:22594–22601
Liu BS, Zhang SH, Yu YG, Liu JH, He X, Sun ZJ, Yu ZQ, Wu YM, Wang ZB (2021) Interface crystal domain regulation via TiO2 surface modification enhancing stability of layered LiNi0.5Co0.2Mn0.3O2 for lithium-ion batteries. Ionics 27:1871–1880
Zhang H, Zhang Y, Xu T, John AE, Li Y, Li W, Zhu B (2016) Poly(m-phenylene isophthalamide) separator for improving the heat resistance and power density of lithium-ion batteries. J Power Sources 329:8–6
He M, Su CC, Peebles C, Feng Z, Connell JG, Liao C, Wang Y, Shkrob IA, Zhang Z (2016) Mechanistic insight in the function of phosphite additives for protection of LiNi0.5Co0.2Mn0.3O2 cathode in high voltage Li-ion cells. ACS Appl Mater Interfaces 8:11450–11458
Liu T, Zhao SX, Wang KZ, Gou LL, Nan CW (2015) Improved rate capability and cycle stability of Li[Ni0.5Co0.2Mn0.3]O2 with Li2MnO3 coating under high cut-off voltage. Appl Surf Sci 355:1222–1228
Liu W, Oh P, Liu X, Lee MJ, Cho W, Chae S, Kim Y, Cho J (2015) Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem Int Ed Engl 54:4440–4457
Li GY, Zhang ZJ, Wang R, Hung ZL, Zuo ZC, Zhou HH (2016) Effect of trace Al surface doping on the structure, surface chemistry and low temperature performance of LiNi0.5Co0.2Mn0.3O2 cathode. Electrochim Acta 212:399–407
Li Y, Liu X, Ren D, Hsu H, Xu GL, Hou J, Wang L, Feng X, Lu L, Xu W, Ren Y, Li R, He X, Amine K, Ouyang M (2020) Toward a high-voltage fast-charging pouch cell with TiO2 cathode coating and enhanced battery safety. Nano Energy 71:104643
Yuan M, Li Y, Chen Q, Chen C, Liu X, Zeng W, Wang R, Xiao S (2019) Surfactant-assisted hydrothermal synthesis of V2O5 coated LiNi1/3Co1/3Mn1/3O2 with ideal electrochemical performance. Electrochim Acta 323:134822
Zhou LJ, Yin ZL, Ding ZY, Li XH, Wang ZX, Guo HJ (2017) CeO2 coating to improve the performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2. Ionics 24:2533–2542
Dai S, Yan G, Wang L, Luo L, Li Y, Yang Y, Liu H, Liu Y, Yuan M (2019) Enhanced electrochemical performance and thermal properties of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material via CaF2 coating. J Electroanal Chem 847:113197
Kim HB, Park BC, Myung ST, Amine K, Prakash J, Sun YK (2008) Electrochemical and thermal characterization of AlF3-coated Li[Ni0.8Co0.15Al0.05]O2 cathode in lithium-ion cells. J Power Sources 179:347–350
Xiong F, Chen Z, Huang C, Wang T, Zhang W, Yang Z, Chen F (2019) Near-equilibrium control of Li2TiO3 nanoscale layer coated on LiNi0.8Co0.1Mn0.1O2 cathode materials for enhanced electrochemical performance. Inorg Chem 58:15498–15506
Huang Y, Jin FM, Chen FJ, Chen L (2014) Improved cycle stability and high-rate capability of Li3VO4-coated Li[Ni0.5Co0.2Mn0.3]O2 cathode material under different voltages. J Power Sources 256:1–7
Wang JH, Wang Y, Guo YZ, Liu CW, Dan LL (2014) Electrochemical characterization of AlPO4 coated LiNi1/3Co1/3Mn1/3O2 cathode materials for high temperature lithium battery application. Rare Met 40:78–83
Liu X, Li H, Li D, Ishida M, Zhou H (2013) PEDOT modified LiNi1/3Co1/3Mn1/3O2 with enhanced electrochemical performance for lithium ion batteries. J Power Sources 243:374–380
Cao G, Jin Z, Zhu J, Li Y, Xu B, Xiong Y, Yang J (2020) A green Al2O3 metal oxide coating method for LiNi0.5Co0.2Mn0.3O2 cathode material to improve the high voltage performance. J Alloys Compd 832:153788
Xiong X, Wang Z, Guo H, Zhang Q, Li X (2013) Enhanced electrochemical properties of lithium-reactive V2O5 coated on the LiNi0.8Co0.1Mn0.1O2 cathode material for lithium ion batteries at 60 ℃. J Mater Chem A 1:1284–1288
Wang D, Li XH, Wang ZX, Guo HJ, Xu Y, Fan YL, Ru JJ (2016) Role of zirconium dopant on the structure and high voltage electrochemical performances of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries. Electrochim Acta 188:48–56
Li Z, Cao S, Xie X, Wu C, Li H, Chang B, Chen G, Guo X, Zhang X, Wang X (2021) Boosting electrochemical performance of lithium-rich manganese-based cathode materials through a dual modification strategy with defect designing and interface engineering. ACS Appl Mater Interfaces 13:53974–53985
Chen Z, Chao D, Chen M, Shen Z (2020) Hierarchical porous LiNi1/3Co1/3Mn1/3O2 with yolk-shell-like architecture as stable cathode material for lithium-ion batteries. RSC Adv 10:18776–18783
Habibi A, Jalaly M, Rahmanifard R, Ghorbanzadeh M (2020) Microwave-reduced graphene oxide wrapped NCM layered oxide as a cathode material for Li-ion batteries. J Alloy Compd 834:155014
Chen XL, Lu WZ, Chen C, Xue MZ (2018) Improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode with different carbon additives for lithium-ion batteries. Int J Electrochem Sci 13:296–304
Huang B, Wang M, Yang X, Xu G, Gu Y (2019) Enhanced electrochemical performance of the layered nickel-rich oxide cathode by KMnO4 treatment precursor. J Alloy Compd 808:151683
Feng L, Liu Y, Qin W, Yang Z, Liu J (2021) A novel double modification to enhance electrochemical performance of LiNi0.5Co0.2Mn0.3O2 by substituting Ce for Co site. Electrochim Acta 391:138904
Zhang M, Wang C, Zhang J, Li G, Gu L (2021) Preparation and electrochemical characterization of La and Al Co-doped NCM811 cathode materials. ACS Omega 6:16465–16471
Li YC, Zhao WM, Xiang W, Wu ZG, Yang ZG, Xu CL, Xu YD, Wang EH, Wu CJ, Guo XD (2018) Promoting the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode via LaAlO3 coating. J Alloy Compd 766:546–555
Zheng H, Chen X, Yang Y, Li L, Li G, Guo Z, Feng C (2017) Self-assembled LiNi1/3Co1/3Mn1/3O2 nanosheet cathode with high electrochemical performance. ACS Appl Mater Interfaces 9:39560–39568
Li Z, Cao S, Wu C, Li H, Chen J, Guo W, Chang B, Shen Y, Bai Y, Wang X (2022) A facile and high-effective oxygen defect engineering for improving electrochemical performance of lithium-rich manganese-based cathode materials. J Power Sources 536:231456
Chen GR, An J, Meng YM, Yuan CZ, Matthews B, Dou F, Shi LY, Zhou Y, Song PG, Wu G, Zhang DS (2019) Cation and anion Co-doping synergy to improve structural stability of Li- and Mn-rich layered cathode materials for lithium-ion batteries. Nano Energy 57:157–165
Zhao LN, Chen GR, Weng YH, Yan TT, Shi LY, An ZX, Zhang DS (2020) Precise Al2O3 Coating on LiNi0.5Co0.2Mn0.3O2 by atomic layer deposition restrains the shuttle effect of transition metals in Li-ion capacitors. Chem Eng J 401:126138
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Zhang, H. & Zhao, W. Improvement of electrochemical performance of LiNi0.5Co0.2Mn0.3O2 by LaF3 coating at high cut-off voltage. Ionics 29, 1335–1345 (2023). https://doi.org/10.1007/s11581-023-04911-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04911-5