Abstract
A model based on a cubic equation of state and on the geometric similitude concept is proposed to calculate the surface tension of pure substances and mixtures containing organic substances, water, and ionic liquids. The geometric similitude is observed between surface tension and density for liquid mixtures at different temperatures and concentrations. The parameters of the model have been estimated by fitting experimental pure component data obtained from the literature. The model has been extended to binary and ternary mixtures using simple mixing and combining mixing rules without interaction parameters, so the predictive capabilities of the model are guaranteed. Data for 90 binary mixtures (2660 data) and 12 ternary mixtures (467 data) are considered. The mixtures are composed of organic solvent + ionic liquid and water + ionic liquids. The temperature varies from 278.15 to 348.15 K in the whole composition range at atmospheric pressure. Various form of estimating the necessary pure component parameters are analyzed. Results show that the model is accurate for primary estimation in process design and process simulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The surface tension is an important property of especial interest in phase transition phenomena found in processes such as catalysis, adsorption, distillation, extraction, and biological membranes [1,2,3]. Examples of these types of processes are those associated with environmental engineering, liquid chromatography, development of compound materials, drug carriers, chemical reactions that occur in fluid surfaces, and microelectronics [2]. Ionic liquids, on the other hand, have emerged as intriguing modern fluids in science and technology so the knowledge of their properties has given origin to extensive research during the last 25 years [3, 4]. Not only experimental data on ionic liquid properties have been measured but also models for correlating and predicting those properties have been proposed [5,6,7]. Ionic liquids, due to their unique molecular architecture and properties, have helped enhancing the capabilities and potential applications of many solvents [8]. In this regard, the surface tension of ionic liquid provides access to understand the intrinsic energetics of ions interactions, an important feature in surface thermodynamics [8, 9]. Experimental data on surface tension are limited and for mixtures is still more reduced. Therefore, having good correlating models but especially predictive models is of paramount importance for many applications involving ionic liquids, especially mixtures containing these fluids [2].
Different theories for modeling the surface tension have been proposed during the last decade such as the gradient theory, perturbation theory, van der Waals theory of fluid interface [10], the significant liquid structure theory, structure-based methods such as quantitative structure property relationship (QSPR) [11, 12], and other empirical models based on the Parachor [13]. Also, other predictive methods such as group contribution approaches [14, 15] and computational intelligence schemes such as artificial neural networks, have been proposed in the literature [16, 17].
The empirical models most commonly used are those based on the Parachor [13, 18]. The most interesting contribution on this line of research is that developed by Gardas and Coutinho [19], who use the Parachor approach to predict the surface tension of 38 imidazolium-type ionic liquids (ILs) (361 data). The mean absolute relative deviation is around 5.75% with maximum deviations of 25.20%. Also, these authors proposed another empirical model based on the molecular volume of the ion pair [19]. This model shows a mean absolute relative deviation of 4.50% when it was applied to 22 imidazolium-type ILs (47 data). Koller et al. [20] developed a new empirical surface tension model based on that suggested by Gardas and Coutinho [19] and proposed an exponential relation between the surface tension and the molar volume. This model with three adjustable parameters per ionic liquid shows deviations lower than 7.00%. More recently, Esmaeili and Hashemipour [21] proposed an empirical exponential model obtained by Multi-Gene Genetic Programming (MGP). This model with three adjustable parameters was applied to ammonium, imidazolium, pyridinium, pyrrolidinium, and phosphonium-type ILs.
In the literature, there are some few methods available to predict the surface tension mixtures, especially binary mixtures of ILs. However, most of them have been applied for a limited number of ILs and are not accurate enough, most probably due to the fact that surface phenomena in mixtures are highly complex because of the structure of the ionic liquids and ion interactions [20]. Experimental data of surface tension for the IL family 1-alkyl-3-methyl imidazolium tetrafluoroborate with water and with ethanol have been presented by Rilo et al. [22]. The authors provide experimental values that had been fitted to a two-parameter linear equation for each mixture. The overall mean absolute relative deviation using the lineal model for the IL + ethanol is below 5.90%, while in the IL + water the mean deviations are below 27.60%. Another interesting contribution on this line of research is that of Varela et al. [23], who applied the Bahe–Varela pseudolattice theory to estimate the surface tension of aqueous binary mixture of ILs. The theoretical predictions show good agreement with experimental data of several IL mixtures for ILs of different hydrophobic strengths in the low to intermediate concentration range. Better overall results were obtained at high IL concentrations.
Other works provide general information about the structure at the interface, based on understanding the energy forces between ions. On the basis of the above developments, different studies have been presented such as nanosized cellulose fibers by electrospinning or acid-catalyzed saccharification of woody materials [8, 24]. However, these studies have been applied to some few mixtures only, specially containing imidazolium-based ionic liquids and in a limited composition range. Oliveira et al. [25] were the first researchers who modeled the surface tension in binary mixtures, especially those containing bis(trifluoromethylsulfonyl) imide-type ionic liquids at 298.15 K and at atmospheric pressure. These authors used the soft-SAFT equation of state coupled with the density gradient theory (DGT) to predict the surface tension using a molecular-based approach. Later, these authors [26] extended the experimental data and modeling to other ILs including pyridinium, pyrrolidinium, and piperidinium. The authors use the Redlich–Kister equation with three adjustable parameters per mixture to estimate the surface tension with a standard deviation lower than 0.0012. Shojaeian and Asadizadehb [27] applied artificial neural networks (ANN) to predict the surface tension of the 33 binary mixtures containing different types of ionic liquids (1537 data points). The correlation coefficients R2 in the training, validation, and testing steps are above 0.98. Also, these authors compared their results with those determined by other methods and concluded that ANN gives the most accurate results.
Another approach that has been explored by several authors is the use of equations of state by comparing the similarities between the PρT diagram of a substance and the PXT diagram of the same substance, being X another property such as viscosity [6]. These similarities gave origin to the concept of geometric similitude, pointed out by Philips [28] at the beginnings of the XX century while studying and analyzing the behavior of the viscosity of carbon dioxide. During the last years, several models have been presented using the concept of geometric similitude and different equations of state have been employed [6, 7, 29,30,31,32]. However, to the best of the author’s knowledge, the correlation and prediction of surface tension in mixtures containing ionic liquids using the geometric similitude model have not been presented in the open literature, in the form done in this paper. The most novel and outstanding aspect of the present paper is the acceptable prediction of surface tension in mixtures containing ionic liquids.
Empirical model based on a cubic equation of state
The geometric similitude concept for the surface tension is more complex to visualize than those for viscosity or density, previously presented by the authors [6]. As known, the visual similitude between two diagrams representing the variation of two different properties with pressure and temperature (plus concentrations in the case of mixtures) gives origin to this method. If one plots density vs temperature at constant pressure (1 bar) for organic substances, a simple plot such as that shown in Fig. 1 is obtained. Similar plot is obtained for the surface tension vs temperature at constant pressure (1 bar) as can be observed in Fig. 1. By simple plots, we mean that one can observe a smooth behavior of the properties which decrease as the temperature increases, for both density and surface tension.
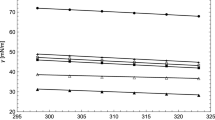
For ionic liquids, being structurally more complex substances than organic alkanes or alcohols, similar plots can be obtained as presented in Fig. 2. Again, the variation of density and of surface tension follows a smooth, steady decrease as the temperature increases. This observation indicates that there is a geometric similitude and that the equation describing these curves should be simple. Although this might be true, the problem of relating variables and mixture properties, of greater importance for industrial applications, gets more complicated. Therefore, the influence of the concentration on the value of the properties is now more complex to observe and to explain. An example of this fact is shown in Fig. 3.
Density and surface tension of mixtures of ethanol and water and [C2mim][Ac] and 1-propanol at different concentrations (expressed as mole fraction x of ethanol or [C2mim][Ac]). Data for water + ethanol [43]; data for [C2mim][Ac] + 1-propanol from [34]. (1) water + (2) ethanol, (1) [C2mim][Ac] + (2) 1-propanol
Figure 3 compares the properties of two mixtures composed of organic systems and ionic liquid mixtures. The curves indicate that for a fixed composition the density and surface tension decrease as the temperature increases. However, the dependency of the properties on concentration (mole fraction) is not clear (separation between lines and slopes of the lines). Thus, a more sophisticated model must be considered to include this third variable, the concentration. Considering that some equations of state can be acceptably applied to the liquid phase, for both pure substances and mixtures, the following hypothesis may be established: an equation of state that can well represent the effects of pressure, temperature, and concentration on density will also be able to represent the effect of these variables on the surface tension, using the concept of geometric similitude. This hypothesis is to be demonstrated in this work through the application of the equation of state (EoS) model to binary and ternary mixtures for predicting the surface tension. It should be noticed that once the geometric similitude is established, the meaning of the parameters is totally lost. Therefore, seeking any relation between the order of magnitude of the parameters, their behavior, or the sign of the parameters, has no sense.
The Valderrama–Patel–Teja cubic equation of state [44, 45] is the selected equation of state that provides the relation between the variables pressure, temperature, and concentration with the surface tension. This equation of state has been used in several applications [46, 47] and has also been successfully applied to the prediction of viscosity of ionic liquid in a wide range of temperature and pressure [6]. The EoS model applied in this paper is shown in Table 1. The table gives details on the dependency of the surface tension σ on pressure, temperature, and concentration. Also, some parameters (constant values and functions) included in the model are defined.
It should be noticed that even surface tension and density decrease with temperature and both behave more or less monotonically within a binary mixture, what is important when the similitude concept is invoked is to have a flexible model that can represent other types of behavior. The cubic equation used in this paper has that flexibility. This equation has the flexibility of representing geometric similitude in the whole range of pressure and temperature, although the particular study is done in a narrower area, just the liquid phase. Therefore, there is no restriction for applying the concept to other pressures and temperatures, including different fluid phases [6].
In Table 1, P is the pressure in bar, T is the temperature in Kelvin, σ is the surface tension in newton per meter, and Zc is the critical compressibility factor (dimensionless). The subscript m, c, i, and j indicate the mixture, the critical properties, and the components “i” and “j,” respectively. The alpha function described in Table 1, row 3, is that proposed by Heyen [48]. The use this alpha function has shown to be appropriate in applications done by the authors in modeling viscosity [6]. The parameter H1 varies for each ionic liquid, while H2 is a constant value for all ionic liquids. The terms ϕ0, ϕ1, β0, β1, γ0, γ1, and R* that appear in the equation of state (Table 1 row 3) are constants for all the ionic liquids and are obtained from an optimization procedure. It is important to clarify that although in the original PTρ equation the parameters may have certain physical meaning, in the extended model based on the geometric similitude concept the model parameters do not necessarily have a physicochemical sense.
For mixtures, the simple mixing rules proposed by the authors for the prediction of thermal conductivity and speed of sound in mixtures containing ionic liquids were employed [7]. The authors stated that the parameter kij in the classical van der Waals mixing [49] rule is of the order of − 2.0, so they fixed this parameter for all mixtures. Clearly, this value for kij has no physical meaning but is just an adjustable parameter that resulted to approximately assume a constant value, keeping the model as a predictive tool for estimating the properties of mixtures. This assumption of kij = − 2.0 for all interactions explains the minus sign in the term − 2xi ∙ xj of the mixing rule for the proposed model (rows 5 and 6 in Table 1). Finally, the units for each variable that present the model are T and Tc in Kelvin, P and Pc in bar, σ in newton per meter, R* in bar · g · m/(K·mol·N), ac in bar·g2 · m2/(mol2 · N2), b and c in g · m/(N · mol), M in g/mol, and H1, H2, α(T), Zc, ϕ0, ϕ1, β0, β1, γ0, and γ1 are all dimensionless.
To solve the model, a cubic polynomial is constructed to then apply the analytical solution of Cardano [50]. In Table 2, f0, f1, f2, p, q, and y are auxiliary parameters of Cardano’s method. The cubic equation provides three real roots, and since we are correlating and predicting a property of the liquid phase, the highest root is always selected as the true solution.
Data selection
Data selected for the surface tension of ionic liquids and mixtures containing ionic liquids were taken from the NIST-IL database that operates online [51, 52]. This database collects data and report uncertainties for each set of experimental data. For surface tension, 2026 data at 1 bar for 130 ionic liquids are included in the database, distributed in the following way: 93 imidazolium ILs (1481 data), 15 pyridinium ILs (322 data), eight pyrrolidinium ILs (73 data), nine ammonium ILs (107 data), three phosphonium ILs (29 data), and two sulfonium ILs (14 data). The uncertainties reported by NIST-ILs are below 4.32%, with the highest uncertainties belonging to the data sets of mixtures containing ILs with the anions [BF4] and [PF6]. For these types of ILs, uncertainties vary from 0.12 to 4.32%, being 0.84% the average uncertainties for [Cxmim][PF6] and 1.36% for [Cxmim][BF4].
Table 3 shows the ranges of temperature, pressure, and surface tension of the data selected for analysis, besides the critical properties for all the substances involved in the study. The molar mass and the critical properties (Tc, Pc, and Zc) were taken from the data given by Valderrama et al. [53]. Also, N in this table is the number of data in a data set. This table is included in the supplementary material that accompanies this paper. It is important to clarify that the critical properties of ILs are impossible to determinate experimentally because ILs start to decompose even at temperature below the normal boiling point [54], so the use of these critical properties is just an extension of the methods used to treat molecular fluids using equations of state. The estimated values are considered to be pseudo experimental and must be interpreted as ″these would be the values of Tc, Pc, Vc, Tb, and ω, if the properties were possible to be measured” [53].
The extension to mixtures is done for three types of binary mixtures, ILs + organic solvents, ILs + water and ILs + ILs, and also for ternary mixtures ILs + water + organic substances. The experimental data are also taken from NIST-IL database [51, 52]. For organic substances, the critical properties (Tc, Pc, and Zc) are taken from DIPPR database [69], and for the organic substances they were taken from other sources [55,56,57,58,59,60,61,62,63,64,65,66,67,68] in the temperature range from 288.15 to 348.15 K at 1 bar. Tables 4 and 5 show the ranges of temperature, concentration, and surface tension for the data: Table 4 for binary mixtures and Table 5 for ternary mixtures. For the 90 binary mixtures (2660 data) and 12 ternary mixtures (467 data), the temperature range goes from 278.15 to 348.15 K at 1 bar, in different concentration ranges.
Parameter estimation
The parameters to be estimated are those related to the mixture parameters am, bm, and cm (ϕ0, ϕ1, β0, β1, γ0, γ1) and to the parameter H2 of the temperature function α(T) (row 2 of Table 1); all these parameters are constant values for all substances. The parameter H1 varies from one fluid to another and is also determined during the optimization process. The above parameters are estimated by using experimental data of pure ILs from the NIST-ILs database [51, 52]. For mixtures, no interaction parameters are included, with the aim of evaluating the predictive capabilities of the model. The generalized reduced gradient [70] optimization method is used to find the optimum values of the model constants incorporated in Solver of MS Excel [70, 71]. This method converges to acceptable accurate solutions according to results presented in the literature [71, 72]. The objective function to be minimized is the average absolute relative deviation between values determined by the EoS model and experimental data, as follows:
In Eq. (1), the superscript “exp” means experimental data, superscript “cal” indicates calculated, and σ is the surface tension in newton per meter. Also, N is the number of data in a data set. When the optimum parameters have been found, a statistical analysis is done to define the goodness and robustness of an EoS model. For these reasons, besides the average absolute relative deviation (Eq. 1), the estimation of the average relative deviation %Δσ and the maximum absolute relative deviation ∣%Δσ∣max are additional adequate statistical parameters for fluid property estimation [73] and are described as follows:
Some of the data reported in Table 3 (for pure ILs only) have been used to test the model, while a fraction of the data was kept for testing. In the case of mixtures, experimental data were not used for modeling, so the values calculated can be regarded as totally predictive.
Results and discussion
The model has eight constants (R*, ϕ0, ϕ1, β0, β1, γ0, γ1, H2), which are valid for ionic liquids and organic and inorganic substances, and one adjustable parameter (H1) to be calculated for each substance. Table 6 shows the final model developed in this work applied to pure substances and mixtures. The values of the parameters are estimated using 1810 data (89% of the dataset), while 216 data (11% of the dataset) were left for analyzing the predictive capabilities of the model.
Pure substances
The results have been classified into three types according to the form in which the only adjustable parameter of the model (H1) was determined: (i) H1 by regression, determined by regression analysis of experimental data of surface tension, (ii) H1 using M, obtained according to a general equation using the molar mass M in g/mol); and (iii) H1 using one σ value, determined using one experimental data of surface tension.
Case 1: H1 by regression
Table 7 presents values of H1 by regression for some selected fluids. Also, the mean deviations (absolute, relative, and maximum values) with respect to experimental data of surface tension are shown. A complete table is provided as supplementary material. Similar deviations for correlation and testing below 3.12% (maximum deviation below 27.88%) are obtained. Also, 45 values have a deviation higher than 10% obtained in the correlation procedure (45 of the 1810 data), while 5 data in the testing procedure (5 of the 216 data). No datum presents deviation higher than 30%.
Table 8 shows the H1 by regression and its deviations for selected organic substances. The average absolute relative deviation is around 3.95%, and its average relative deviations are around − 0.18%. These results obtained for pure fluids are considered to be accurate enough to extend the model for correlation and predicting the surface tension in mixtures. The complete table of the data used is provided as supplementary material. It can be observed in Table 8 that the parameter H1 for water resulted to be negative (− 2.36), while for all ionic liquids and organic substances the value is positive and varied between 0.50 and 2.77. Clearly this value for H1 has no physical meaning but is just an adjustable parameter that provides accurate values of the surface tension and keeps the predictive capabilities of the model when it is applied to mixtures.
Case 2: H1 using M
The values of H1 by regression, presented in Case 1 above, correlate in an acceptable form (with some few exceptions) with the molar mass M (g/mol) of the ionic liquid, so a model that relates these two variables is proposed (Fig. 4). A quadratic equation is acceptable for these purposes: H1 = 3.32 · 10−1 + 7.54 · 10−3 M–4.54 · 10−6 M2. The coefficient of determination (R2) is 0.99. With this estimated coefficient, the values of surface tension are calculated giving variable results. The results using the generalization of H1 in terms of the molar mass can be observed in Table 9. As seen in this table, during the correlation procedure the average absolute relative deviation is about 9.05% (maximum 48.60%), 35.08% of the data (635 of the 1810 data) presents deviations above 10%, while 3.09% (56 of the 1810 data) gives deviations above 30%. Also, during the testing process, the average absolute relative deviation is about 8.49% (maximum 48.04%), while for 28.24% of the data (61 of the 216 data) deviations are above 10% and for 2.31% (5 of the 216 data) deviations are above 30%. For most of the cases, deviations are below 10%.
Case 3: H1 using one σ value
Since the model has only one adjustable parameter, this value could be estimated using a single experimental value of the surface tension, say σ = σo at T = To and P = Po. Table 6, row 4, shows the equation to estimate H1, obtained by conveniently arranging the model. This equation required the critical properties (Tc, Pc, and Zc) and the reference temperature and pressure (T0 = 298.15 K and P0 = 1 bar, for instance). In the supplementary material, the authors provide the complete table for all the data evaluated in this research. Table 7 shows the average deviations (relative, absolute, and maximum values) for a set of selected ionic liquids, while Table 9 shows an overall picture of all the cases considered in the analysis. In this case, for 130 ionic liquids (2026 data), the average absolute relative deviation is 4.64% (maximum 38.76%). Also, 10.91% of the data (221 of the 2026 data) presents deviations higher than 10% and for only six data points of the 2026 total data (0.30%) deviations are higher than 30%. A graphical picture clarifies these numbers and allows getting a better understanding of the accuracy and magnitude of deviations of the proposed model. Figure 5 presents the calculated surface tension plotted against experimental values. The coefficient of determination (R2) is around 0.91.
Table 9 provides a good summary of all these cases for each of the calculations. Average deviations, maximum deviations, and some other statistical parameters are listed for the three cases. As observed in the table, results are as expected, in the sense that the more we generalize the model, the higher are deviations with respect to experimental data. What is important, however, is that even the generalized model proposed in this work provides values of acceptable accuracy for primary estimations, when no other data are available. Also, important are how these cases work when the model is extended to mixtures, as discussed in what follows.
Extension to mixtures
The extension of a model to calculate properties of mixtures is a severe test for a model, especially if no mixture data are used for calculating the surface tension for the mixture, as done in this work. In many applications of equations of state, binary interaction parameters are introduced into de mixing rules to obtain more accurate results. However, in this work no interaction parameters are used, so the calculations for mixtures are totally predictive, and pure component properties only are used to predict the surface tension of mixtures.
The presentation of results for the case of mixtures has been divided into two groups: (i) results for binary mixtures and (ii) results for ternary mixtures. In both cases, and similar to the presentation of results for pure substances, results have been classified into three types according to the form in which the only pure component parameter of the model (H1) was determined: (i) H1 by regression, (ii) H1 using M, and (iii) H1 using one σ value.
Results for binary mixtures
Table 10 presents detailed deviations for the binary mixtures considered in this work for the three cases, while an overall picture of deviations is presented in Table 11. As it can be observed in these tables, the predicting model with optimum H1 gives average absolute relative deviation around 6.46% and 23.27% of the data (619 of 2660 data) presents deviations higher than 10%. Also, for only 1.05% of the data (28 of 2660 data) deviations are higher than 30%. There are three binary mixtures (3 of 90 mixtures) whose deviation is around 20% and are composed of [C4mim][BF4] + ethanol, [C1mim][MSO4] + methanol and [mpy][BF4] + ethanol. According to the authors who provide the experimental data [22, 74], the modeling of this system produced errors that vary between 1.52% and 18.72%, similar to those obtained in this work. However, if one interaction parameter is estimated the deviation gets down around 7.06%, similar to the mean deviations of the other mixtures. In general, these results are surprisingly accurate for an equation of state model that does not use any mixture interaction parameter.
Figure 6 shows a graphical view of these results for selected binary mixtures. For a model of this type, the figures show that correlation between experimental data and correlated results is acceptable. Of course, that there are some few outliers, but those do not alter the general tendency presented in Fig. 7. More than 80% of the results are predicted with deviations lower than 10%.
Results for ternary liquid mixtures
A final and still more severe test for a general model as the one proposed here is its extension to predict the surface tension of ternary mixtures, without using any interaction parameters, nor employing binary mixture information; just pure component information. Table 12 presents detailed deviations for the ternary mixtures considered in this work for the three cases, while an overall picture of deviations is presented in Table 13. As observed in the tables, the average absolute deviations are similar for the three same cases studied, separated according to the form in which the pure component parameter H1 was calculated: (i) by regression, (ii) by using one experimental data of σ for each component in the mixture (H1 using one σ value), or (iii) according to the general equation in terms of the molar mass (H1 using M). Average absolute relative deviation is below 12% for most cases, while the average mean relative deviations are similar and below 3.93%. Only two of the 12 mixtures analyzed in this work give higher deviations: 2-propanol + water + [C2mim][DEP] and 1-propanol + water + [C2mim][DEP] (data taken from NIST-ILs [51, 52] from Ref. [75]). We could not find any explanation from the modeling point of view. It could happen that interactions are different between the three components in the mixture, and for better modeling, interaction parameters may be needed. If one adjustable parameter is included, the deviations are similar to the other ternary mixtures (around 13%), so an interaction parameter may be appropriate for mixtures in which deviations are not too high and more accurate results are needed.
Final discussion
Comparison of the results of the proposed model and others presented in the literature is needed to properly evaluate the goodness and usefulness of a model. The characteristics and results of several models reported in the open literature are presented in Table 14. All the models are of empirical nature [19, 76,77,78,79,80,81,82,83,84], and few studies are done of models with several ionic liquids and reasonable amounts of data [19]. In Table 14, the second column indicates the model and their characteristics (mathematical model, number of adjustable parameters). Also, the third column shows the temperature ranges of the data used. Column 5 shows literature results, column 6 results using H1 by regression, column 7 results using H1 using one σ value, and column 8 results with H1 using M. Also, in this table, models 1 to 4 are for pure ionic liquid, models 6 to 8 are for binary mixtures, and model 9 is for ternary mixtures. Most of these models require between 1 to 5 adjustable parameters per mixture besides the experimental values of pure substances and other transport property estimations (such as viscosity). Another characteristic of most literature models is that they cannot be applied for temperatures other than those used in developing the model.
For pure ionic liquids Table 14 shows that the model proposed in this paper provides the lowest deviations in comparison to other molecular theories (model 1) or group contribution method (model 2). Also, the empirical models 3 and 4 of Table 14 provide lower deviations than the proposed model, situation that can be explained by these two facts: (i) these literature models require other properties such as viscosity and melting point and (ii) these models are applied for imidazolium-type ILs only. Our general model can be used for any type of ILs and organic substances and does not need any other property.
When the extension to mixtures is done with the model proposed in this work, deviations increase as it can be expected from a predictive model. For instance, model 5 in Table 14, which does not use any interaction parameter, gives an average absolute relative deviation of about 19% using an empirical mixing rule that depends on the pure components, while the present model deviations are below 10%. On the other hand, model 6 of Table 14 produces deviations that are of the same order as the present model. However, model 6 requires the molar volume as an additional input parameter. As mentioned above, the model developed in this work does not require other physical and transport property estimation and also does not require any interaction parameter for mixtures. Model 7 of Table 14 is based on Connors and Wright’s polynomial equation with two adjustable parameters giving deviations of around 2.22% in comparison to the present model, which produces deviations below 1.58%. The phenomenological equation presented as model 8 in Table 14 employs four adjustable parameters per mixture producing an average absolute relative deviation of around 0.24%. Certainly, this deviation is lower than that obtained in this work, in which no interaction parameters are included. Finally, model 9 corresponds to a model for ternary mixtures in which five parameters are included. Obviously, the deviation of this five-parameter model (from 1 to 2%) is lower than 5.01% given by the predictive model proposed in this work, which is also acceptable for a totally predictive model.
To the best of the author’s knowledge, there are no models in the literature that can be extended to mixtures in the simple way presented in this work, that can give such low deviations for mixtures using pure component data only, that can be applied to different types of mixtures, and that cover wide ranges of temperature. These results verify the hypothesis stated at the beginning of this work.
Conclusions
A generalized empirical model with only one adjustable parameter, determined from pure substance data, is proposed for calculating the surface tension of pure ionic liquids and for predicting the surface tension of binary and ternary mixtures containing ionic liquids. Based on the study, the following main conclusion may be drawn: (i) the model, based on the geometric similitude concept, can correlate surface tension data and can predict the surface tension of pure substances with acceptable accuracy; (ii) the model can be extended to predict surface tension of mixtures containing ionic liquids using simple mixing rules; (iii) the extension to mixtures shows the predictive capabilities of the model, since values are determined using pure component data only; and (iv) in all cases using a pure component parameter H1 calculated from a set of experimental data provides the best results. Deviations increase a little if a value of the parameter H1 is obtained from one experimental data, and higher deviations are found using the generalized model for H1.
Abbreviations
- EoS:
-
Equation of Statqe
- Eq.:
-
Equation
- Fig.:
-
Figure
- IL:
-
Ionic liquid
- MS:
-
Microsoft
- NIST-ILs:
-
National Institute of Standards and Technology - Ionic Liquids Database
- VPT:
-
Valderrama-Patel-Teja
- α :
-
Temperature function defined in Table 1
- %Δ :
-
Percent deviation
- ρ:
-
Mass density (g/cm3)
- σ:
-
Surface tension (N/m)
- Ø, β, γ :
-
Constant for all substances described in Table 1
- cal:
-
Calculated
- c:
-
Critical property
- exp:
-
Experimental
- i, j :
-
Components i and j
- L:
-
Liquid phase
- m:
-
Mixture
- max:
-
Maximum value
- mim:
-
Minimum value
References
Zheng M, Tian J, Mulero Á (2013) New correlations between viscosity and surface tension for saturated normal fluids. Fluid Phase Equilib 360:298–304. https://doi.org/10.1016/j.fluid.2013.09.045
Tariq M, Freire MG, Saramago B, Coutinho JA, Lopes JNC, Rebelo LPN (2012) Surface tension of ionic liquids and ionic liquid solutions. Chem Soc Rev 41:829–868. https://doi.org/10.1039/C1CS15146K
Minea AA, Murshed SS (2018) A review on development of ionic liquid based nanofluids and their heat transfer behavior. Renew Sust Energ Rev 91:584–599. https://doi.org/10.1016/j.rser.2018.04.021
Yan X, Anguille S, Bendahan M, Moulin P (2019) Ionic liquids combined with membrane separation processes: a review. Sep Purif Technol 222:230–253. https://doi.org/10.1016/j.seppur.2019.03.103
Wang X, Chi Y, Mu T (2014) A review on the transport properties of ionic liquids. J Mol Liq 193:262–266. https://doi.org/10.1016/j.molliq.2014.03.011
Valderrama JO, Cardona LF, Rojas RE (2019) Correlation and prediction of ionic liquid viscosity using Valderrama-Patel-Teja cubic equation of state and the geometric similitude concept. Part I: pure ionic liquids. Fluid Phase Equilib 497:164–177. https://doi.org/10.1016/j.fluid.2019.04.031
Cardona LF, Valderrama JO (2020) Physical and transport properties of ionic liquids using geometric similitude and a cubic equation of state. Part 2: thermal conductivity, and speed of sound of water+ ionic liquid mixtures. J Mol Liq 317:113926. https://doi.org/10.1016/j.molliq.2020.113926
Plechkova NV, Seddon KR (2008) Applications of ionic liquids in the chemical industry. Chem Soc Rev 37:123–150. https://doi.org/10.1039/B006677J
Roosta A, Sadeghi B (2016) Surface tension estimation of binary mixtures of organic compounds using artificial neural networks. Chem Eng Commun 203:1349–1358. https://doi.org/10.1080/00986445.2016.1194273
Baidakov VG, Khotienkova MN (2016) Surface tension of methane–nitrogen solutions: 2. Description in the framework of the van der Waals gradient theory. Fluid Phase Equilib 425:402–410. https://doi.org/10.1016/j.fluid.2016.06.038
Shang Q, Yan F, Xia S, Wang Q, Ma P (2013) Predicting the surface tensions of ionic liquids by the quantitative structure property relationship method using a topological index. Chem Eng Sci 101:266–270. https://doi.org/10.1016/j.ces.2013.05.053
Mirkhani SA, Gharagheizi F, Farahani N, Tumba K (2013) Prediction of surface tension of ionic liquids by molecular approach. J Mol Liq 179:78–87. https://doi.org/10.1016/j.molliq.2012.11.018
Deetlefs M, Seddon KR, Shara M (2006) Predicting physical properties of ionic liquids. Phys Chem Chem Phys 8:642–649. https://doi.org/10.1039/B513453F
Shahsavari S, Mesbah M, Soroush E, Farhangian H, Alizadeh S, Soltanali S (2018) A simple group contribution correlation for modeling the surface tension of pure ionic liquids. J Mol Liq 265:292–298. https://doi.org/10.1016/j.molliq.2018.06.004
Gharagheizi F, Ilani-Kashkouli P, Mohammadi AH (2012) Group contribution model for estimation of surface tension of ionic liquids. Chem Eng Sci 78:204–208. https://doi.org/10.1016/j.ces.2012.06.052
Atashrouz S, Mirshekar H (2014) Phase equilibrium modeling for binary systems containing CO2 using artificial neural networks. Bulg Chem Commun 46:104–116
Ojaki HA, Lashkarbolook M, Movagharnejad K (2020) Correlation and prediction of surface tension of highly non-ideal hydrous binary mixtures using artificial neural network. Colloids Surf A Physicochem Eng Asp 590:124474. https://doi.org/10.1016/j.colsurfa.2020.124474
Poling BE, Prausnitz JM, O’Connell JP (2000) The properties of gases and liquids, 5th edn. McGraw-Hill, New York
Gardas RL, Coutinho JA (2008) Applying a QSPR correlation to the prediction of surface tensions of ionic liquids. Fluid Phase Equilib 265:57–65. https://doi.org/10.1016/j.fluid.2008.01.002
Koller TM, Steininger C, Rausch MH, Fröba AP (2017) A simple prediction method for the surface tension of ionic liquids as a function of temperature. Int J Thermophys 38:167. https://doi.org/10.1007/s10765-017-2301-z
Esmaeili H, Hashemipour H (2018) A simple correlation for determining ionic liquids surface tension. J Mol Liq 272:692–696. https://doi.org/10.1016/j.molliq.2018.10.011
Rilo E, Pico J, García-Garabal S, Varela LM, Cabeza O (2009) Density and surface tension in binary mixtures of CnMIM-BF4 ionic liquids with water and ethanol. Fluid Phase Equilib 285:83–89. https://doi.org/10.1016/j.fluid.2009.07.010
Varela LM, Carrete J, Turmine M, Rilo E, Cabeza O (2009) Pseudolattice theory of the surface tension of ionic liquid− water mixtures. J Phys Chem B 113:12500–12505. https://doi.org/10.1021/jp9057065
Matsushita Y, Yasuda S (2003) Reactivity of a condensed–type lignin model compound in the Mannich reaction and preparation of cationic surfactant from sulfuric acid lignin. J Wood Sci 49:166–171. https://doi.org/10.1007/s100860300026
Oliveira MB, Domínguez-Pérez M, Freire MG, Llovell F, Cabeza O, Lopes-da-Silva JA, Vega LF, Coutinho JA (2012) Surface tension of binary mixtures of 1-alkyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide ionic liquids: experimental measurements and soft-SAFT modeling. J Phys Chem B 116:12133–12141. https://doi.org/10.1021/jp3059905
Oliveira MB, Dominguez-Perez M, Cabeza O, Lopes-da-Silva JA, Freire MG, Coutinho JA (2013) Surface tensions of binary mixtures of ionic liquids with bis (trifluoromethylsulfonyl) imide as the common anion. J Chem Thermodyn 64:22–27. https://doi.org/10.1016/j.jct.2013.04.014
Shojaeian A, Asadizadeh M (2020) Prediction of surface tension of the binary mixtures containing ionic liquid using heuristic approaches; an input parameters investigation. J Mol Liq 298:111976. https://doi.org/10.1016/j.molliq.2019.111976
Phillips P (1912) The viscosity of carbon dioxide. Proc R Soc 87A:48–52. https://doi.org/10.1098/rspa.1912.0058
Khosharay S, Pierantozzi M, Di Nicola G (2018) Modeling investigation on the viscosity of pure refrigerants and their liquid mixtures by using the Patel–Teja viscosity equation of state. Int J Refrig 85:255–267. https://doi.org/10.1016/j.ijrefrig.2017.10.004
Bonyadi M, Rostami M (2017) A new viscosity model based on Soave-Redlich-Kwong equation of state. Fluid Phase Equilib 451:40–47. https://doi.org/10.1016/j.fluid.2017.07.009
Cardona LF, Forero LA, Velásquez JA (2019) Correlation and prediction of thermal conductivity using the Redlich–Kwong cubic equation of state and the geometric similitude concept for pure substances and mixtures. Ind Eng Chem Res 58:23417–23437. https://doi.org/10.1021/acs.iecr.9b04974
Cardona LF, Forero LA, Velásquez JA (2019) Modelamiento de la Viscosidad con Base en una Ecuación Cúbica μTP del Tipo Peng-Robinson. Inf Tecnol 30:259–272. https://doi.org/10.4067/S0718-07642019000400259
Ye CW, Li J (2012) Density, viscosity, and surface tension of n-octanol-phosphoric acid solutions in a temperature range 293.15–333.15 K. Russ J Phys Chem A 86:1515–1521. https://doi.org/10.1134/S0036024412100263
Castro MC, Arce A, Soto A, Rodríguez H (2016) Thermophysical characterization of the mixtures of the ionic liquid 1-ethyl-3-methylimidazolium acetate with 1-propanol or 2-propanol. J Chem Eng Data 61:2299–2310. https://doi.org/10.1021/acs.jced.5b01023
Luning Prak DJ, Alexandre SM, Cowart JS, Trulove PC (2014) Density, viscosity, speed of sound, bulk modulus, surface tension, and flash point of binary mixtures of n-dodecane with 2, 2, 4, 6, 6-pentamethylheptane or 2, 2, 4, 4, 6, 8, 8-heptamethylnonane. J Chem Eng Data 59:1334–1346. https://doi.org/10.1021/je5000132
Jasper JJ, Kring EV (1955) The isobaric surface tensions and thermodynamic properties of the surfaces of a series of n-alkanes, C5 to C18, 1-alkenes, C6 to C16, and of n-decylcyclopentane, n-decylcyclohexane and n-dcylbenzene. J Phys Chem 59:1019–1021. https://doi.org/10.1021/j150532a006
Ahosseini A, Sensenich B, Weatherley LR, Scurto AM (2010) Phase equilibrium, volumetric, and interfacial properties of the ionic liquid, 1-hexyl-3-methylimidazolium bis (trifluoromethylsulfonyl) amide and 1-octene. J Chem Eng Data 55:1611–1617. https://doi.org/10.1021/je900697w
Luning Prak DJ, Cowart JS, Trulove PC (2014) Density, viscosity, speed of sound, bulk modulus, and surface tension of binary mixtures of n-heptane+ 2, 2, 4-trimethylpentane at (293.15 to 338.15) K and 0.1 MPa. J Chem Eng Data 59:3842–3851. https://doi.org/10.1021/je5007532
Liu QS, Tong J, Tan ZC, Welz-Biermann U, Yang JZ (2010) Density and surface tension of ionic liquid [C2mim][PF3 (CF2CF3) 3] and prediction of properties [Cnmim][PF3 (CF2CF3) 3](n= 1, 3, 4, 5, 6). J Chem Eng Data 55:2586–2589. https://doi.org/10.1021/je901035d
Liu QS, Yang M, Yan PF, Liu XM, Tan ZC, Welz-Biermann U (2010) Density and surface tension of ionic liquids [Cnpy][NTf2](n= 2, 4, 5). J Chem Eng Data 55:4928–4930. https://doi.org/10.1021/je100507n
Bandrés I, Royo FM, Gascón I, Castro M, Lafuente C (2010) Anion influence on thermophysical properties of ionic liquids: 1-butylpyridinium tetrafluoroborate and 1-butylpyridinium triflate. J Phys Chem B 114:3601–3607. https://doi.org/10.1021/jp9120707
Jiang H, Zhao Y, Wang J, Zhao F, Liu R, Hu Y (2013) Density and surface tension of pure ionic liquid 1-butyl-3-methyl-imidazolium L-lactate and its binary mixture with alcohol and water. J Chem Thermodyn 64:1–13. https://doi.org/10.1016/j.jct.2013.04.015
Khattab IS, Bandarkar F, Fakhree MAA, Jouyban A (2012) Density, viscosity, and surface tension of water+ ethanol mixtures from 293 to 323K. Korean J Chem Eng 29:812–817. https://doi.org/10.1007/s11814-011-0239-6
Valderrama JO, Faúndez CA, Campusano R (2019) An overview of a thermodynamic consistency test of phase equilibrium data. Application of the versatile VPT equation of state to check data of mixtures containing a gas solute and an ionic liquid solvent. J Chem Thermodyn 131:122–132. https://doi.org/10.1016/j.jct.2018.09.019
Valderrama JO (1990) A generalized Patel-Teja equation of state for polar and nonpolar fluids and their mixtures. J Chem Eng Jpn 23:87–91. https://doi.org/10.1252/jcej.23.87
Danesh A, Xu DH, Todd AC (1991) Comparative study of cubic equations of state for predicting phase behaviour and volumetric properties of injection gas-reservoir oil systems. Fluid Phase Equilib 63:259–278. https://doi.org/10.1016/0378-3812(91)80036-U
Danesh A (1998) PVT and phase behaviour of petroleum reservoir fluids. Elsevier, Amsterdam
Heyen, G. (1980) A cubic equation of state with extended range of application, in: Proceedings of the 2nd World Congress of Chemical Engineering, Frankfurt
Valderrama JO (2003) The state of the cubic equations of state. Ind Eng Chem Res 42:1603–1618. https://doi.org/10.1021/ie020447b
Cardano G (1968) Ars magna or the rules of algebra. Dover Publications, New York
Kazakov A, Magee JW, Chirico RD, Paulechka E, Diky V, Muzny CD, Kroenlein K, Frenkel M, NIST Standard Reference Database 147: NIST Ionic Liquids Database (ILThermo), Version 2.0, National Institute of Standards and Technology, Gaithersburg MD, 20899, http://ilthermo.boulder.nist.gov.
Dong Q, Muzny CD, Kazakov A, Diky V, Magee JW, Widegren JA, Chirico RD, Marsh KN, Frenkel M (2007) ILThermo: a free-access web database for thermodynamic properties of ionic liquids. J Chem Eng Data 52:1151–1159. https://doi.org/10.1021/je700171f
Valderrama JO, Forero LA, Rojas RE Extension of a group contribution method to estimate the critical properties of ionic liquids of high molecular mass. Ind Eng Chem Res 54:3480–3487. https://doi.org/10.1021/acs.iecr.5b00260
Maia FM, Calvar N, González EJ, Carneiro AP, Rodriguez O, Macedo EA (2013) Modeling of ionic liquid systems: phase equilibria and physical properties. In: Ionic liquids—new aspects for the future InTech. https://doi.org/10.5772/51812
Antón V, Artigas H, Muñoz-Embid J, Artal M, Lafuente C (2016) Thermophysical properties of two binary aqueous mixtures containing a pyridinium-based ionic liquid. J Chem Thermodyn 99:116–123. https://doi.org/10.1016/j.jct.2016.04.003
Zdziennicka A, Szymczyk K, Krawczyk J, Jańczuk B (2012) Activity and thermodynamic parameters of some surfactants adsorption at the water–air interface. Fluid Phase Equilib 318:25–33. https://doi.org/10.1016/j.fluid.2012.01.014
Enders S, Kahl H, Winkelmann J (2007) Surface tension of the ternary system water+ acetone+ toluene. J Chem Eng Data 52:1072–1079. https://doi.org/10.1021/je7000182
Gonçalves FAMM, Trindade AR, Costa CSMF, Bernardo JCS, Johnson I, Fonseca IMA, Ferreira AGM (2010) PVT, viscosity, and surface tension of ethanol: new measurements and literature data evaluation. J Chem Thermodyn 42:1039–1049. https://doi.org/10.1016/j.jct.2010.03.022
Muhammad A, Mutalib MIA, Wilfred CD, Murugesan T, Shafeeq A (2008) Viscosity, refractive index, surface tension, and thermal decomposition of aqueous N-methyldiethanolamine solutions from (298.15 to 338.15) K. J Chem Eng Data 53:2226–2229. https://doi.org/10.1021/je800282a
Vazquez G, Alvarez E, Navaza JM (1995) Surface tension of alcohol water+ water from 20 to 50°C. J Chem Eng Data 40:611–614. https://doi.org/10.1021/je00019a016
Kao YC, Tu CH (2011) Densities, viscosities, refractive indices, and surface tensions for binary and ternary mixtures of 2-propanol, tetrahydropyran, and 2, 2, 4-trimethylpentane. J Chem Thermodyn 43:216–226. https://doi.org/10.1016/j.jct.2010.08.019
Vázquez G, Alvarez E, Rendo R, Romero E, Navaza JM (1996) Surface tension of aqueous solutions of diethanolamine and triethanolamine from 25° C to 50° C. J Chem Eng Data 41:806–808. https://doi.org/10.1021/je960012t
Santos BMS, Ferreira AGM, Fonseca IMA (2003) Surface and interfacial tensions of the systems water+ n-butyl acetate+ methanol and water+ n-pentyl acetate+ methanol at 303.15 K. Fluid Phase Equilib 208:1–21. https://doi.org/10.1016/S0378-3812(02)00320-5
Wandschneider A, Lehmann JK, Heintz A (2008) Surface tension and density of pure ionic liquids and some binary mixtures with 1-propanol and 1-butanol. J Chem Eng Data 53:596–599. https://doi.org/10.1021/je700621d
Bonnet JC, Pike FP (1972) Surface properties of nine liquids. J Chem Eng Data 17:145–150
Vogel AI (1948) 366. Physical properties and chemical constitution. Part XX. Aliphatic alcohols and acids. Journal of the chemical society (Resumed) 1814-1819
Kinart CM, Kinart WJ, Bald A (1999) The measurements of the surface tension of mixtures of dimethyl sulfoxide with methyl, ethyl and propyl alcohols. Phys Chem Liq 37:317–321. https://doi.org/10.1080/00319109908031436
Geppert-Rybczyńska M, Lehmann JK, Heintz A (2014) Physicochemical properties of two 1-alkyl-1-methylpyrrolidinium bis [(trifluoromethyl) sulfonyl] imide ionic liquids and of binary mixtures of 1-butyl-1-methylpyrrolidinium bis [(trifluoromethyl) sulfonyl] imide with methanol or acetonitrile. J Chem Thermodyn 71:171–181. https://doi.org/10.1016/j.jct.2013.12.009
Daubert TE (1998) Evaluated equation forms for correlating thermodynamic and transport properties with temperature. Ind Eng Chem Res 37:3260–3267. https://doi.org/10.1021/ie9708687
Lasdon LS, Waren AD, Jain A, Ratner M (1978) Design and testing of a generalized reduced gradient code for nonlinear programming. ACM Trans Math Softw 4:34–50
Raubenheimer J (2017) Excellence in data visualization?: the use of Microsoft Excel for data visualization and the analysis of big data. In: Theodosia P (ed) Data visualization and statistical literacy for open and big data. IGI Global, Pennsylvania
El-Awad MM (2015) A multi-substance add-in for the analyses of thermo-fluid systems using Microsoft excel. J Eng Appl Sci 2:63–69
Valderrama JO, Alvarez VH (2005) Correct way of reporting results when modeling supercritical phase equilibria using equations of state. Can J Chem Eng 83:578–581. https://doi.org/10.1002/cjce.5450830323
García-Mardones M, Cea P, Gascón I, Lafuente C (2014) Thermodynamic study of the surface of liquid mixtures containing pyridinium-based ionic liquids and alkanols. J Chem Thermodyn 78:234–240. https://doi.org/10.1016/j.jct.2014.07.001
Wan Normazlan WMD, Sairi NA, Alias Y, Udaiyappan AF, Jouyban A, Khoubnasabjafari M (2014) Composition and temperature dependence of density, surface tension, and viscosity of EMIM DEP/MMIM DMP+ water+ 1-propanol/2-propanol ternary mixtures and their mathematical representation using the Jouyban–acree model. J Chem Eng Data 59:2337–2348. https://doi.org/10.1021/je400576e
Lemraski EG, Zobeydi R (2014) Applying parachor method to the prediction of ionic liquids surface tension based on modified group contribution. J Mol Liq 193:204–209. https://doi.org/10.1016/j.molliq.2013.12.043
Mousazadeh MH, Faramarzi E (2011) Corresponding states theory for the prediction of surface tension of ionic liquids. Ionics. 17:217–222. https://doi.org/10.1007/s11581-011-0536-4
Akbari F, Alavianmehr MM, Ardakani RB, Mohammad-Aghaie D (2018) Thermophysical properties of ionic liquids and their mixtures from a new equation of state. Ionics. 24:1357–1369. https://doi.org/10.1007/s11581-017-2310-8
Ren NN, Gong YH, Lu YZ, Meng H, Li CX (2014) Surface tension measurements for seven imidazolium-based dialkylphosphate ionic liquids and their binary mixtures with water (methanol or ethanol) at 298.15 K and 1 atm. J Chem Eng Data 59:189–196. https://doi.org/10.1021/je400004j
Hong M, Sun A, Liu C, Guan W, Tong J, Yang JZ (2013) Physico-chemical properties of 1-alkyl-3-methylimidazolium propionate ionic liquids {[C n mim][pro](n= 3, 4, 5, 6)} from 288.15 K to 328.15 K. Ind Eng Chem Res 52:15679–15683. https://doi.org/10.1021/ie402890r
Connors KA, Wright JL (1989) Dependence of surface tension on composition of binary aqueous-organic solutions. Anal Chem 61:194–198. https://doi.org/10.1021/ac00178a001
Geppert-Rybczyńska M, Lehmann JK, Safarov J, Heintz A (2013) Thermodynamic surface properties of [BMIm][NTf2] or [EMIm][NTf2] binary mixtures with tetrahydrofuran, acetonitrile or dimethylsulfoxide. J Chem Thermodyn 62:104–110. https://doi.org/10.1016/j.jct.2013.02.021
Rilo E, Domínguez-Pérez M, Vila J, Varela LM, Cabeza O (2012) Surface tension of four binary systems containing (1-ethyl-3-methyl imidazolium alkyl sulphate ionic liquid+ water or+ ethanol). J Chem Thermodyn 49:165–171. https://doi.org/10.1016/j.jct.2012.01.023
Ghani NA, Sairi NA, Aroua MK, Alias Y, Yusoff R (2014) Density, surface tension, and viscosity of ionic liquids (1-ethyl-3-methylimidazolium diethylphosphate and 1,3-dimethylimidazolium dimethylphosphate) aqueous ternary mixtures with MDEA. J Chem Eng Data 59:1737–1746. https://doi.org/10.1021/je400562z
Acknowledgments
The authors thank their respective institutions for permanent support. In particular, JOV is grateful for the support of the National Agency for Research and Development (ANID), formerly known as National Commission for Scientific and Technological Research (CONICYT. Chile) through the Grant Proyecto Anillo ACM 170005; LFC thanks the Ministry of Science, Technology, and Innovation in Colombia formerly known as Administrative Department of Science Technology and Innovation (Colciencias-Colombia), for the support through the program National Doctorates 727 of 2015.
Funding
This study did not have any direct funding
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cardona, L.F., Valderrama, J.O. Surface tension of mixtures containing ionic liquids based on an equation of state and on the geometric similitude concept. Ionics 26, 6095–6118 (2020). https://doi.org/10.1007/s11581-020-03697-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03697-0