Abstract
The Neotropics are among the least explored regions from a mycological perspective. A few recent molecular studies in South America have shown high fungal diversity as well as numerous groups of mostly undescribed taxa. Through soil metabarcoding analysis we compared richness and species composition among macrofungal communities, belonging to Agaricales, Russulales, Boletales and Phallomycetidae groups, in three elevational forests types in the subtropical Yungas of Northwestern Argentina (Piedmont forest; Montane forest, Montane cloud forest). The aims of this study were to assess richness of taxonomic and functional groups along the elevation gradient and to assess the relationships between environmental variables and species composition in the studied fungal communities. The results have shown rich Agaricomycetes communities, diversely structured among forests habitats. The elevation gradient differentially affected the richness and distribution of Agaricales, Russulales, Boletales and Phallomycetidae. Based on fungal trophic modes and guilds, the gradient also affected the ectomycorrhizal taxa distribution. When considering the basidiomata growth forms (agaricoid, boletoid, gasteroid, etc.), only the secotioid type showed significant elevational differences. Additional analyses indicated that saprotrophic nutritional mode was dominant along the entire gradient, being partially replaced by biotrophic modes at higher elevations. Fungal communities in the Montane cloud forests are most dissimilar when compared with communities at the Piedmont forest and Montane forest, which is consistent with the different biogeographic origins of these forests. DNA metabarcoding sequence analysis provided detailed information on the diversity and taxonomic and functional composition of macrofungal communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungi represent one of the largest groups of living organisms with approximately 100,000 described species (Blackwell 2011). Recent estimates suggest there may be between 0.8 and 5.1 million fungal species (Blackwell 2011; Schmit and Mueller 2007), which emphasizes the need to improve our knowledge about fungal diversity. In particular, tropical and subtropical ecosystems are comparatively under sampled in relation to temperate forests and are predicted to be the most diverse in soil-inhabiting fungal species (O’Brien et al. 2005; Taylor et al. 2014; Tedersoo et al. 2014). Recently, various studies have been conducted in order to characterize the diversity and structure of fungal communities as well as their functional roles in tropical and subtropical forests (Lodge 1997; Lodge and Cantrell 1995a, b; Meier et al. 2010; Piepenbring et al. 2012; Rosa and Capelari 2009; Schmit and Mueller 2007; Swapna et al. 2008; Young et al. 2002). Moreover, many authors have unveiled that various biotic and abiotic environmental factors such as plant identity and soil composition, precipitation and elevation differentially affect the distribution of fungal groups in tropical systems and other biomes (Bahram et al. 2012; Geml 2017; Geml et al. 2014, 2017; Gómez-Hernández and Williams-Linera 2011; Gómez-Hernández et al. 2012; Masayuki et al. 2008; McGuire et al. 2012; Peay et al. 2013; Swapna et al. 2008; Tedersoo et al. 2014). Recent mycological studies in South American tropical forests have revealed high fungal diversity and numerous groups of mostly undescribed taxa (Geml et al. 2014; Henkel et al. 2012; López-Quintero et al. 2012; Peay et al. 2013).
Fungi play essential roles in ecosystems due to their nutritional modes as parasites, symbionts and saprotrophs, comprising various taxa that evolved convergently as specialists in the exploitation of various substrates and/or symbiotic partners (Mueller et al. 2007; Oberwinkler 1994). These nutritional modes and the ecosystem function of the target fungal taxa can be used to describe functional groups of fungi (Oberwinkler 1994), but other groups of organisms, e.g. guilds of plants and insects have been also classified based on their roles in ecosystem functioning (Gitay and Noble 1997; Lavorel and Garnier 2001). Recent studies have depicted the robustness of a trait-based approach as a useful framework to describe some of aspects of fungal ecology, and traits that can be related to major ecosystem processes, such as resource acquirement, soil aggregation, host growth, decomposition, etc. (Aguilar-Trigueros et al. 2015; Boddy 2001; Chase and Leibold 2003; Mitchell 2003; Rillig et al. 2014). Also, it has been showed that environmental variables related to elevation, and the responses of certain fungal functional groups may be structuring communities of fungi along elevation gradients (Bahram et al. 2012; Coince et al. 2014; Geml 2017; Looby et al. 2016).
This work focused on the macrofungal (i.e., fungi with macroscopic reproductive structures) communities in the Tucuman-Bolivian montane forests (hereafter, Yungas), situated on the eastern slopes of the Andes. The Yungas are floristically distinct from the tropical northern Andean forests (Brown et al. 2001) and, together with the adjacent seasonally dry piedmont forests, constitute the southern limit of the Amazonian biogeographic domain (Cabrera 1976; Prado 2000). The flora and fauna of the region have been relatively well studied and are considered very diverse and rich in endemics (Blake and Rougés 1997; Hueck 1978; Lavilla and Manzano 1995; Legname 1982; Meyer 1963; Ojeda and Mares 1989). Unfortunately, the Yungas and especially the piedmont forests have been severely affected by agriculture, losing over 90% of the original area, and are being replaced by sugar cane and transgenic cultivars of soybean (Malizia et al. 2012). In addition, extensive livestock farming and forest fires are important disturbance factors in this ecosystem (Brown et al. 2002; Grau and Brown 2000; Malizia et al. 2012; Pacheco et al. 2010). Deforestation has been found to influence litter decomposition, reducing or changing fungal diversity as well as the production of fruiting bodies of ligninolytic basidiomycetes (Ishikawa et al. 2007; Kinoshita and Fukuda 2004; Looby et al. 2016). It has been showed that forest soils with high C:N ratios may have a higher prevalence of basidiomycetes (i.e. white rot decomposers) than those crop soils with lower C:N ratios, thus shifting the overall distribution of fungi across these land use types (Lauber et al. 2008). In addition to anthropogenic disturbance, Pacheco et al. (2010) and Wicaksono et al. (2017) have recently predicted, based on modelled present and future distributions of tree species that suitable habitat area for piedmont and montane cloud forests will decrease due to climate change. Considering that fungal communities in subtropical ecosystems of Argentina are undersampled and their forests structure and distributions are rapidly changing through anthropogenic influences, it is important to characterize the fungal communities in the remaining preserved areas of the Yungas.
Since the 1900s, taxonomic studies on various groups of macrofungi have been done in the Yungas by Spegazzini (1912, 1919), Singer (1953), and Singer and Morello (1960). Most recently, additional studies have focused on the systematics and distribution of Xylariaceae (Hladki and Romero 2001, 2010), polypores (Robledo et al. 2003; Robledo and Rajchenberg 2007; Urcelay and Robledo 2004), and selected agaricoid groups (Baroni et al. 2012; Niveiro 2012; Niveiro et al. 2012, 2014a, b), thus increasing the number of known taxa. Further studies using molecular techniques have focused on the ectomycorrhizal fungi associated with Alnus acuminata in the montane cloud forests (Becerra et al. 2002, 2005a, b, 2007, 2009, 2011; Nouhra et al. 2005, 2015; Pritsch et al. 2010; Wicaksono et al. 2017). Despite these important contributions, we still know little about how richness and distribution of specific macrofungal groups are affected by environmental factors, many of which relate to elevation and respective vegetation types that are characteristic of the Yungas region.
We focused on macrofungal taxa in Agaricales, Russulales, Boletales and various highly diverse lineages within Phallomycetidae, with a diverse set of nutritional modes, such as saprotrophic, mycorrhizal, plant or animal parasitic, muscicolous and fungicolous (Kirk et al. 2008). These groups exhibit enormous morphological plasticity and ecological diversity and, due to their diversity and abundance, are among the most ecologically important fungi in forest ecosystems. The order Agaricales in itself includes more than half of all known species of the homobasidiomycetes (Hibbett et al. 1997; Hibbett and Thorn 2001), accounting for more than 13,000 species and 413 genera (Kirk et al. 2008). Russulales and Boletales are also characterized by a diverse array of species, however, these groups are less represented in ecosystems, roughly accounting for 1800 and 1300 species, respectively. The Phallomycetidae, which includes the orders Geastrales, Gomphalles, Hysterangiales and Phallales, are represented by around 600 species (Kirk et al. 2008), and new species are continuously being described in all orders. In addition, these macrofungi contain some of the most conspicuous and familiar types of mushrooms, and thus, can have prominent roles in fungal conservation efforts.
The Yungas represent a particularly interesting system to study these fungal groups. The three elevational forest types in the Yungas differ in their plant diversity and composition along the gradient. At the upper vegetation belt, the ectomycorrhizal Alnus acuminata forms extensive monodominant forests. These montane cloud forests exhibit lower productivity and slower nutrient cycling rates compared with the lowland tropics (Looby et al. 2016), and are usually characterized by cooler climate conditions, and particular edaphic characteristics, e.g., with soil pH decreasing and organic matter and N increasing with elevation (Geml et al. 2014). The mid- and low-elevation vegetation belts in the Yungas exhibit taller average canopy with highly diverse plant communities of tropical and subtropical species, warmer temperatures and higher soil pH values. They also present a dense shrub understory, as well as many lianas, climbers and epiphytes (Brown et al. 2001). Given that the diversity of plants and animals and, consequently, the variety of organic substrates are higher in the warmer lower elevations (Brown et al. 2001; Geml et al. 2014; Meier et al. 2010), taxonomic fungal groups possessing symbiotrophic, endophytic, wood and litter-decaying, and pathogenic taxa are expected to be differentially represented along of the elevational gradient in the Yungas.
In this study, we analyzed DNA metabarcoding data from multiple sites representing the three major forest types in the Yungas. Considering that the different habitats along the elevational gradient are susceptible to current variations of land use and climate change effects, the aims of this study were to (1) describe the soil macrofungal communities, and to (2) explore correlations among local environmental factors and richness and composition of macrofungi at taxonomic and functional levels.
Materials and methods
The study region
The 24 sampled sites are located within preserved forested areas in The Yungas UNESCO Biosphere Reserve (Lomáscolo et al. 2010), Parque Nacional Campo de los Alisos and Reserva Provincial La Florida. The sites are not affected by sugar cane and soybean monocultures, and, therefore, represent almost intact forest remnants along the entire latitudinal extent of the Yungas in Argentina (ca. 22.2–27.4°S) as well as the three different elevational forest types (Brown et al. 2001; Grau and Brown 2000; Prado 2000): the piedmont forest (400–700 m.a.s.l.), the montane forest (700–1500 m.a.s.l.), and the montane cloud forest (1500–3000 m.a.s.l.), hereafter named as PF, MF, and MCF, respectively. Detailed information on the elevational forest types as well as the localities and edaphic data on the sampling sites are provided in Geml et al. (2014). Sampling sites consisted of approximately 50 × 50 m plots situated at a distance of at least 500 m from each other within a forest type, with most sites being chosen to be at least 1 km from the nearest one, whenever possible.
Sampling and molecular work
Communities of terrestrial macrofungal Agaricomycetes in all elevational forest types in the Yungas were characterized and compared after re-analyzing soil DNA metabarcoding data generated by Geml et al. (2014), using an updated set of bioinformatic tools. The data generation process is described in detail in Geml et al. (2014), in the present study we only include a brief methodological description. At each sampling site, 40 soil cores (2 cm in diameter and ca. 20 cm deep) were taken and pooled, resulting in a composite soil sample for each site. Genomic DNA was extracted from 1 g of dry soil using a NucleoSpin® Soil kit (Macherey–Nagel GmbH & Co., Düren, Germany), according to the manufacturers protocol. For each sample, DNA extraction was carried out in duplicate and the extracts were combined. The remaining parts of the pooled soil samples were kept for soil chemical analyses that were carried out at the Laboratorio de Suelos y Aguas of the Facultad de Ciencias Agropecuarias (Universidad Nacional de Córdoba) following protocols described in Sparks et al. (1996). The ITS2 region (ca. 250 bp) of the nuclear ribosomal DNA repeat was PCR amplified and later sequenced using Ion Torrent as described in detail in Geml et al. (2014).
The raw sequence data (deposited in Dryad: https://doi.org/10.5061/dryad.8fn8j) contained 7,489,045 sequences with a modal read length of 287 bp. The primers were removed and poor quality ends were trimmed off based on 0.02 error probability limits in Geneious Pro 5.6.1 (BioMatters, New Zealand). Subsequently, sequences were filtered using MOTHUR v. 1.32.1 (Schloss et al. 2009) based on the following settings: no ambiguous bases (maxambig = 0), homopolymers no longer than 10 nucleotides (maxhomop = 10), and length range from 150 to 400 bp (minlength = 150; maxlength = 400), resulting in 4,760,162 quality-filtered sequences with an average read length of 272.4 ± 49.9 (mean ± SD). The remaining sequences were grouped into 9144 operational taxonomic units (OTUs) at 97% sequence similarity using USEARCH v.8.0 (Edgar 2010), while simultaneously removing putative chimeric OTUs. We assigned sequences to taxonomic groups based on pairwise similarity searches against the curated UNITE fungal ITS sequence database containing identified fungal sequences with assignments to Species Hypothesis groups using dynamic assignment based on periodically updated, phylogeny-based species delimitations (Kõljalg et al. 2013). After excluding OTUs with < 70% similarity or < 150 bp pairwise alignment length to a fungal sequence, 7634 fungal OTUs were retained, comprising a total of 1,416,245 high-quality sequences with an average of 59,010 reads per sample. Since all the OTUs in the Phallomycetidae (Geastrales, Gomphalles, Phallales and Hysterangiales) were identified as soil saprotrophs, and the majority represented by Geastrales, we decided to use the Phallomycetidae as a whole in the ecological analyses.
The trophic modes (pathotroph, saprotroph, symbiotroph), guilds (ectomycorrhizal, mycoparasite, plant pathogen, soil saprotroph, lignicolous saprotroph), and growth forms (agaricoid, boletoid, gasteroid, resupinate, hydnoid, clavarioid, etc.) were assigned to OTUs using FUNGuild (Nguyen et al. 2016). For OTUs that matched taxa with no information available in FUNGuild, the trophic modes/guilds and growth forms were assigned individually by retrieving functional data from UNITE and NCBI databases of the matching Species Hypotheses. Lepiota-like fungi that matched sequences from fungi cultivated in gardens of Neotropical leaf-cutting ants were separated from other saprotrophs in a new guild, because these ant symbionts represent a highly specialized functional group within saprotrophs.
Because the metabarcoding analyses was methodologically oriented to soil fungi, we excluded orders mostly dominated by wood-decay taxa (Auriculariales, Trechisporales, Hymenochaetales, Gloeophyllales, Polyporales, and Corticiales), that, by definition, are restricted to woody substrates (i.e. logs, tree trunks and branches of standing or fallen trees). Therefore, OTUs within the above mentioned orders obtained from our soil sampling likely represent spores or mycelial fragments fallen from woody substrates, and likely comprises only a fraction of their naturally occurring communities in the Yungas.
Statistical analyses
The observed number of OTUs (richness) and Chao 2 estimates (Chao et al. 2005) were calculated for each forest type using sample-based rarefaction in EstimateS 9.1.0 (Colwell et al. 2012) with 100 randomizations, without resampling and without shuffling data among the 8 replicate plots per vegetation type. These indices were tested across all sites using analysis of variance (ANOVA), with means compared with Tukey’s HSD test. We also compared species turnover within and between elevational forest types calculated by pairwise comparisons of communities in the corresponding sampling sites. The beta diversity measure was Sørensen similarity index that was calculated from presence–absence data.
Non-metric multidimensional scaling (NMDS) was applied with metaMDS() function of the ‘vegan’ package (Oksanen et al. 2012) in R 2.13.2 (R Development Core Team 2011). NMDS were performed on a presence/absence matrix of sites by OTUs. PerMANOVA analyses were conducted to determine if the fungal communities were influenced by the forest types, PF, MF, and MCF, with the function adonis() of the ‘vegan’ package with 999 permutations and using Bray–Curtis dissimilarity as the measure of distance. Orthogonal rotation of the resulting NMDS solution was used to maximize correlation between elevation and the major axes. The possibility that significant effects were due to either differences of multivariate location (between group variability) or to dispersion (within group variability) rather than by the compositional change was discarded from PerMANOVA analyses with the betadisper() function of vegan. A secondary matrix of sites by environmental variables, presence of Alnus and OTU richness values for genera, trophic modes/guilds, and growth forms were included in the NMDS analyses. The significance of the vectors was determined with the envfit() function of the ‘vegan’ package with 999 permutations. We determined the indicator OTUs as an additional descriptor for the fungal community of each forest type with function indval() of the R package ‘labdsv’ (Dufrêne and Legendre 1997; Roberts 2013).
Results
Macrofungal diversity
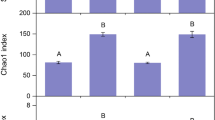
Of the 7634 non-singleton fungal OTUs, 1254 were assigned to the Agaricales, Boletales, Russulales and Phallomycetidae. In total, OTUs belonging to 123 genera of Agaricomycetes were detected along the elevational gradient (Table 1). The order Agaricales was the most diverse, accounting for 1076 OTUs (85.8%), followed by Phallomycetidae (146 OTUs, 11.6%), Russulales (19 OTUs, 1.5%) and Boletales (13 OTUs, 1.03%). We found that the total observed OTU richness of terrestrial Agaricomycetes was significantly different among forest types (F = 177.8, p < 0.001) and decreased with elevation from 607 (± 17.4), 569 (± 19.6) and 449 (± 15.2) for the PF, MF, and MCF vegetation types, respectively. Total estimated OTU richness was substantially higher compared to the observed values for the Agaricales and Phallomycetidae along the whole gradient, and by a lesser degree for the Russulales and Boletales (Fig. 1). When considering individual fungal groups, the elevation patterns were somewhat different. In Agaricales, the observed and estimated OTUs richness differed significantly among forest types (F = 81.0, p < 0.001 and F = 102.5, p < 0.001, respectively) with the MCF having the lowest observed and estimated richness. While the PF and MF had similar observed richness, estimated richness of Agaricales was highest in the MF (Fig. 1). The Boletales showed a very different distribution pattern, both observed and estimated OTUs richness values were significantly lower in the MF than in the PF and MCF (observed: F = 22.6, p < 0.001; estimated: F = 10.4, p = 0.001). In Phallomycetidae, observed OTUs richness was also significantly different among elevational forest types (F = 275.4, p < 0.001), strongly decreasing with elevation. However, this trend was less marked in the estimated richness values (F = 5.7, p = 0.01), only the MCF showed significantly lower richness than the MF and PF (Fig. 1). The observed OTU richness of Russulales was not significantly different among forest types (F = 2.5, p = 0.105), while the estimated richness was highest in the MF (F = 7.3, p = 0.004).
Observed and estimated (Chao 2) OTUs richness in Agaricales, Boletales, Phallomycetidae and Russulales for the three elevational forest types in the Andean Yungas forest of Argentina with standard deviation. Different letters above bars represent significant differences (p ≤ 0.05) in the observed (lower case) and estimated (upper case) values in pairwise comparisons between forest types. PF Piedmont forest, MF Montane forest, MCF Montane cloud forest
Beta diversity measures indicated that sampled fungal communities within the elevational zones were most similar to one another in the MCF, suggesting lower community turnover at high elevations than in the mid elevation (Sørensen: F = 7.8, p < 0.001). Meanwhile, the similarity of fungal communities within PF were intermediate and was not significantly different from either of the two other elevational forest types (Table 2). Pairwise comparisons among the forest types showed that similarity in fungal communities composition was highest between PF and MF sites, followed by similarity values between the MCF and MF (Sørensen: F = 17.5, p < 0.001; Table 2).
OTU richness in each particular genus was highly variable throughout the sampling sites and forests types as well (Table 1). In terms of OTU richness, the lower elevation zone (PF) was characterized by the dominance of saprotrophic genera, such as Chlorophyllum, Entoloma, Geastrum, Gymnopus, Lepiota, Leucoagaricus, Marasmius, Neopaxillus, Pterula and Ramaria. Similarly, in the MF the dominant genera were the saprotrophs Arachnion, Entoloma, Geastrum, Leucoagaricus, Micropsalliota, Mycena, Neopaxillus and Psilocybe. On the other hand, in the MCF, the most dominant were the ectomycorrhizal taxa Alnicola, Cortinarius, Inocybe, and saprotrophic Geastrum, Lepista, Ramaria and Mycena, the later was dominant in all three forest types, but particularly abundant in the PF (Table 1).
Community composition and functional roles along the elevational gradient
The NMDS analysis returned a two-dimensional ordination with a final stress of 0.127 and indicated that the studied Agaricomycetes communities were strongly structured according to elevational forest type (Fig. 2). The PerMANOVA analyses indicated that the composition of Agaricomycetes OTUs was significantly different among forest types (pseudo-F = 2.29, r2 = 0.18, p = 0.001).
Non-metric multidimensional scaling (NMDS) ordination based on community composition of terrestrial Agaricomycetes in the three elevational forest types in the Andean Yungas forest of Argentina. Ordination plots correspond to a environmental variables, b fungal genera, c trophic modes/guilds, and d fruit body morphology types. Due the high number of vectors, in (a, b) only variables with a significance level of p ≤ 0.06 are shown. For further details see Table S1
In the NMDS ordination plots, the vectors of elevation, N, organic matter and the presence of Alnus correlated positively, while pH and P correlated negatively with elevation (Fig. 2a, Table S1). OTU richness in twelve genera correlated significantly with fungal community composition along the elevational gradient (Fig. 2b, Table S1). Of these, Alnicola, Alpova, and Lactarius showed an increasing richness with elevation, while richness in Lacrymaria and Clitopilus were negatively correlated with elevation (Fig. 2b, Table S1). Of the trophic modes, the symbiotroph taxa were significantly correlated with forest types (Table S1). Among them, ECM OTUs showed a greater richness in the MCF than in the other forest types (Fig. 2c). Likewise, when considering the basidiomata growth morphology, the clavarioid type vector (marginally correlated with NMDS axis 1), was positively associated with the MCF sites (Fig. 2d, Table S1). A significant negative correlation between the secotiod type and axis 2 of the NMDS was observed (Table S1).
Indicator OTU analyses revealed 31 OTUs as significant indicators of forest type (p < 0.05, Table S2). Of these, 19 were related to MCF, 1 with MF and 11 with PF. Most indicators OTUs for MCF forest type belonged to ECM symbiotrophs such as Alnicola sphagneti, Cortinarius sp., Inocybe jacobi, Lactarius sp. and Lactarius rufus, five saprotrophs: Mycena aff. pura, Henningsomyces candidus, Clavaria sp., Conocybe alboradicans, Clavulinopsis sp. and Entoloma rhidopolium. The nutritional mode of the latter species is not clearly defined, and considered in the literature either ECM or saprotroph (Tedersoo et al. 2010). Most indicator species were of the agaricoid type with the exception of clavarioid Clavaria and Clavulinopsis. For the MF forest, only one OTU was selected as an indicator, but remained as an unidentified Agaricales.
Indicator species (OTUs) of PF forest type were all saprotrophs (Fig. 3), and corresponded to Clitopilus sp., Coprinopsis cinerea, Phallus sp., Geastrum sp., G. quadrifidium, Lepiota sp., Chlorophyllum agaricoides, Macrolepiota sp., and Coprinellus aureogranulatus. Most indicator species belonged to the agaricoid type and few to the gasteroid type.
Discussion
Macrofungal diversity
The analyzed Agaricomycetes groups were unevenly distributed across the elevational forest types in the Argentinian Yungas. The decreasing richness with elevation showed the effect of the forest type variation along the gradient, and estimation of species richness by Chao 2 indicated overlapping values depending on the fungal group and elevational forest type considered. Agaricales and Russulales showed an observed richness mostly uniform for the three forest types. However, the estimated richness was significantly higher in the MF type, suggesting that additional sampling effort in the intermediate forest type may reveal more species at this elevation. This higher estimated richness in Agaricales and Russulales in the MF is in agreement with the mid-elevation peak in tree diversity recorded in the Yungas (Morales et al. 1995), and the pattern is partially consistent with previous estimates of macrofungal richness based on sporocarps along an elevational gradient in Mexico (Gómez-Hernández and Williams-Linera 2011). On the other hand, most taxa in Phallomycetidae and Boletales appear to prefer habitats at lower elevation, and at both extremes, respectively. Again, the comparison of observed and estimated richness suggests that further sampling is necessary, particularly in the lower vegetation types. Our findings are in agreement with Rahbek (1995), who compared data from numerous studies carried out in elevational gradients and concluded that various groups of organisms are influenced differently by biological and environmental factors. Most of the studies indicated a mid-elevation peak in the number of species in disagreement with the widely acknowledged general pattern of decline in species richness with elevation.
The above-mentioned differences in elevational trends of alpha diversity measures among taxonomic groups suggest that they might be differentially affected by several factors such as resource abundance, host and habitat diversity (Lodge and Cantrell 1995a, b). The beta diversity measures indicated high community turnover among the elevational zones. The mid-elevation MF shared more OTUs with either the PF or the MCF zones (representing the extremes of the elevational gradient) than these latter shared between each other. In addition, the results suggest that MF fungal communities show higher affinity to PF communities, which is in agreement with floristic data and the pronounced climatic differences between the PF and the MCF (Brown et al. 2001; Morales et al. 1995). The high-elevation MCF are mostly dominated by cold-adapted taxa with Holartic and Gondwanan origin (i.e. Viburnum sp., Ilex sp., Juglans sp., Alnus sp., Podocarpus sp., Prunus sp., among others), while the MF and PF mostly comprise Neotropical and Pantropical elements, such as Anacardiaceae, Bignoniaceae, Boraginaceae, Lauraceae, Leguminosae, Moraceae, Myrtaceae, Rhamnaceae, and Rubiaceae etc. (Brown et al. 2001; Grau and Brown 2000; Malizia et al. 2012; Prado 2000).
As expected, the Agaricales was by far the most diverse group in our study, followed by Phallomycetidae, Boletales and Russulales. Despite the different methodological approaches and the overall number of taxa recorded, studies based on sporocarps sampling in many regions and in particular in tropical forests of South America have shown similar patterns in relation to the distribution and number of taxa within Agaricomycetes groups. Franco-Molano et al. (2010) in Colombia, accounted for a total of 813 species distributed within the Agaricales (679 spp.), Boletales (51 spp.), Cantharellales (23 spp.) and Russulales (60 spp.). Similarly, Agaricales was the most species rich taxonomic group in the Atlantic rain forest fragments in Minas Gerais (Brazil) as well, with 109 species representing 39 genera in 8 families (Rosa and Capelari 2009).
Previous sporocarp samplings in the Yungas of Argentina have also provided important information on the fungal composition associated to this region. Niveiro (2012) compiled all described macrofungal taxa for the Yungas of Argentina, totaling 629 species. However, many of the taxonomic names are duplicated due to existence of wrongly assigned species or unresolved synonymy, causing an overestimation of the number of “described” species for the region. Niveiro (2012) recorded and studied 125 “Agaricales sl.” species in the Yungas, and described 12 new to science within the genera Pouzarella, Inocybe, Clitocybula, Mycena, Pluteus, Pholiota and Hemimycena (Niveiro 2012; Niveiro et al. 2012, 2014a, b). Based on richness estimators, Niveiro (2012) concluded that only 23–46% of the Agaricales were recorded, and that a high diversity of taxa remained to be studied. In our study, soil metabarcoding analyses provided 1254 OTUs for the considered Agaricomycetes group, then based on the number of described species for the Yungas of Argentina, we can predict that many more species are still undescribed.
Macrofungal communities and functional roles along the gradient
Based on our results, the composition of the macrofungal communities is strongly influenced by the elevational gradient, more specifically by the presence of specific plant species that act as symbiotic hosts of ECM taxa in the upper forest type, as well as the variation in soil characteristics. Changes in tree composition and, as a consequence, in the quality and quantity of woody substrates for decomposer taxa may be affecting the fungal community composition along the elevational gradient (Meier et al. 2010). As revealed by previous studies, the combination of biotic and abiotic factors affects the distribution of species and the structure of fungal communities. The compositional shifts are accompanied by varying responses of dominant fungal functional groups (Looby et al. 2016), and determine their relationships to specific ecosystems (Braga-Neto et al. 2008; Gómez-Hernández and Williams-Linera 2011; Lodge and Cantrell 1995a, b; López-Quintero et al. 2012). Important factors influencing fungal communities in this study were primarily those at the habitat scale such as forest type and soil characteristics (i.e. concentration of N, P, pH and amount of organic matter). Similar factors correlated with fungal composition such as soil pH, N, P, C:N ratio and temperature have been previously described also for temperate forests (Bahram et al. 2012; Coince et al. 2014).
The symbiotroph trophic guild (e.g., ECM) was significantly associated with the different forest types. The composition of terrestrial Agaricomycetes was significantly different among forest types and beta diversity measures, NMDS ordinations, and the indicator species analyses suggest that the best-defined vegetation belts and associated fungal communities are the high-elevation MCF and low-elevation PF. The mid-elevation MF constitutes a transition zone between MCF and PF. The data suggest that the distributional range of the predominant taxa at low- and high-elevations overlaps at the intermediate forest type, and this may contribute to the mid-elevation peak in Agaricales and Russulales.
As previously stated, macrofungal communities were correlated with soil organic matter and N content, which is likely influencing the various ECM and “tomentelloid” fungi adapted to the MCF, rich in soil C and N (Becerra et al. 2005a; Geml et al. 2014; Nouhra et al. 2015). This vegetation belt is also characterized by the low diversity of plant species, soil strongly influenced by the dominant ECM and nitrogen fixing Alnus acuminata, as well as by the wettest and coldest climate conditions.
In contrast, PF and MF harbor diverse communities of tropical and subtropical plants species restricted to frost-free habitats, without dominant ECM hosts (Brown et al. 2001; Geml et al. 2014). The turnover of decomposing organic matter occurs faster at higher temperatures at lower elevations (Osono 2007; Rastin et al. 1990), given the wide variety of soil and litter decomposers, which are dominant at these two vegetation belts (Fig. 3) as supported by the indicator OTUs for this set of macrofungal groups. Moreover, mid- and low-elevation forest types have highly diverse floristic composition with a varied array of phenological and functional strategies (Brown et al. 2001; Malizia et al. 2012), providing a wide spectrum of suitable substrata for the saprotrophs. It has been observed that a great diversity of lignified materials can affect the diversity of decomposer basidiomycete assemblages, and this effect is likely to be produced by the different capabilities of fungi to decompose, the synergistic decomposing activities among basidiomycete species, or the interactions of fungi with other microorganisms such as endophytes, mites, bacteria, etc. (Addison et al. 2003; Fujita 1989; Hintikka 1988; Iwabuchi et al. 1994; Osono 2007).
Within Agaricales, the litter decomposer taxa recovered for this study, such as Agaricus, Mycena, Lepiota and Clavaria have activity related to the lignin decomposition process, i.e. bleaching activity; manganese peroxidases and laccase production; loss of lignin in leaves, etc. (Osono 2007). In addition, it is noteworthy the presence of numerous OTUs corresponding to species of Agaricaceae, Lepiotaceae and Clavariaceae that matched with > 95% sequence similarity to fungal saprotrophic taxa associated to diverse symbiotic leaf-cutting ants. OTUs identified as Agaricus and Leucoagaricus were abundant at the lower elevation forests types. Saprotrophic litter species belonging to Leucoagaricus, Leucocoprinus and Pterula have been described in association with Neotropical leaf-cutting ants (Chapela et al. 1994; Dentinger et al. 2009; Mueller et al. 2001; North et al. 1997). Little is known about the diversity of this group of fungi and their contribution to the cycling of nutrients in the lowland forests of the Yungas. In this sense, obtaining new data on functional diversity will contribute to the understanding on how biodiversity affects the ecosystem processes (Aguilar-Trigueros et al. 2015). Other abundant OTUs were recorded within Gymnopus, Pterula, and Psilocybe which are all well-known decomposers of organic matter, i.e., litter and wood inhabitants. Within Mycena, at least 22 species have been previously described morphologically from the region (Niveiro 2012). However, from our metabarcoding soil data, out of 68 OTUs accounting for Mycena, only few OTUs matched a previously described species with 94–99% similarity to M. pura; a similar pattern was shown by morphological species of Leucoagaricus, with 6 species previously recorded, in comparison with the 94 OTUs recovered from the analysis. However, none matched previously described taxa. These results highlight the need for additional studies to adequately characterize the macrofungal communities in each forest type, and also to provide much-needed reference data for Neotropical fungi public DNA fungal sequence databases.
Indicator OTUs analyses exclusively selected saprotrophs taxa for the lower vegetation belts, PF and MF. Within the Boletales, estimated OTU richness showed higher values at both extremes of the gradient, partitioned by the ectomycorrhizal genera such as Alpova and resupinate saprotrophs (i.e. Leucogyrophana and Amylothelia) at higher elevations, and the saprotrophic resupinate and agaricoid taxa (i.e. Hydnomerulius, Serpula and Hygrophoropsis) in the low-elevation forests. Phallomycetidae, as previously mentioned, showed higher richness values at intermediate and lower elevations, mostly represented by numerous OTUs of saprotrophic taxa such as Geastrum, Ramaria and Phallus, with diverse basidioma growth morphology types. In the Russulales, the distribution of OTUs along the gradient is partitioned, displaying the symbiotrophic ectomycorrhizal taxa (i.e. Russula and Lactarius) in the MCF sites dominated by Alnus, and saprotrophic wood decomposer and plant pathogens at lower elevations. Some of the wood decomposers, such as the distinctly hydnoid Hericium, seems to have some substrate preference, usually growing on the central core of standing trees, and rarely producing fruit bodies when the invasion zone is exposed in fallen trees (Hallenberg et al. 2013). Plant pathogenic taxa such as Peniophora, and Cristinia have resupinate basidiomata. Some Peniophora species are considered pioneer species capable of invading living or recently dead wood and can be restricted to certain trees (Boddy and Rayner 1983; Oberwinkler 1994), while the ecology of Cristinia is poorly known.
The secotioid growth morphology was negatively correlated with elevation, and represented by a Podaxis species that appear in one site of the PF at the south-eastern distribution of the Calilegua National Park, bordering the ecotone with Chaquean vegetation type. This ecotone area is dry and characterized by even higher temperatures, and pronounced differences among seasons. Podaxis as well as some gasteroid taxa are abundant in the forests of the Chaco phytogeographic region (Domínguez de Toledo 1989). Their richness and composition seem to be affected by a precipitation gradient decreasing from the northeast to the southwest of northern Argentina (Hernandez Caffot 2013). The Phallomycetidae was mostly represented by the Geastraceae with 111 Geastrum OTUs that were abundant in all forest types, but most diverse in the PF forest type with several indicator OTUs. It is noteworthy that Geastrum spp. is one of the dominant genera of gasteroid taxa in the adjacent Chaco forests (Hernandez Caffot 2013).
Gómez-Hernández and Williams-Linera (2011) determined that the variation in the environmental factors along an elevation gradient differentially affected macrofungal functional groups in a tropical mountain ecosystem in Mexico. They found that models for alpha and beta diversity for all macromycetes and ectomycorrhizal communities displayed peaks in the mid-elevation section of the gradient, whereas saprotrophs, such as the xylophagous and litter fungi, displayed a peak in the lower and mid-elevation sections. This pattern seems to be partially concordant for the Yungas, although less pronounced, perhaps partly due to the lower number of vegetation belts along the elevational range. Studies in the Amazon basin, in which the elevational gradient was not an influencing variable, have shown also a correlation between plant composition and fungal richness for biotrophic (pathogens and mycorrhizal fungi), but not for saprotrophic fungi, suggesting that the effect of vegetation on fungal communities is in part due to direct plant–fungal interactions (Peay et al. 2013).
Conclusion
Our results described highly diverse macrofungal communities as well as groups previously not recorded for the Yungas, such as the Agaricales taxa associated with ants. In addition, this study provides further insights into previously described patterns of turnover of taxa between different forests habitats along elevational gradients, which is considered one of the dominant factors affecting their richness at landscape scales (Braga-Neto et al. 2008; Nantel and Neumann 1992). The variation of environmental and microclimatic factors related to elevation is affecting species richness and the distribution of specific Agaricomycetes taxa and functional groups in the Yungas. The saprotrophic nutritional mode is highly dominant along the gradient, being partially replaced by biotrophic (ECM) modes at higher elevations. Fungal communities in the MCF are most dissimilar when compared with communities at the PF and MF, which is consistent with the different biogeographic origins of these forests. Our study shows that the use of DNA metabarcoding sequences can provide a detailed first insight into the diversity and composition of macrofungal communities of highly diverse tropical ecosystems, thus offering a baseline for a series of more in-depth mycological and ecological studies in these ecosystems.
References
Addison JA, Trofymow JA, Marshall VG (2003) Functional role of collembola in successional coastal temperate forests on Vancouver Island, Canada. Appl Soil Ecol 24:247–261. https://doi.org/10.1016/S0929-1393(03)00090-8
Aguilar-Trigueros C, Hempel S, Powell J, Anderson I, Antonovics J, Bergmann J, Cavagnaro T, Chen B, Hart M, Klironomos J, Petermann J, Verbruggen E, Veresoglou S, Rillig M (2015) Branching out: towards a trait-based understanding of fungal ecology. Fungal Biol Rev 29:34–41. https://doi.org/10.1016/j.fbr.2015.03.001
Bahram M, Põlme S, Kõljalg U, Zarre S, Tedersoo L (2012) Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytol 193:465–473. https://doi.org/10.1111/j.1469-8137.2011.03927.x
Baroni TJ, Albertó E, Niveiro N, Lechner B (2012) New species and records of Pouzarella (Agaricomyetes, Entolomataceae) from northern Argentina. Kurtziana 37:41–63
Becerra A, Daniele G, Domínguez L, Nouhra E, Horton T (2002) Ectomycorrhizae between Alnus acuminata H.B.K. and Naucoria escharoides (Fr.:Fr.) Kummer from Argentina. Mycorrhiza 12:61–66. https://doi.org/10.1007/s00572-001-0148-3
Becerra A, Pritsch K, Arrigo N, Palma M, Bartoloni N (2005a) Ectomycorrhizal colonization of Alnus acuminata Kunth in northwestern Argentina in relation to season and soil parameters. Ann For Sci 62:325–332. https://doi.org/10.1051/forest:2005027
Becerra A, Zak MR, Horton T, Micolini J (2005b) Ectomycorrhizal and arbuscular mycorrhizal colonization of Alnus acuminata from Calilegua National Park (Argentina). Mycorrhiza 15:525–531. https://doi.org/10.1007/s00572-005-0360-7
Becerra A, Cabello M, Chiarini F (2007) Arbuscular mycorrhizal colonization of vascular plants from the Yungas forests, Argentina. Ann For Sci 64:765–772. https://doi.org/10.1051/forest:2007056
Becerra AG, Cabello M, Zak MR, Bartoloni N (2009) Arbuscular mycorrhizae of dominant plant species in Yungas forests, Argentina. Mycologia 101:612–621. https://doi.org/10.3852/08-176
Becerra AG, Cabello M, Bartoloni N (2011) Native arbuscular mycorrhizal fungi in the Yungas forests, Argentina. Mycologia 103:273–279. https://doi.org/10.3852/10-193
Blackwell M (2011) The fungi: 1, 2, 3… 5.1 million species? Am J Bot 98:426–438. https://doi.org/10.3732/ajb.1000298
Blake JG, Rougés M (1997) Variation in capture rates of understory birds in El Rey National Park, Northwestern Argentina. Ornitol Neotrop 8:185–193
Boddy L (2001) Fungal community ecology and wood decomposition processes in angiosperms: from standing tree to complete decay of coarse woody debris. Ecol Bull 49:43–56
Boddy L, Rayner A (1983) Ecological roles of basidiomycetes forming decay communities in attached oak branches. New Phytol 93:77–88. https://doi.org/10.1111/j.1469-8137.1983.tb02694.x
Braga-Neto R, Luizao RCC, Magnusson WE, Zuquim G, Castilho CV (2008) Leaf litter fungi in a Central Amazonian forest: the influence of rainfall, soil and topography on the distribution of fruiting bodies. Biodivers Conserv 17:2701–2712. https://doi.org/10.1007/s10531-007-9247-6
Brown AD, Grau HR, Malizia LR, Grau A (2001) Argentina. In: Kappelle M, Brown AD (eds) Bosques nublados del Neotrópico. INBio, Cambridge, pp 623–659
Brown AD, Grau A, Lomáscolo T, Gasparri NI (2002) Una estrategia de conservación para las selvas subtropicales de montaña (Yungas) de Argentina. Ecotropicos 15:147–159
Cabrera A (1976) Regiones fitogeográficas de la República Argentina. Enciclopedia de Agricultura, Jardinería y Fruticultura 2:1–85
Chao A, Chazdon RL, Colwell RK, Shen TJ (2005) A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol Lett 8:148–159. https://doi.org/10.1111/j.1461-0248.2004.00707.x
Chapela I, Rehner S, Schultz T, Mueller U (1994) Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science 266:1691–1694. https://doi.org/10.1126/science.266.5191.1691
Chase JM, Leibold MA (2003) Ecological niches: linking classical and contemporary approaches. University of Chicago Press, Chicago
Coince A, Cordier T, Lengellé J, Defossez E, Vacher C, Robin C, Buée M, Benoît M (2014) Leaf and root-associated fungal assemblages do not follow similar elevational diversity patterns. PLoS ONE 9:e100668. https://doi.org/10.1371/journal.pone.0100668
Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation, and comparison of assemblages. J Plant Ecol 5:3–21. https://doi.org/10.1093/jpe/rtr044
Dentinger B, Lodge J, Munkacsi A, Desjardin D, Mclaughlin D (2009) Phylogenetic placement of an unusual coral mushroom challenges the classic hypothesis of strict coevolution in the Apterostigma pilosum group ant–fungus mutualism. Evolution 63:2172–2178. https://doi.org/10.1111/j.1558-5646.2009.00697.x
Domínguez de Toledo LS (1989) Gasteromycetes (Eumycota) del centro y oeste de la Argentina. Doctoral Dissertation. Universidad Nacional de Córdoba
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Franco-Molano AE, Corrales A, Vasco-Palacios AM (2010) Macrohongos de Colombia II. Listado de especies de los órdenes Agaricales, Boletales, Cantharellales y Russulales (Agaricomycetes, Basidiomycota). Actual Biol 32:89–114
Fujita H (1989) Succession of higher fungi in a forest of Pinus densiflora. Trans Mycol Soc Jpn 30:125–147
Geml J (2017) Altitudinal gradients in mycorrhizal symbioses—the current state of knowledge on how richness and community structure change with elevation. Ecol Stud 230:107–123. https://doi.org/10.1007/978-3-319-56363-3_5
Geml J, Pastor N, Fernandez L, Pacheco S, Semenova T, Becerra A, Wicaksono C, Nouhra E (2014) Large-scale fungal diversity assessment in the Andean Yungas forests reveals strong community turnover among forest types along an altitudinal gradient. Mol Ecol 23:2452–2472. https://doi.org/10.1111/mec.12765
Geml J, Morgado LN, Semenova-Nelsen TA, Schilthuizen M (2017) Changes in richness and community composition of ectomycorrhizal fungi among altitudinal vegetation types on Mount Kinabalu in Borneo. New Phytol 215:454–468. https://doi.org/10.1111/nph.14566
Gitay H, Noble IR (1997) What are plant functional types and how should we seek them? In: Smith TM, Shugart HH, Woodward FI (eds) plant functional types. Cambridge University Press, Cambridge, pp 3–19
Gómez-Hernández M, Williams-Linera G (2011) Diversity of macromycetes determined by tree species, vegetation structure, and microenvironment in tropical cloud forest in Veracruz, Mexico. Botany 89:203–216. https://doi.org/10.1139/B11-007
Gómez-Hernández M, Williams-Linera G, Guevara R, Lodge DJ (2012) Patterns of macromycetes community assemblage along an elevation gradient: options for fungal gradient and metacommunity analyses. Biodiv Conserv 21:2247–2268. https://doi.org/10.1007/s10531-011-0180-3
Grau A, Brown AD (2000) Development threats to biodiversity and opportunities for conservation in the mountain ranges of the Upper Bermejo River Basin, NW Argentina and SW Bolivia. Ambio 29:445–450. https://doi.org/10.1579/0044-7447-29.7.445
Hallenberg N, Henrik Nilsson R, Robledo G (2013) Species complexes in Hericium (Russulales, Agaricomycota) and a new species—Hericium rajchenbergii—from southern South America. Mycol Prog 12:413–420. https://doi.org/10.1007/s11557-012-0848-4
Henkel TW, Aime MC, Chin MML, Miller SL, Vilgalys R, Smith ME (2012) Ectomycorrhizal fugal sporocarp diversity and discovery of new taxa in Dicymbe monodominant forests of the Guiana Shield. Biodivers Conserv 21:2195–2220. https://doi.org/10.1007/s10531-011-0166-1
Hernandez Caffot L (2013) Diversidad y ecología de Agaricomycetes (Phallomycetidae y Agaricomycetidae, Basidiomycota) asociados a relictos de bosque Chaqueño en Argentina. PhD Thesis Dissertation, Universidad Nacional de Córdoba, Argentina
Hibbett DS, Thorn RG (2001) Basidiomycota: Homobasidiomycetes. In: McLaughlin DJ et al (eds) The Mycota. Systematics and evolution. 7B. Springer, Berlin, pp 121–168
Hibbett DS, Pine EM, Langer E, Langer G, Donoghue MJ (1997) Evolution of gilled mushrooms and puffballs inferred from ribosomal DNA sequences. PNAS 94:12002–12006
Hintikka V (1988) On the macromycete flora in oligotrophic pine forests of different ages in south Finland. Acta Bot Fenn 136:89–94
Hladki AI, Romero AI (2001) The genus Kretzschmaria from Tucumán (Argentina). Mycotaxon 79:481–496
Hladki AI, Romero AI (2010) A preliminary account of Xylaria in the Tucumán province, Argentina, with a key to species from the Northern Provinces. Fungal Divers 42:79–96. https://doi.org/10.1007/s13225-009-0008-6
Hueck K (1978) Los bosques de Sudamérica: ecología, composición e importancia económica. Sociedad Alemana de Cooperación Técnica (GTZ), Eschborn, Germany
Ishikawa H, Osono T, Takeda H (2007) Effects of clear-cutting on decomposition processes in leaf litter and the nitrogen and lignin dynamics in a temperate secondary forest. J For Res 12:247–254. https://doi.org/10.1007/s10310-007-0013-0
Iwabuchi S, Sakai S, Yamaguchi O (1994) Analysis of mushroom diversity in successional young forests and equilibrium evergreen broad-leaved forests. Mycoscience 35:1–14
Kinoshita A, Fukuda H (2004) Difference of fruiting bodies of higher fungi between the sites with and without understory management (in Japanese with English abstract). Jpn J For Environ 46:29–34
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Dictionary of the fungi, 10th edn. CABI, Wallingford
Kõljalg U, Nilsson RH, Abarenkov K et al (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277. https://doi.org/10.1111/mec.12481
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415. https://doi.org/10.1016/j.soilbio.2008.05.021
Lavilla EO, Manzano AS (1995) La batraco fauna de las selvas de montaña del noroeste argentino. In: Brown AD, Grau HR (eds) Investigación, conservación y desarrollo en las selvas subtropicales de montaña. Laboratorio de Investigaciones Ecológicas de las Yungas, UNT, Tucumán, pp 157–162
Lavorel S, Garnier E (2001) Aardvarck to Zyzyxia—functional groups across kingdoms. New Phytol 149:360–364. https://doi.org/10.1046/j.1469-8137.2001.00048.x
Legname P (1982) Los árboles indígenas del noroeste argentino. Opera Lilloana 34:1–226
Lodge DJ (1997) Factors related to diversity of decomposer fungi in tropical forests. Biodivers Conserv 6:681–688. https://doi.org/10.1023/A:1018314219111
Lodge DJ, Cantrell S (1995a) Fungal communities in wet tropical forests: variation in time and space. Can J Bot 73:1391–1398. https://doi.org/10.1139/b95-402
Lodge DJ, Cantrell S (1995b) Diversity of litter agarics at Cuyanero, Ecuador: calibrating sampling efforts in tropical rainforest. Mycologist 9:149–151. https://doi.org/10.1016/s0269-915x(09)80003-7
Lomáscolo T, Brown AD, Malizia LR (2010) Reserva de Biósfera de las Yungas. Ediciones del Subtrópico, Fundación ProYungas, Yerba Buena, Tucumán
Looby C, Maltz MR, Treseder K (2016) Below-ground responses to elevation in a changing cloud forest. Ecol Evol 6:1996–2009. https://doi.org/10.1002/ece3.2025
López-Quintero CA, Straatsma G, Franco-Molano AE, Boekhout T (2012) Macro-fungal diversity in Colombian Amazon forest varies with regions and regimes of disturbance. Biodivers Conserv 21:2221–2243. https://doi.org/10.1007/s10531-012-0280-8
Malizia L, Pacheco S, Blundo C, Brown AD (2012) Caracterización altitudinal, uso y conservación de las Yungas subtropicales de Argentina. Ecosistemas 21:53–73
Masayuki U, Rota W, Balser TC, Kanehiro K (2008) Variations in the soil microbial community composition of a tropical montane forest ecosystem: does tree species matter? Soil Biol Biochem 40:2699–2702. https://doi.org/10.1016/j.soilbio.2008.06.023
McGuire KL, Fierer N, Bateman C, Treseder KK, Turner BL (2012) Fungal community composition in neotropical rain forests: the influence of tree diversity and precipitation. Microb Ecol 63:804–812. https://doi.org/10.1007/s00248-011-9973-x
Meier CL, Rapp J, Bowers RM, Silman M, Fierer N (2010) Fungal growth on a common wood substrate across a tropical elevation gradient: temperature sensitivity, community composition, and potential for above-ground decomposition. Soil Biol Biochem 42:1083–1090. https://doi.org/10.1016/j.soilbio.2010.03.005
Meyer T (1963) Estudios sobre la selva tucumana: la selva de mirtáceas de las Pavas. Opera Lilloana 10:1–144
Mitchell CE (2003) Trophic control of grassland production and biomass by pathogens. Ecol Lett 6:147–155
Morales JM, Sirombra M, Brown AD (1995) Riqueza de árboles en las Yungas argentinas. In: Brown AD, Grau HR (eds) Investigación, conservación y desarrollo en las selvas subtropicales de montaña. Laboratorio de Investigaciones Ecológicas de las Yungas, UNT, Tucumán, pp 163–174
Mueller UG, Schultz TR, Currie CR, Adams MM, Malloch D (2001) The origin of the attine ant–fungus mutualism. Q R Biol 76:169–197. https://doi.org/10.1086/393867
Mueller GM, Schmit JP, Leacock PR et al (2007) Global diversity and distribution of macro-fungi. Biodivers Conserv 16:37–48. https://doi.org/10.1007/s10531-006-9108-8
Nantel P, Neumann P (1992) Ecology of ectomycorrhizal-basidiomycete communities on a local vegetation gradient. Ecology 73:99–117. https://doi.org/10.2307/1938724
Nguyen NH, Song Z, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248. https://doi.org/10.1016/j.funeco.2015.06.006
Niveiro N (2012) Agaricales sensu lato (Agaricomycetes) de las Selvas del Dominio Amazónico de la Argentina. Diversidad, Distribución y Abundancia. Tesis Doctoral. Universidad Nacional de Córdoba
Niveiro N, Popoff O, Desjardin D, Albertó E (2012) Mycena moconensis, a new species of section Polyadelphia from Argentina. Mycotaxon 119:167–173. https://doi.org/10.5248/119.167
Niveiro N, Popoff O, Lechner B, Albertó E (2014a) Pholiota oblita, new species in sect. Adiposae stirps Subflammans (Strophariaceae, Agaricomycetes), from the Argentinean Yungas. Phytotaxa 167:276–282. https://doi.org/10.11646/phytotaxa.167.3.6
Niveiro N, Popoff O, Albertó E (2014b) Hemimycena longipleurocystidiata (Mycenaceae, Agaricomycetes), a new species from the Argentinean Atlantic Forest. Phytotaxa 177:49–55. https://doi.org/10.11646/phytotaxa.177.1.4
North R, Jackson C, Howse P (1997) Evolutionary aspects of ant–fungus interactions in leaf-cutting ants. Tree 12:386–389. https://doi.org/10.1016/S0169-5347(97)87381-8
Nouhra E, Domínguez L, Becerra A, Trappe J (2005) Morphology, molecular analysis and some ecological aspects of the hypogeous fungi Alpova austroalnicola sp. nov. Mycologia 97:598–604. https://doi.org/10.3852/mycologia.97.3.598
Nouhra E, Pastor N, Becerra A, Sarrionandia Areitio E, Geml J (2015) Greenhouse grown seedlings of Alnus species showed lack of specificity and a strong preference for Tomentella ectomycorrhizal associates. Microb Ecol 69:813–825. https://doi.org/10.1007/s00248-014-0522-2
O’Brien H, Parrent J, Jackson J, Moncalvo J, Vilgalys R (2005) Fungal community analysis by large-scale sequencing of environmental samples. Appl Environ Microb 71:5544–5550. https://doi.org/10.1128/AEM.71.9.5544-5550.2005
Oberwinkler F (1994) Evolution of functional groups in Basidiomycetes (Fungi). In: Schulze ED, Mooney HA (eds) Biodiversity and ecosystem function. Springer, Berlin, pp 143–163. https://doi.org/10.1007/978-3-642-58001-7_7
Ojeda RA, Mares MA (1989) A biogeographic analysis of the mammals of Salta Province, Argentina: patterns of species assemblage in the Neotropics. Texas Tech University Press, Lubbock
Oksanen J, Blanchet FGK, Roeland Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2012) vegan: Community Ecology Package, Version 2.0-5. http://cran.r-project.org/package=vegan
Osono T (2007) Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol Res 22:955–974. https://doi.org/10.1007/s11284-007-0390-z
Pacheco S, Malizia L, Cayuela L (2010) Effects of climate change on subtropical forests of South America. Trop Conserv Sci 3:423–437
Peay K, Baraloto C, Fine P (2013) Strong coupling of plant and fungal community structure across western Amazonian rainforests. The ISME Journal 7:1852–1861. https://doi.org/10.1038/ismej.2013.66
Piepenbring M, Hofmann TA, Unterseher M, Kost G (2012) Species richness of plants and fungi in western Panama: towards a fungal inventory in the neotropics. Biodivers Conserv 21(9):2181–2193. https://doi.org/10.1007/s10531-011-0213-y
Prado DE (2000) Seasonally dry forests of tropical South America: from forgotten ecosystems to a new phytogeographic unit. Edinb J Bot 57:437–461. https://doi.org/10.1017/S096042860000041X
Pritsch K, Becerra A, Põlme S, Tedersoo L, Schloter M, Agerer R (2010) Description and identification of Alnus acuminata ectomycorrhizae from Argentinean alder stands. Mycologia 102:1263–1273. https://doi.org/10.3852/09-311
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Rahbek C (1995) The elevational gradient of species richness: a uniform pattern? Ecography 18:200–205. https://doi.org/10.1111/j.1600-0587.1995.tb00341.x
Rastin N, Schlechte G, Hüttermann A (1990) Soil macro-fungi and some soil biological, biochemical and chemical investigations on the upper and lower slope of a spruce forest. Soil Biol Biochem 22:1039–1047. https://doi.org/10.1016/0038-0717(90)90029-Y
Rillig MC, Aguilar-Trigueros CA, Bergmann J, Verbruggen E, Veresoglou SD, Lehmann A (2014) Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol 205:1385–1388. https://doi.org/10.1111/nph.13045
Roberts DW (2013) labdsv: ordination and multivariate analysis for ecology package, Version 1.6-1. URL: http://cran.r-project.org/web/packages/labdsv
Robledo G, Rajchenberg M (2007) South American polypores: first annotated checklist from Argentinean Yungas. Mycotaxon 100:5–9
Robledo G, Urcelay C, Rajchenberg M, Domínguez L (2003) Políporos (Aphyllophorales, Basidiomycota) parásitos y saprófitos de Alnus acuminata en el noroeste argentino. Bol Soc Argent Bot 38:207–224
Rosa LH, Capelari M (2009) Agaricales fungi from Atlantic rain forest fragments in Minas Gerais, Brazil. Braz J Microbiol 40:846–851. https://doi.org/10.1590/S1517-83822009000400015
Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microb 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Schmit JP, Mueller GM (2007) An estimate of the lower limit of global fungal diversity. Biodiver Conserv 16:99–111. https://doi.org/10.1007/s10531-006-9129-3
Singer R (1953) Four years of mycological work in southern South America. Mycologia 45:865–891
Singer R, Morello JH (1960) Ectotrophic forest tree mycorrhizae and forest communities. Ecology 41:549–551
Sparks DL, Page AL, Helmke PA, Loeppert RH (1996) Methods of soil analysis, part 3—chemical methods. Soil Society of America Book Series. American Society of Agronomy, Madison
Spegazzini C (1912) Mycetes argentinenses (series VI). Anales del Museo Nacional de Historia Natural de Buenos Aires 23:167–244
Spegazzini C (1919) Los Hongos del Tucumán. Primera Reunión Nacional de la Sociedad Argentina de Ciencias Naturales, Tucumán, pp 254–274
Swapna S, Syed A, Krishnappa M (2008) Diversity of macro-fungi in semi-evergreen and moist deciduous forest of Shimoga district-Karnataka, India. J Mycol Plant Pathol 38:21–26
Taylor D, Hollingsworth T, McFarland J, Lennon N, Nusbaum C, Ruess R (2014) A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol Monogr 84:3–20. https://doi.org/10.1890/12-1693.1
Tedersoo L, May T, Smith M (2010) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263. https://doi.org/10.1007/s00572-009-0274-x
Tedersoo L et al (2014) Global diversity and geography of soil fungi. Science 346(1256688):1–10. https://doi.org/10.1126/science.1256688
Urcelay C, Robledo G (2004) Community structure of polypores (Basidiomycota) in Andean alder wood in Argentina: functional groups among wood-decay fungi? Austral Ecol 29:471–476. https://doi.org/10.1111/j.1442-9993.2004.01387.x
Wicaksono CY, Aguirre Guiterrez J, Nouhra E, Pastor N, Raes N, Pacheco S, Geml J (2017) Contracting montane cloud forests: a case study of the Andean alder (Alnus acuminata) and associated fungi in the Yungas. Biotropica 49:141–152. https://doi.org/10.1111/btp.12394
Young AM, Forster PI, Booth R (2002) A preliminary checklist of the macro-fungi of the Wet Tropics and Einasleigh Uplands Bioregions of Queensland, Australia. Austral Mycol 21:16–20
Acknowledgements
Financial support was provided by Secretaría de Ciencia y Técnica (SECYT), Universidad Nacional de Córdoba, and by the Alberta Mennega Foundation. The molecular work was sponsored by a Research Initiative grant awarded to J. G. by the Naturalis Biodiversity Center. The authors thank the personnel at the protected areas sampled for providing permits for the scientific work, as well as to Alejandro Brown (President of the Fundación ProYungas) for his insightful guidance in selecting the sampling sites and for offering logistical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Brearley.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Environmental variables, Alnus sp. presence, fungal genera, trophic modes and fruit body morphology types correlation with NMDS dimensions 1 and 2 of the NMDS plot of fungal OTU composition in the three forest types in the Andean Yungas forest of Argentina. P values are based on 999 permutations (DOCX 31 kb)
Supplementary Material 2
Significantly indicator OTU (p < 0.05) indicator values, p-values and closest matchs for fungal OTU composition in the three forest types in the Andean Yungas forest of Argentina. MCF (Montane cloud forest), MF (Montane forest), PF (Piedmont forest) (DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Eduardo, N., Florencia, S., Nicolás, P. et al. Richness, species composition and functional groups in Agaricomycetes communities along a vegetation and elevational gradient in the Andean Yungas of Argentina. Biodivers Conserv 27, 1849–1871 (2018). https://doi.org/10.1007/s10531-018-1512-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-018-1512-3