Abstract
Endophytic fungi comprise a group of microorganisms of great diversity, which can be modulated by biotic and abiotic factors. The present study aims to understand how environmental factors, blooming and cultivars change the diversity of the endophytic fungal communities in sorghum leaves in two forest areas in Brazil. Our results showed that Sorghum bicolor has a rich and diverse community of endophytic fungi distributed in Ascomycota, Basidiomycota and Mucoromycota, with members of Dothideomycetes and Sordariomycetes as dominant groups. We found 581 endophytic fungi (filamentous and yeasts) identified in 55 species of 37 genera. A greater diversity and richness of endophytic fungi were found in sorghum leaves from the Atlantic Forest (diversity = H′ = 2.68 and richness = 46), differing significantly from the Caatinga forest (diversity = H′ = 2.51 and richness = 25). No difference in richness or diversity was observed between the two crops or between the phenological stages. We detected different species indicator for the two areas, nine taxa were associated with sorghum in the Atlantic Forest (Colletotrichum sp. 2, Epicoccum sorghinum, Curvularia sp., Colletotrichum sp. 1, Fusarium thapsinum, Nigrospora oryzae, Phyllosticta capitalensis, Fusarium oxysporum and Amesia nigricolor) and five taxa were indicators of sorghum in the Caatinga Forest (Meyerozyma sp., Talaromyces pinophilus, Metschnikowia sp., Rhodosporidiobolus sp. and Acremonium pinkertoniae). One species was an indicator of the cultivar IPA 2502 and no species were related to the cultivar IPA SF15. In relation to pre-blooming and post-blooming stages, two species were considered indicators for the former while six were considered for the latter. We observed that the number of filamentous fungi increases with the post-blooming stage and higher rainfall, while the diversity of endophytic yeasts was influenced by smaller rainfall and pre-blooming stage. Our data improves the understanding of factors that may influence the endophytic fungal community in ecologically and economically important grass species. The endophytic fungal diversity associated with sorghum is important to the mycodiversity estimation and may be useful in studies of biocontrol of pathogens and promotion of crops growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sorghum bicolor (grain sorghum) is the fifth most produced cereal in the world (Venkateswaran et al. 2019). A typical crop of tropical climate presents characteristics of xerophilic plants (Shakoor et al. 2014), and it is well adapted to hot and dry conditions, being mainly cultivated in the arid and semiarid regions of the world (Menz et al. 2002). Brazil is the 9th largest world producer, with 2.8 million tons grown in an area of 0.82 million hectares (Foreign Agricultural Service 2019). Cultivation of sorghum is extremely important in semiarid regions of Asia, Africa and Central America, mainly for human consumption (Shakoor et al. 2014; Proietti et al. 2015). In Brazil, the production of sorghum is destined for animal (forage and sweet sorghum, other sorghum type, not grain sorghum, used also to animal feed) feed and biofuels production (Taleon et al. 2012). The productivity of sorghum grain is susceptible to abiotic factors and attacks by various pathogens such as bacteria, viruses and mainly fungi; fungal contamination constitutes an important restriction for the increase of sorghum production worldwide (Astoreca et al. 2019; Choi et al. 2021; Poudel et al. 2022).
The beneficial symbiotic microbes, such as endophytic fungi, have been increasingly appreciated for the useful impact on plant physiology (Dastogeer 2018). Endophytes can promote plant growth and induce resistance to adverse abiotic and biotic effects, such as drought and nutrient deficiency in semiarid regions, and even the capacity to resist against pathogens, thus having potential to protect agricultural crops (Harman and Uphoff 2019; Quach et al. 2022). Among the endophytic fungal diversity, yeasts represent an important component and share a unique niche along with filamentous fungi (Joubert and Doty 2018). Due to capacity of endophytes act in the promotion of plant growth, these microorganisms are considered potential to applications in agriculture (Joubert and Doty 2018). Studies on fungal endophytes and their interaction with different hosts have recently shown important information about the benefits of this ecological relationship (Giauque and Hawkes 2013; Collinge et al. 2022). However, studies on the community composition of endophytic fungi in sorghum crops are still scarce (Zida et al. 2014; Rodrigues et al. 2018). To the best of our knowledge, no study has simultaneously investigated the contributions of environmental factors, phenological stages and cultivars of sorghum in the establishment of the endophytic fungal community in sorghum leaves.
Brazil has different biomes and among the tropical forests, the Atlantic Forest is considered a region with high biodiversity and endemism; however, it is highly threatened and intensely fragmented (Rodrigues et al. 2009). Another Brazilian Forest threatened by anthropic impacts is the Caatinga (da Silva and Barbosa 2017), a tropical dry forest with high richness and endemic species (Fernandes and Queiroz 2018). Environmental conditions and plant composition in the Atlantic Forest and in the Caatinga can have a significant influence on the endophytic community of plants cultivated in their areas. Several factors, such as temperature, humidity, precipitation, nutritional characteristics of the plant (Pieterse et al. 2018), host species (Saikkonen 2007) and phenological stage (Dalal and Kulkarni 2014) can change the structure of the endophytic fungal community. The understanding of the correlation between the endophytic community and plant growth stages is also considered an important factor in ecological studies of these fungi in plants of agronomic interest (Zida et al. 2014; Schlemper et al. 2018).
Phenological stage of the plants and different cultivars are significant factors that may influence the abundance, diversity and composition of endophytic fungi (da Silva et al. 2016; Schlegel et al. 2018). In order to better understand the structure of the endophytic fungal community in sorghum leaves cultivated in different forests in Brazil, we analysed the diversity and distribution of endophytes and correlated their communities with environmental factors, plant phenological stages and cultivars. Our objective was to understand how these factors change the endophytic fungal communities in sorghum leaves. We focused on three main hypotheses: (I) the distribution of fungal endophytes is modulated by the phenological stages of the plant, (II) the community of endophytic fungi in sorghum is influenced by the change of rainfall and temperature in areas with different climatic conditions and (III) the species of endophytic fungi varies among different cultivars of sorghum.
Material and methods
Study areas
The study was carried out in two sorghum crops located in the Experimental Station of the Agronomic Institute of Pernambuco (IPA) in Serra Talhada and Goiana municipalities, Pernambuco state, Northeast region, Brazil. The two areas studied are about 450 km distant from each other. The Serra Talhada area (7°55′48′′S 38°17′18′′W) is located in one of the Brazilian tropical dry forest (Caatinga). The climate tropical semiarid (BSh) according to the classification of Köppen (Koppen 1923) characterized by long hot and arid periods. The rainy season begins in November and ends in April. The mean annual temperature is 25.8 °C, mean annual precipitation is 640 mm and the predominant soils are Argisols (Pereira et al. 2017). The Goiana area (7°38′20′′S 34°57′22′′W) is located in the Atlantic Forest biome. The climate in the region is classified as tropical Atlantic (Aw) according to the Koppen classification. This region has low temperature variations during the year and high rainfall, with annual average temperatures of 25 °C, and annual mean rainfall of 1800 mm (Carvalho et al. 2015); the soil type is a Ultisol (Pereira et al. 2014).
Isolation and morphological identification of endophytic fungi
Sorghum leaves were collected in August and October 2014 in the Atlantic Forest domain area (Goiana), and in December 2014 and March 2015 in the Caatinga domain area (Serra Talhada). In each area, the leaves were collected from individuals of two different cultivars of S. bicolor, IPA SF15 and IPA 2502. The cultivar IPA SF15 has a high dry matter yield, besides adapting to different climatic and soil situations (Tabosa et al. 2002), and the IPA 2502 has stems rich in fermentable sugars, high production of biomass, resistant to water stress, adapting well to the scarcity of rainfall in the semiarid regions (Durães 2011).
The experimental design consisted of two areas, two sorghum cultivars and two plant phenological stages: before reproductive differentiation (pre-blooming) and after grain differentiation (post-blooming). For each area, 48 random points were delimited, and three individuals of sorghum were chosen at each point, from which nine leaves were collected, totalizing 864 leaves (432 from sorghum growing in the Caatinga forest area and 432 from the Atlantic Forest area). The leaves were washed in tap water, fragmented into 5 mm discs, using a sterile perforator, and under aseptic conditions the fragments were disinfested using 70% alcohol for 30 s and 2% sodium hypochlorite (NaClO) for 120 s. The fragments were then washed twice in sterile distilled water (Araújo et al. 2002; modified). Overall, 1728 leaves discs were placed in Petri dishes containing malt extract agar (MEA) supplemented with chloramphenicol (50 mg L−1), incubated at room temperature (28 ± 2 °C) and observed daily during 15 days for the fungal isolation. After isolation and purification of endophytes, a previous morphological identification of endophytic fungi was performed using microcultures and macro- and micro-morphological analyses and reproductive structures observation using several mycological literatures (e.g. Ellis 1971, 1976; Sutton 1980; Domsch et al. 1993; Seifert et al. 2011). For endophytic fungi without sporulation in culture, it was necessary to use different culture media to stimulate the fungal sporulation, such as potato-dextrose agar (PDA), wheat germ agar, oatmeal agar and V8-juice agar. For the relative frequency, the number of isolates of each species was divided by the total number of isolates (Bezerra et al. 2013).
DNA extraction, PCR and sequencing
After previous morphological identification, at least one representative of each fungal species was used to perform DNA extraction. All filamentous isolates that did not sporulated in culture were also included to the molecular study, as well as all yeasts. The fungal biomass was obtained from cultures grown on MEA after 7 days, the mycelium was transferred to 2 mL micro-tubes with screw cap, and to each tube was added 0.5 g of glass beads of two different diameters on the proportion of 1:1 (150–212 μm and 425–600 μm). The material was crushed by stirring at high speed in a FastPrep homogenizer.
The extraction of the genomic DNA was performed by the CTAB method based on the protocol described by Oliveira et al. (2016). For amplification of the rDNA ITS region, the primers ITS1 and ITS4 were used (White et al. 1990). Polymerase chain reaction (PCR) that contained PCR reactions were carried out in a volume of 25 μL containing 75 mM Tris-HCl (pH8.8), 200 mM (NH4)2SO4, 0.01% Tween 20, 2 mM MgCl2, 200 μM each dNTPs, 1 μM each primer and 2 units Taq DNA polymerase. Thermal cycling parameters were temperature an initial preheat 95 °C for 3 min; followed by 39 cycles of denaturation at of 94 °C for 45 s, annealing at 58 °C for 1 min and extension 72 °C for 1 min; 72 °C for 7 min. The products of DNA extractions and reactions (5 μL) of PCR were visualized under UV light in 1% agarose gel stained with GelRed. Amplification products were purified with the PureLink PCR Purification Kit (Invitrogen), following the manufacturer’s instructions, and the sequences were obtained using the Multipurpose DNA Sequencing Platform of the Bioscience Center/UFPE.
Phylogenetic analyses
The ITS rDNA sequences obtained in this study were used to search for similar sequences deposited in the GenBank database of the NCBI using the BLASTn tool. The sequences selected along the sequences from endophytes were aligned using the MAFFT v.7 interface (Katoh and Standley 2013) and adjusted using MEGAv.7 (Kumar et al. 2016). For the phylogenetic analyses, a matrix for filamentous fungi and another for yeasts was constructed. Bayesian inference (BI) and maximum likelihood (ML) analyses were conducted with MrBayes on XSEDE and RAxML-HPC BlackBox (8.2.8), respectively, both in the CIPRES science gateway (https://www.phylo.org/) as described by Silva et al. (2019). The best nucleotide model for each matrix was obtained using MrModelTest v.2.3 (Nylander 2004) which proposed GTR+I+G for both datasets. The ITS sequences were deposited in GenBank (Table 1).
Statistical analysis
To evaluate the species of endophytic fungi between areas, analyses of cultivars and phenological stages were carried out of the diversity index of Shannon-Weiner and richness per sample. The richness (S) was the number of species in each sample and the Shannon-Weiner diversity index was calculated through to the equation H′ = -Σ(Xi/Xo) × log(Xi/Xo), Xi = number of endophytic isolates of each species and Xo = total number of endophytic isolates of all species. To test whether there was statistical significance between richness and diversity, the Kruskal-Wallis non-parametric test was used (p ≤ 0.05). This test was used because the samples did not reach normality.
An indicator species analysis was performed to verify which species are related to each area, phenological stages and sorghum cultivars. The indicator value (IndVal) was calculated and the significance was determined by the Monte Carlo test. The species with IndVal ≥ 25% and p < 0.05 were considered to be indicators. Venn diagrams were constructed to verify the shared and exclusive species between forest types and cultivars using the tool http://bioinformatics.psb.ugent.be/webtools/Venn/.
Number of isolates and species richness of the samples were modelled using generalized linear models, using rainfall, temperature, pre- and post-blooming and sorghum variety as explanatory variables. Number of isolates and richness are count data, then Poisson regressions were performed, but signs of overdispersions were detected (i.e. residual deviance much larger than the degrees of freedom) affecting the interpretability of the models coefficients, which is resulted from the response variable variance to be larger than the mean (up to 4.7 times larger in the acquired data) (Dean and Lawless 1989). To circumvent the problems caused by overdispersion, negative-binomial models were constructed for count data instead (Gardner et al. 1995). Collinearity was assessed with variance inflation factor analysis, and high values (higher than 4) between rainfall and area, and between pre-post blooming and mean temperature were observed. Rainfall was chosen instead of the area, and pre- and post-blooming instead of temperature, as mean temperature variation in the studied areas are very low (23.9–25.7 °C), and probably biologically with low significance. Goodness-of-fit of the negative binomial models were performed using Pearson’s chi-squared test of the probability of the residual deviance, given the degrees of freedom. The best model with a good fit were chosen based on the lowest Akaike Information Criterion (AIC) value.
To infer if the endophytic fungal community composition changes is related to any of the measured variables, a principal coordinate analysis (PCoA) was performed, with the resulting sample scores correlated with the measured variables. Numerical variables were fitted to the sample scores as vectors, and categorical variables as factors using the “envfit ()” function of the “vegan” package (Oksanen et al. 2016). The p value for each variable was accessed with 999 permutations. The test of indicator species was performed with the command “multipatt” function in the “indicspecies” package (Cáceres and Legendre, 2009) using RStudio (R Core Team, 2017).
Results
Fungal isolation rate and identification
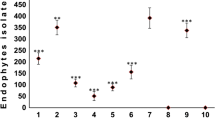
From 581 endophytes isolated, 477 were filamentous fungi and 104 were yeasts. Members of the phylum Ascomycota (540 isolates, 93%) were dominant, followed by members of Basidiomycota (35 isolates, 6%) and Mucoromycota (6 isolates, 1%) which had fewer representatives. The ascomycetous isolates were represented by four classes, Dothideomycetes (185 isolates, 31.84%), Eurotiomycetes (32 isolates, 5.51%), Saccharomycetes (69 isolates, 11.88%), Sordariomycetes (254 isolates, 43.72%) and 11 orders, Botryosphaeriales (13 isolates) Capnodiales (6), Diaporthales (13), Eurotiales (33), Glomerellales (107), Hypocreales (86), Magnaporthales (2), Pleosporales (166), Saccharomycetales (69), Sordariales (5), Xylariales (37) and members classified as Ascomycota incertae sedis (3 isolates). The basidiomycetous endophytes were represented by three classes, Microbotryomycetes (12 isolates, 2%), Tremellomycetes (20 isolates, 3.4%), Ustilaginomycetes (3 isolates, 0.5%), distributed in Sporidiobolales (12 isolates), Tremellales (20) and Ustilaginales (3), and the mucoromycetous endophytic fungi were represented by Mucorales (6 isolates, 1%) (Table 1). The largest number of endophytes (430 isolates, 74%) was found in sorghum cultivated in the Atlantic Forest area while in the Caatinga Forest 151 (26%) fungal were recovered. The endophytic species most abundant were Curvularia hawaiiensis (77 isolates, 13.25%), Colletotrichum sp. 2 (68 isolates, 11.7%) and E. sorghinum (68 isolates, 11.7%). From all species found, 34 were represented by one, two or three isolates and were considered as rare isolation.
Diversity and species composition
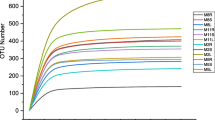
Fifty-five endophytic species distributed in 37 genera, only 16 taxa were shared between the two crop areas (Fig. 1A). Greater richness of endophytic fungi was found in sorghum leaves from Atlantic Forest (46 species) compared to the Caatinga (25 species) and differed significantly between areas (p < 0.01). We also observed statistical differences in the diversity of endophytic fungi in sorghum leaves between the different areas (p < 0.01), the Atlantic Forest showed greater diversity (H′ = 2.68) compared to the Caatinga (H′ = 2.51).
We detected different species indicator for some studied area; nine species were associated with the sorghum cultivation in the Atlantic Forest area: Colletotrichum sp. 2, Epicoccum sorghinum, Curvularia sp., Colletotrichum sp. 1, Fusarium thapsinum, Nigrospora oryzae, Phyllosticta capitalensis, Fusarium oxysporum and Amesia nigricolor (Table 2). While five species were considered indicators of sorghum cultivation in the Caatinga Forest area: Meyerozyma sp., Talaromyces pinophilus, Metschnikowia sp., Rhodosporidiobolus sp. and Acremonium pinkertoniae. Seven genera were considerate indicators of the Atlantic Forest: Colletotrichum, Curvularia, Epicoccum, Fusarium, Nigrospora, Phyllosticta and Mucor; five genera were indicators of the Caatinga which are Acremonium, Meyerozyma, Metscnikowia, Rhodosporidiobolus and Talaromyces.
When comparing the two cultivars in the study (IPA SF15 and IPA 2502), no difference in richness or diversity was observed (p > 0.05) between the two crops (IPA SF15: H′ = 2.85 and IPA 2502: H′ = 3.08). In general, a total of 38, 27, 16 e 15 taxa were present in the cultivars IPA 2502, IPA SF15 (Atlantic Forest), IPA 2502 e IPA SF15 (Caatinga), respectively. Higher number of unique species was found in the cultivar IPA 2502 in the Atlantic Forest area (Fig. 1B). Venn diagrams showed that the cultivars (IPA 2502 and IPA SF15) in Atlantic Forest shared exclusively 11 taxa; however, there were no taxa shared exclusively between cultivars (IPA 2502 and IPA SF15) in the Caatinga (Fig. 1B). Only four species were shared between all cultivars: Exserohilum rostratum, Fusarium dlaminii, Talaromyces pinophilus and Rhodosporidiobolus sp. Only the species Acremonium pinkertoniae was an indicator of the cultivar IPA 2502. Acremonium was indicative of the cultivar IPA 2502 and no genus was related to the cultivar IPA SF15.
There was no statistical difference (p > 0.05) between the average richness and diversity per sample between the phenological stages: pre-blooming (H′ = 3.17) and post-flowering stage (H′ = 2.48). However, indifferent of the site of cultivation, in the pre-blooming stage we observed a high richness of endophytic fungi. In relation to abundance, the Atlantic Forest showed a greater number of isolates in the post-blooming period, while in the Caatinga, greater abundance was found in the pre-blooming stage.
In the Atlantic Forest, a total of 114 endophytes were found during the host pre-blooming stage, while 316 were recorded during the host post-blooming stage. Although the pre-blooming stage had a lower number of isolates, the richness was higher (38 taxa in pre-blooming and 24 species in post-blooming). The Atlantic Forest during the pre-blooming stage exhibited as dominant species Colletotrichum sp. 1 and F. thapsinum, whereas in post-blooming Colletotrichum sp. 2, C. hawaiiensis and E. sorghinum were dominant species. On the other hand, endophytic species of the Caatinga area differ from the Atlantic Forest area, mainly because it had a larger number of endophytic yeasts (85 isolates, 56%). During the pre-blooming stage were found 17 species, wherein Metschnikowia sp. and Meyerozyma sp. were dominant species, whereas in post-blooming of the 14 species, A. pinkertoniae and T. pinophilus were dominant. For the pre-blooming stage, two species and Meyerozyma and Papiliotrema were considered indicator genera while for the post-blooming stage, six species were indicators and Epicoccum, Curvularia, Fusarium and Nigrospora were the associated genera (Table 2).
Analysis of environmental factors and endophytic fungal diversity
The regression models that best described the variation in species richness and number of isolates are presented in the Table 3. All constructed models are shown in the Supplementary material S1. There was no effect of cultivar types on endophytic species; however, rainfall had a positive effect on the number of isolates and species, whereas the post-blooming period had a positive effect only on the number of isolates (Fig. 2). This pattern was driven by filamentous fungi, when analysed separately it was possible to observe the effect of these variables, where rainfall and post-blooming period have a positive effect on the number of isolates (Fig. 2, Table 4). Nevertheless, endophytic yeast responded differently, with a lower number of species and isolates on higher rainfall and post-blooming period. Sorghum cultivars have no predictive power on the number of species and isolates on both groups of fungi.
The relationship between sample’s species composition is shown in the principal coordinate analysis (PCoA) ordination plots (Fig. 3). Excepting for sorghum cultivars, all variables are significantly related with the sample scores in the ordination. The stronger correlations are with rainfall followed by the blooming period (Table 5). The filamentous fungi communities displayed a similar pattern, whereas the yeast community composition seems to be affected only by the blooming period and temperature.
Principal coordinate analysis (PCoA) ordination of the endophytic fungi samples composition, based on the Jaccard’s dissimilarity. Statistically significant vectors and factors of the measured variables are displayed (Table 2)
Discussion
Characteristics of community composition and dominant groups
In the present study, the composition, diversity and distribution of the endophytic fungal community in sorghum leaves were investigated, correlating with environmental factors, phenological stage and cultivar. Members of three phyla, eight classes, fifteen orders, twenty-eight families, thirty-five genera and fifty-five species were identified, showing a rich diversity of endophytes fungi in sorghum leaves. The rich endophytic fungal community belonging to the phylum Ascomycota observed in the present study is according to previous studies on fungal endophytes in grasses and other plant species (Loro et al. 2012; Sánchez Márquez et al. 2010; García et al. 2013; Zida et al. 2014; Santamaria et al. 2018, Ren et al. 2019, Kinge et al. 2019, Pambuka et al. 2021). Species of Dothideomycetes and Sordariomycetes were dominant in the present study; both were already found associated with species of Poaceae, including Sorghum (Crouch and Beirn 2009; Higgins et al. 2011). Members of these classes are cosmopolitan, and they are often found as endophytes, pathogens or saprobes in woody debris, decaying leaves or dung (Schoch et al. 2006; Hongsanan et al. 2017). Combinations of these niches suggest that it is possible that these fungi start their life cycle as endophytes and change their mode of life to a saprobic state when the plant dies (Schoch et al. 2006; Herre et al. 2007).
The isolation of 55 species distributed in fifteen taxonomic orders shows that sorghum harbours a rich and diverse endophytic mycobiota compared with data obtained in other studies in crop Sorghum (Zida et al. 2014; Rodrigues et al. 2018). The dominant fungal genera were Colletotrichum, Curvularia and Epicoccum. The presence of a large number of isolates shows to be a characteristic of the endophytic mycobiota of grasses (Neubert et al. 2006; Sánchez Márquez et al. 2010; Loro et al. 2012; Zida et al. 2014). Similar results were previously found by Zida et al. (2014) who studied S. bicolor in a semiarid region in Burkina Faso, Africa. Studies showed that Colletotrichum endophytic species are considered biocontrol agents, confer protective benefits to plants, reduce incidence of disease and increase fertility under nutritional conditions (Arnold et al. 2003; Mejía et al. 2008; Salunkhe et al. 2011; Hiruma et al. 2016; Khiralla et al. 2018; Bengyella et al. 2019, Casas et al. 2021). Although Colletotrichum and Curvularia species are pathogens known worldwide for a wide variety of hosts, mainly grass species (Manamgoda et al. 2011; Dean et al. 2012). At present study, it apparently does not cause disease in the host, suggesting that future studies are needed to better understand the ecological function of sorghum-fungus interaction.
Influence of environment on endophytic fungi
The results indicate that both factors (biotic and abiotic) drive the community of endophytic fungi in S. bicolor. In general, the abundance of filamentous fungi increases with higher rainfall and post-blooming period, whereas both abundance and richness of yeasts decreases. The contrasting response of yeasts and filamentous fungi community is probably an indication of niche partitioning. However, the introduction of different fungal species into a niche increases competition for nutrients, enzyme production and niche colonization, creating competitive conditions that may have been responsible for decreasing the community of endophytic yeasts (Joubert and Doty 2018). Both occupy the same plant tissue, but they present particular physiological properties and probably they are adapted to different environments, that is, present different responses for the plant physiology, water availability, and resource allocation during reproduction. Little information is known about the ecology of endophytic yeasts, and more studies are needed to elucidate in detail the relationship which they establish with plants (see Joubert and Doty 2018).
In the present study, the rainfall was the environmental factor that had determined the density and influenced the composition of the endophytic fungi community in sorghum leaves. High humidity conditions are more favourable to sporulation and fungal infection, so it is therefore likely that the greater rainfall present in the Atlantic Forest influence the increase of endophytic colonization associated with the host species. The precipitation and the level of endophytic colonization of leave tissues are positively correlated (Rodrigues 1994; Wilson and Carroll 1994; Rajagopal and Suryanarayanan 2002; Mishra et al. 2012, Li et al. 2018). Precipitation could possibly also be associated with the greater richness and diversity of endophytic fungi in crops in the Atlantic Forest. For example, Naik et al. (2008) studying medicinal plants collected in southern India obtained more endophytes with increasing rainfall during the rainy season than during the summer season. Suryanarayanan et al. (2002) verifying the diversity of endophytic fungi in four different types of tropical forests under a precipitation gradient recovered a higher number of fungal isolates during the rainy season. Massimo et al. (2015) evaluating the endophytic fungi distributions associated with tree and shrub leaf tissues of the Sonoran Desert (Arizona) observed that the isolation frequency increased in the period characterized by high humidity and rainfall. On the other hand, when the plant is subjected to a narrow band of water potential, a wide range of responses is displayed at the molecular and cellular levels, such as inhibition of shoot growth and closure of the stomata as an attempt to maintain favourable water content in the tissues by greater possible time (Martínez-Vilalt and Garcia-Forner 2016, Lata et al. 2018). This regulation in the opening of the stomatal pore may restrict the endophytic fungal infection during the dry period.
The detection of sorghum indicator genera and species for the study areas shows that the differences in environmental conditions, including temperature and rainfall, as well as the host physiology favored the distribution of these species. We observed that some species occurred exclusively in a cultivated area, such as Colletotrichum sp. 1 and Epicoccum sorghinum that were indicator species for sorghum crop in the Atlantic Forest and Talaromyces pinophilus for sorghum crop in the Caatinga area. Similarly to our study, the genera Colletotrichum and Phyllosticta were also reported as indicators for Atlantic Forest ecosystem (brejo de altitude), while Talaromyces was indicative for Caatinga, by Pádua et al. (2019) who investigated the fungal endophyte community of the leaves of Myracrodruon urundeuva in Brazil. Olmo-Ruiz et al. (2014) also reported Colletotrichum sp. as indicator species for Elaphoglossum doanense in the tropical moist forest, Costa Rica. Although the species belonging to these genera are found colonizing a variety of hosts in Poaceae (Wong and Hyde, 2001; Sánchez Márquez et al. 2007; Angelini et al. 2012; Manamgoda et al. 2013), the preference of these taxa can be correlated with the ideal conditions that the environment provides for growth and the function of these fungi in each environment.
Influence of host including species and flowering period
Although environmental variation between sites has a very large influence on endophytic communities, genetic differences within the same plant species can modulate fungal diversity (Ahlholm et al. 2002). However, in the present study, there was no effect of the different sorghum cultivars on the community of endophytic fungi. In despite of some species were observed only in one of the cultivars. This result was in agreement with other studies (Pancher et al. 2012; Whitaker et al. 2018; Oliveira et al. 2021). Further studies are needed to explore the ecological and evolutionary consequences of host species on the composition of the endophyte community.
We observe that the increase in the filamentous fungi abundance is correlated with the phenological stages of the plant. This result is consistent with the observations made by Martins et al. (2021) that in cultivars of Olea europaea an increase is recorded in the abundance of fungi along the different phenological stages. Similarly, Schlemper et al. (2018) found growth stage as a dominant factor determining the fungal community in the sorghum rhizosphere. We suggest that the influence of the phenological stage at fungal community composition occurs due to the better habitat condition with the physiological changes of the sorghum, aging of the leaf, decrease of the content of antifungal substances and increase of microscopic wounds. In addition, leaf chemistry also may influence endophytic fungal infection (Arnold and Herre 2003). Plants produce different chemical exudates during the different growth stages (Berg and Smalla 2009), which may have greater effects on the fungal community. Zahn and Amend (2019) suggested that fungi are responsible for directly modifying the phenology of host’s flowering and are capable of producing abundant secondary metabolites that may act on flowering phenology. Other researchers suggested that endophytic fungi are effective in altering hormone levels and controlling stomatal behaviour and osmotic adjustment, improving turgor maintenance (Malinowski and Belesky 2000; Mandyam and Jumpponen 2005).
Despite the growing studies of host interactions with the endophytic fungal assembly, it is not clear yet how the plants can select the endophytic mycobiota over biotic and abiotic conditions (García et al. 2013). The present study revealed that endophytic fungal communities are driven by the plant growth stages and rainfall, showing that several factors modulate the composition of the endophytic fungal community in sorghum leaves. Although the cultivar did not influence the endophytic community, the interaction between endophyte-host needs to be studied in detail to better understand the biology and ecology of these fungi associated with sorghum crops and other economically important plants. Our results improve our understanding of the dominant factors that influence the community of endophytic fungi in ecologically and economically important grass species. It is suggested that future studies test the biocontrol potential of these fungi and the potential to promote the growth of plants such as sorghum. Furthermore, these taxa can be used in future studies considering their agricultural and biotechnological potential.
Data availability
The datasets generated during the current study are available in the Genbank repository.
References
Araújo WL, Lima AOS, Azevedo JL, Marcon J, Sobral JLP (2002) Manual: isolamento de microrganismos endofíticos. ESALQ, Piracicaba, p 86
Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA (2003) Fungal endophytes limit pathogen damage in a tropical tree. PNAS 100:15649–15654. https://doi.org/10.1073/pnas.2533483100
Arnold AE, Herre EA (2003) Canopy cover and leaf age affect colonization by tropical fungal endophytes: ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia 95:388–398. https://doi.org/10.1080/15572536.2004.11833083
Angelini P, Rubini A, Gigante D, Reale L, Pagiotti R, Venanzoni R (2012) The endophytic fungal communities associated with the leaves and roots of the common reed (Phragmites australis) in Lake Trasimeno (Perugia, Italy) in declining and healthy stands. Fungal Ecology 5:683–693. https://doi.org/10.1016/j.funeco.2012.03.001
Astoreca AL, Emateguy LG, Alconada TM (2019) Fungal contamination and mycotoxins associated with sorghum crop: its relevance today. Eur J Plant Pathol 155:381–392. https://doi.org/10.1007/s10658-019-01797-w
Ahlholm JU, Helander M, Henriksson J, Metzler M, Saikkonen K (2002) Environmental conditions and host genotype direct genetic diversity of Venturia ditricha, a fungal endophyte of birch trees. Evolution 56:1566–1573
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13. https://doi.org/10.1111/j.1574-6941.2009.00654.x
Bezerra JDP, Santos MGS, Barbosa RN, Svedese VM, Lima DMM, Fernandes MJS, Gomes BS, Paiva LM, Almeida-Cortez JS, Souza-Motta CM (2013) Fungal endophytes from cactus Cereus jamacaru in Brazilian tropical dry forest: a first study. Simbiose 60:53–63. https://doi.org/10.1007/s13199-013-0243-1
Bengyella L, Iftikhar S, Nawaz K, Fonmboh DJ, Yekwa EL, Jones RC, Njanu YMT, Roy P (2019) Biotechnological application of endophytic filamentous Bipolaris and Curvularia: a review on bioeconomy impact. World J Microbiol Biotechnol 35:69. https://doi.org/10.1007/s11274-019-2644-7
de Carvalho AL, Menezes RSC, Nóbrega RS, Pinto AS, Ometto JPHB, Randow C, Giarolla A (2015) Impact of climate changes on potential sugarcane yield in Pernambuco, Northeastern region of Brazil. Renew Energy 78:26–34. https://doi.org/10.1016/j.renene.2014.12.023
Casas LL, Almeida LND, Pereira JO, Costa Neto PDQ, Azevedo JLD (2021) Colletotrichum siamense, a mycovirus-carrying endophyte, as a biological control strategy for anthracnose in guarana plants. Braz Arch Biol Technol 64. https://doi.org/10.1590/1678-4324-2021200534
Cáceres MD, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574
Crouc JA, Beirn LA (2009) Anthracnose of cereals and grasses. Fungal Divers 39:19–44
Collinge DB, Jensen B, Jørgensen HJ (2022) Fungal endophytes in plants and their relationship to plant disease. Curr Opin Microbiol 69:102177
Choi HW, Hong SK, Lee YH, Yoon YN (2021) First report of Colletotrichum sublineola causing Anthracnose on Sorghum bicolor in South Korea. Plant Disease 105:05–1559. https://doi.org/10.1094/PDIS-03-20-0637-PDN
da Silva FMC, Barbosa LCF (2017) Impact of human activities on the Caatinga. In: Silva JMC, Leal IR, Tabarelli M (eds) Caatinga the largest Tropical Dry Forest Region in South America. Springer, Switzerland, pp 359–368
da Silva KJ, de Armas RD, Soares CRFS, Ogliari JB (2016) Communities of endophytic microorganisms in different developmental stages from a local variety as well as transgenic and conventional isogenic hybrids of maize. World J Microbiol Biotechnol 32:189. https://doi.org/10.1007/s11274-016-2149-6
Dean C, Lawless JF (1989) Tests for detecting overdispersion in Poisson regression models. J Am Stat Assoc 84:467–472. https://doi.org/10.1080/01621459.1989.10478792
Dean R, Kan VJAL, Pretorius ZA, Hammond-Kosack KE, Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD (2012) Review the top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430. https://doi.org/10.1111/J.1364-3703.2011.00783.X
Domsch KH, Gams W, Anderson TH (1993) Compendium of soil fungi. Volume 1. Academic press, London
Durães FOM (2011) Sorgo sacarino: desenvolvimento de tecnologia agronômica. Agroenergia em Revista 3:7
Dalal JM, Kulkarni NS (2014) Population variance and diversity of endophytic fungi in Soybean (Glycine max (L) Merril). Res Rev J Bot Sci 3:33–39
Del Olmo-Ruiz M, Arnold AE (2014) Interannual variation and host affiliations of endophytic fungi associated with ferns at La Selva, Costa Rica. Mycologia 106:8–21. https://doi.org/10.3852/13-098
Dastogeer KM (2018) Influence of fungal endophytes on plant physiology is more pronounced under stress than well-watered conditions: a meta-analysis. Planta 248:1403–1416
Ellis MB (1971) Dematiaceous hyphomycetes. CMI, Kew
Ellis MB (1976) More dematiaceous hyphomycetes. CMI, Kew
Gardner W, Mulvey EP, Shaw EC (1995) Regression analysis of counts and rates. Quant Meth Psych 118:392–404
García E, Alonso Á, Platas G, Sacristán S (2013) The endophytic mycobiota of Arabidopsis thaliana. Fungal Divers 60:71. https://doi.org/10.1007/s13225-012-02190
Giauque H, Hawkes CV (2013) Climate affects symbiotic fungal endophyte diversity and performance. Am J Bot 100:1435–1444
Harman GE, Uphoff N (2019) Symbiotic root-endophytic soil microbes improve crop productivity and provide environmental benefits. Scientifica. https://doi.org/10.1155/2019/9106395
Herre EA, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Butler A, Bael SAV (2007) Ecological implications of antipathogen effects of tropical fungal endophytes and mycorrhizae. Ecology 88:550–558. https://doi.org/10.1890/05-1606
Higgins KL, Coley PD, Kursar TA, Arnold AE (2011) Culturing and direct PCR suggest prevalent host generalism among diverse fungal endophytes of tropical forest grasses. Mycologia 103:247–260. https://doi.org/10.3852/09-158
Hiruma K, Gerlach N, Sacristán S, Nakano RT, Hacquard S, Kracher B, Neumann U, Ramírez D, Bucher M, Connell RJO, Schulze-Lefert P (2016) Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status-dependent. Cell 165:464–474. https://doi.org/10.1016/j.cell.2016.02.028
Hongsanan S, Maharachchikumbura SSN, Hyde KD, Samarakoon MC, Jeewon R, Zhao Q, Al-Sadi AM, Bahkali AH (2017) An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers 84:25–41. https://doi.org/10.1007/s13225-017-0384-2
Fernandes MF, Queiroz LP (2018) Vegetação e flora da Caatinga. Ciência e Cultura 70:51–56
Foreign Agricultural Service, USDA (2019) Grain world markets and trade. Foreign Agricultural Service, USDA, Washington, DC
Joubert PM, Doty SL (2018) Endophytic yeasts: Biology, ecology and applications. In: Pirttilä A, Frank A (eds) Endophytes of Forest Trees. Forest Sci. https://doi.org/10.1007/978-3-319-89833-9_1
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Kinge TR, Cason ED, Valverde Portal Á, Nyaga M, Gryzenhout M (2019) Endophytic seed mycobiome of six sorghum (Sorghum bicolor) cultivars from commercial seedlots using an Illumina sequencing approach. Mycosphere 10:739–756. https://doi.org/10.5943/mycosphere/10/1/16
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Khiralla A, Spina R, Saliba S, Laurain-Mattar D (2018) Diversity of natural products of the genera Curvularia and Bipolaris. Fungal Biol Rev 33:101–112. https://doi.org/10.1016/j.fbr.2018.09.002
Köppen W (1923) Die Klimate der Erde-Grundriss der Klimakunde. Walter de Gruyter and Co., Berlin, Leipzig, p 369
Lata R, Chowdhury S, Gond SK, White JF Jr (2018) Induction of abiotic stress tolerance in plants by endophytic Microbes. Lett Appl Microbiol 66:268–276. https://doi.org/10.1111/lam.12855
Li X, Wang J, Zhang S, Wang H, Li X, Li X, Zhang H (2018) Distribution of fungal endophytes in roots of Stipa krylovii across six vegetation types in grassland of northern China. Fungal Ecol 31:47–53. https://doi.org/10.1016/j.funeco.2017.11.001
Loro M, Valero-Jiménez CA, Nozawac S, Márquez LM (2012) Diversity and composition of fungal endophytes in semiarid Northwest Venezuela. J Arid Environ 85:46–55. https://doi.org/10.1016/j.jaridenv.2012.04.009
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Science 40:923–940. https://doi.org/10.2135/cropsci2000.404923x
Mandyam K, Jumpponen A (2005) Seeking the elusive function of the root colonising dark septate endophytic fungi. Stud Mycol 53:173–189. https://doi.org/10.3114/sim.53.1.173
Massimo NC, Arendt KR, Riddle JM, Furr SH, Steen C, U’Ren JM, Sandberg DC, Arnold AE (2015) Fungal endophytes in aboveground tissues of desert plants: infrequent in culture, but highly diverse and distinctive symbionts. Microb Ecol 70:61–76. https://doi.org/10.1007/s00248-014-0563-6
Martínez-Vilalta J, Garcia-Forner N (2016) Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant Cell Environ 40:962–976. https://doi.org/10.1111/pce.12846
Manamgoda DS, Cai L, Bahkali AH, Chukeatirote E, Hyde KD (2011) Cochliobolus: an overview and current status of species. Fungal Divers 51:3–42. https://doi.org/10.1007/s13225-011-0139-4
Manamgoda DS, Udayanga D, Cai L, Chukeatirote E, Hyde KD (2013) Endophytic Colletotrichum from tropical grasses with a new species C. endophytica. Fungal Diversity 61:107–115. https://doi.org/10.1007/s13225-013-0256-3
Martins F, Cameirão C, Mina D, Benhadi-Marín J, Pereira JA, Baptista P (2021) Endophytic fungal community succession in reproductive organs of two olive tree cultivars with contrasting anthracnose susceptibilities. Fungal Ecology 49:101003. https://doi.org/10.1016/j.funeco.2020.101003
Mejía LC, Rojas EI, Maynard Z, Bael SV, Arnold AE, Hebbar P, Samuels GJ, Robbins N, Herre EA (2008) Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biological Control 46:4–14. https://doi.org/10.1016/j.biocontrol.2008.01.012
Menz MA, Klein RR, Mulle JE, Obert JA, Unruh NC, Klein PE (2002) A high-density genetic map of Sorghum bicolor (L.) Moench based on 2926 AFLP, RFLP and SSR markers. Plant Mol Biol 48:483–499. https://doi.org/10.1023/A:1014831302392
Mishra A, Gond SK, Kumar A, Sharma VK, Verma SK, Kharwar RN, Sieber TN (2012) Season and tissue type affect fungal endophyte communities of the Indian medicinal plant Tinospora cordifolia more strongly than geographic location. Microb Ecol 64:388–398. https://doi.org/10.1007/s00248-012-0029-7
Neubert K, Mendgen K, Brinkmann H, Wirsel SGR (2006) Only a few fungal species dominate highly diverse mycofloras associated with the common reed. Appl Environ Microbio 7(2):1118–1128. https://doi.org/10.1128/AEM.72.2.1118-1128.2006
Naik BS, Shashikala J, Krishnamurthy YL (2008) Diversity of fungal endophytes in shrubby medicinal plants of Malnad region, Western Ghats, Southern India. Fungal Ecol 1:89–93. https://doi.org/10.1016/j.funeco.2008.05.001
Nylander JAA (2004) MrModeltest 2.2. Computer program and documentation distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala
Oliveira RJV, Bezerra JL, Lima TEF, da Silva GA, Cavalcanti MADQ (2016) Phaeosphaeria nodulispora, a new endophytic coelomycete isolated from tropical palm (Cocos nucifera) in Brazil. Nova Hedwigia 103(1-2):185–192
Oliveira RJVD, Sousa NMFD, Pinto Neto WDP, Bezerra JL, Silva GAD, Cavalcanti MADQ (2021) Seasonality affects the community of endophytic fungi in coconut (Cocos nucifera) crop leaves. Acta Bot Brasilica 34:704–711. https://doi.org/10.1590/0102-33062020abb0106
Oksanen J, Blanchet, FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2016) Vegan: community Ecology Package. E R package version 2.3–4. http://CRAN.R-project.org/package¼vegan.
Pancher M, Ceol M, Corneo P, Longa C, Yousaf S, Pertot I, Campisano A (2012) Fungal endophytic communities in grapevines (Vitis vinifera L.) respond to crop management. Appl Environ Microbiol 78:4308–4317
Pambuka GT, Kinge TR, Ghosh S, Cason ED, Nyaga MM, Gryzenhout M (2021) Baseline data of the fungal phytobiome of three sorghum (Sorghum bicolor) cultivars in South Africa using targeted environmental sequencing. J Fungi 7:978. https://doi.org/10.3390/jof7110978
Pádua APSLD, Freire KTLDS, Oliveira TGLD, Silva LFD, Araújo-Magalhães GR, Agamez-Montalvo GS, Silva IR, Bezerra JDP, Souza-Motta CMD (2019) Fungal endophyte diversity in the leaves of the medicinal plant Myracrodruon urundeuva in a Brazilian dry tropical forest and their capacity to produce L-asparaginase. Acta Bot Brasilica 33:39–49. https://doi.org/10.1590/0102-33062018abb0108
Pereira CMR, da Silva DKA, de Almeida Ferreira AC, Goto BT, Maia LC (2014) Diversity of arbuscular mycorrhizal fungi in Atlantic Forest areas under different land uses. Agric Ecosyst Environ 185:245–252. https://doi.org/10.1016/j.agee.2014.01.005
Pereira PDC, Silva TG, Zolnier S, Silva SM, MJD S (2017) Water balance in soil cultivated with forage cactus clones under irrigation. Rev Caatinga 30:776–785. https://doi.org/10.1590/1983-21252017v30n326rc
Pieterse Z, Aveling TA, Jacobs A, Cowan DA (2018) Seasonal variability in fungal endophytes from Aizoaceae plants in the Succulent Karoo biodiversity hotspot, South Africa. J Arid Environ 156:19–26. https://doi.org/10.1016/j.jaridenv.2018.05.004
Proietti I, Frazzoli C, Mantovani A (2015) Exploiting nutritional value of staple foods in the world’s semi-arid Areas: Risks, benefits, challenges and opportunities of sorghum. Healthcare 3:172–193. https://doi.org/10.3390/healthcare3020172
Poudel B, Purushotham N, Jones A, Nasim J, Adorada DL, Sparks AH, Vaghefi N (2022) The first annotated genome assembly of Macrophomina tecta associated with charcoal rot of sorghum. Genome Biol Evol 14:6–081. https://doi.org/10.1093/gbe/evac081
Quach QN, Thrasher T, Kowalski KP, Clay K (2022) Fungal endophyte effects on invasive Phragmites australis performance in field and growth chamber environments. Fungal Ecol 57:101153. https://doi.org/10.1016/j.funeco.2022.101153
Rajagopal K, Suryanarayanan TS (2002) Isolation of endophytic fungi from leves of neem (Azadirachata indica A. Juss). Curr Sci 78:1375–1378
R CoreTeam (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ren F, Dong W, Sun H, Yan DH (2019) Endophytic mycobiota of Jingbai pear trees in North China. Forests 10:260. https://doi.org/10.3390/f10030260
Rodrigues RT, Silva MMS, Oliveira DM, Simplício JB, Costa CMC, Siqueira VM (2018) Endophytic fungi from Sorghum bicolor (L.) Moench: Influence of genotypes and crop systems and svaluation of antimicrobial Activity. J Agric Sci Technol 8:267–277. https://doi.org/10.17265/2161-6264/2018.05.001
Rodrigues KF (1994) The foliar fungal endophytes of the Amazonian palm Euterpe oleracea. Mycologia 86:376–385. https://doi.org/10.1080/00275514.1994.1202642
Rodrigues RR, Lima RA, Gandolfi S, Nave AG (2009) On the restoration of high diversity forests: 30 years of experience in the Brazilian Atlantic Forest. Biol Conserv 142:1242–1251
Santamaria O, Rodrigo S, Lledó S, Poblaciones MJ (2018) Fungal endophytes associated with Ornithopus compressus growing under semiarid conditions. Plant Ecol Divers 11:5–6. https://doi.org/10.1080/17550874.2018.1540020
Sánchez Márquez S, Bills GF, Domínguez Acuña L (2010) Endophytic mycobiota of leaves and roots of the grass Holcus lanatus. Fungal Divers 41:115–123. https://doi.org/10.1007/s13225-009-0015-7
Sánchez Márquez M, Bills GF, Zabalgogeazcoa I (2007) The endophytic mycobiota of the grass Dactylis glomerata.
Saikkonen K (2007) Review Forest structure and fungal endophytes. Fungal Biol 21:67–74. https://doi.org/10.1016/j.fbr.2007.05.001
Salunkhe RB, Patil SV, Patil CD, Salunke BK (2011) Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol Res 109:823–831. https://doi.org/10.1007/s00436-011-2328-1
Seifert KA, Morgan-Jones G, Gams W, Kendrick B (2011) The genera of Hyphomycetes, 2nd edn. CBS-KNAW Fungal Biodiversity Centre, Utrecht
Silva RM, Oliveira RJ, Bezerra JD, Bezerra JL, Souza-Motta CM, Silva GA (2019) Bifusisporella sorghi gen. et sp. nov. (Magnaporthaceae) to accommodate an endophytic fungus from Brazil. Mycol Prog 18:847–854. https://doi.org/10.1007/s11557-019-01494-2
Shakoor N, Nair R, Crasta O, Morris G, Feltus A, Kresovich S (2014) A Sorghum bicolor expression atlas reveals dynamic genotype-specific expression profiles for vegetative tissues of grain, sweet and bioenergy sorghums. BMC Plant Biology 14:35. https://doi.org/10.1186/1471-2229-14-35
Schlemper TR, van Veen JA, Kuramae EE (2018) Co-variation of bacterial and fungal communities in different sorghum cultivars and growth stages is soil dependent. Microb Ecol 76:205–214. https://doi.org/10.1007/s00248-017-1108-6
Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98:1041–1052. https://doi.org/10.1080/15572536.2006.11832632
Sutton BC (1980) The Coelomycetes. Fungi imperfect with pycnidia, acervuli and stromata. 1st ed Kew: CMI.
Schlegel M, Queloz V, Sieber TN (2018) The endophytic mycobiome of European ash and sycamore maple leaves–geographic patterns, host specificity and influence of ash dieback. Front Microbiol 9:2345
Suryanarayanan TS, Murali TS, Venkatesan G (2002) Occurrence and distribution of fungal endophytes in tropical forests across a rainfall gradient. Can J Bot 80:818–826. https://doi.org/10.1139/B02-069
Tabosa JN, Lima GS, Lira MA, Filho JJT, Brito ARMB (2002) Comportamento de cultivares de sorgo forrageiro em diferentes ambientes agroecológicos dos estados de Pernambuco e Alagoas. Revista Brasileira de Milho e Sorgo 1:47–45. https://doi.org/10.18512/1980-6477/rbms.v1n02p%25p
Taleon V, Dykes L, Rooney WL, Rooney LW (2012) Effect of genotype and environment on flavonoid concentration and profile of black Sorghum grains. J Cereal Sci 56:470475. https://doi.org/10.1016/j.jcs.2012.05.001
Venkateswaran K, Elangovan M, Sivaraj N (2019) Origin, domestication and diffusion of Sorghum bicolor. In Breeding Sorghum for diverse end uses (pp. 15-31). Woodhead Publishing.
Wilson D, Carroll GC (1994) Estudos de infecção de Discula quercina, um endófito de Quercus garryana. Mycologia 86:635–647. https://doi.org/10.1080/00275514.1994.12026463
White TJ, Bruns T, Lee S, Taylor L (1990) Amplification and direct sequencing of fungal RNA genes for phylogenetics. In: Gelfand MA, Sninsky DH, White JJ, Eds TJ (eds) Innis. Academic Press, PCR protocols. A guide to methods and applications, pp 315–322
Whitaker BK, Reynolds HL, Clay K (2018) Foliar fungal endophyte communities are structured by environment but not host ecotype in Panicum virgatum (switchgrass). Ecology 99:2703–2711. https://doi.org/10.1002/ecy.2543
Wong MK, Hyde KD (2001) Diversity of fungi on six species of Gramineae and one species of Cyperaceae in Hong Kong. Mycol Res 105:1485–1491. https://doi.org/10.1017/S0953756201004695
Zahn G, Amend AS (2019) Foliar fungi alter reproductive timing and allocation in Arabidopsis under normal and water-stressed conditions. BioRxiv 51:96–78. https://doi.org/10.1101/519678
Zida EP, Thio IG, Néya BJ, O’Hanlon K, Deleuran LC, Wulff EG, Lund OS, Shetty PH, Boelt B (2014) Fungal Endophytes of sorghum in Burkina Faso: occurrence and distribution. Afr J Microbiol Res 8:3782–3793. https://doi.org/10.5897/AJMR2014.7020
Funding
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing fellowships and financial support to this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design as well as to material preparation, data collection, and discussions. Molecular lab work was performed by Rejane M. F. Silva, Walter P.P., and Rafael J.V. Oliveira. Phylogenetic analyses were performed by Jadson D.P. Bezerra and Rafael J.V. Oliveira. The statistical analyses in this study were performed by Vitor Xavier de Lima and Larissa Cardoso Vieira. Macroscopic and microscopic analyses were performed by Rejane M. F. Silva, José L. Bezerra and Cristina M. Souza-Motta. José Nildo Tabosa was involved in planning and in the collection of material. Gladstone A. Silva designed and supervised the project, revised the manuscript. The manuscript was written by Rejane M. F. Silva. All authors commented on the manuscript as well as read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Claus Baessler
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, R.M.F., Neto, W.P., Oliveira, R.J. et al. Effect of climate and phenological stage on fungal endophytes community in Sorghum bicolor leaves. Mycol Progress 22, 19 (2023). https://doi.org/10.1007/s11557-023-01870-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-023-01870-z