Abstract
Tumor-derived extracellular vesicles (EVs) have a pleiotropic role in cancer, interacting with target cells of the tumor microenvironment, such as fibroblasts, immune and endothelial cells. EVs can modulate tumor progression, angiogenic switch, metastasis, and immune escape. These vesicles are nano-shuttles containing a wide spectrum of miRNAs that contribute to tumor progression. MiRNAs contained in extracellular vesicles (EV-miRNAs) are disseminated in the extracellular space and are able to influence the expression of target genes with either tumor suppressor or oncogenic functions, depending on both parental and target cells. Metastatic cancer cells can balance their oncogenic potential by expressing miRNAs with oncogenic function, whilst exporting miRNAs with tumor suppressor roles out of the cells. Importantly, treatment of cancer cells with specific natural and chemical compounds could induce the elimination of miRNAs with oncogenic function, thereby reducing their aggressiveness. In this review, we discuss the mechanisms by which EV-miRNAs, acting as miRNAs with oncogenic or tumor suppressor functions, could contribute to cancer progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

1 Introduction
Classic cell-to-cell communication is mediated by several molecules, such as cell adhesion components, soluble messengers or extracellular vesicles (EV). EVs are classified by different nomenclatures based on their size, intracellular origin and releasing mechanism. The two classes of EVs better characterized are exosomes and microvesicles [1]. Microvesicles are shed directly from plasma membranes, whereas exosomes are distinct from other EVs due to their origin, size, function, and composition [2]. The term “exosome” was first proposed in the 1980s to describe small (30 to 100 nm) vesicles of endosomal origin that were released during reticulocyte maturation [3]. These nanovesicles originate from multivesicular bodies (MVBs) of the endocytic system and are released into the extracellular space after fusion of MVBs with the plasma membrane. EVs are nanovesicles with a lipid bilayer, representing a complete molecular package containing a plethora of proteins including transmembrane receptors, membrane transporters, adhesion molecules, cytoskeletal and heat shock proteins, cytokines, growth factors, lipids, mRNAs, and miRNAs, able to influence the phenotype and biological functions of recipient cells [4]. EV cargo may be deregulated in disease and used as “snapshots” of their producer cells [5].
The proteins contained within EVs depend on the cell type and reflect the origin and state of the parental cells as outlined in the databases: ExoCarta [6], Vesiclepedia [7] and EVpedia [8]. Proteins involved in cancer pathogenesis, such as oncoproteins, have been found in tumor-derived vesicles. EVs are also able to eliminate molecules from cells, and these discarded cargoes can have consequences on neighboring cells [9].
Experimental evidence suggests that EV-miRNAs play a critical role not only in cancer cells, but also in the tumor microenvironment. EV-miRNAs constitute a bridge between cancer cells and the tumor microenvironment [10]. MiRNAs are selectively packaged in EVs and then transferred to recipient cells, neighboring or distant, to modulate gene expression [11]. Recently, Bayraktar et al. considered miRNAs as hormones, because they influence the phenotype of recipient cells and many distant tissues [12].
2 EVs: Roles in Cancer Progression
EVs play an important role in the different steps of cancer progression such as migration, angiogenesis, immune escape and pre-metastatic niche preparation, transferring oncogenic proteins and nucleic acids, such as mRNAs and miRNAs [5, 13].
Recent findings support that EVs are involved in tumor microenvironment modulation, promoting angiogenesis and preparing the metastatic niche [13, 14]. EVs are mediators between cancer cells and the surrounding vasculature to induce angiogenic responses [15,16,17]. Taverna et al. described that exosomes released from leukemia cells directly affect endothelial cells, modulating the neovascularization process [18]. It was also described that multiple myeloma-derived exosomes are able to induce angiogenesis in recipient endothelial cells [19].
EV-miRNAs released by cancer cells also contribute to formation of the metastatic niche through: I) suppression of an antitumor immune response, II) secretion of miRNAs with tumor-suppressor function and III) induction of epithelial-mesenchymal transition (EMT) [20, 21]. How tumor-derived EVs are able to target neighboring or distant cells, is not completely understood. Metastatic EV-mediated organotropism remains one of cancer’s greatest mysteries; cancer cells derived from a specific metastatic site displayed enhanced abilities to metastasize in preferential organs [22, 23]. Peinado et al. showed that an “exosomal protein signature” may determine the site of distant metastases in melanoma patients. The role of specific integrins present on tumor-derived exosomes, to guide exosomes to specific organs, is emerging [24]. Hoshino et al. analyzed the proteomic profile of exosomes isolated from 28 organ-specific metastatic cell lines. They reported that exosomes containing ITGαvβ5 bind preferentially to Kupffer cells, mediating liver tropism, whereas exosomes containing ITGα6β4 and ITGα6β1 bind lung-resident fibroblasts and epithelial cells, governing lung tropism [22].
In this review, we discuss EV-miRNA functions, focusing on their dual role in intercellular communication between tumor and host cells. The dual role of EV-miRNA involves exosomal shuttling of miRNAs with an oncogenic role, potentially affecting stromal cells to regulate angiogenesis, EMT and immune detection, or eliminating miRNAs with tumor suppressor function”., consequently increasing the expression of cellular oncogenes. Moreover, depending on the cellular origin and target cells, the same miRNA may simultaneously elicit both oncogenic and onco-suppressor functions.
There are different potentially non-exclusive hypotheses regarding how EV-miRNAs can contribute to cancer. Cancer cells use EVs as nano-shuttles to release miRNAs with oncogenic and tumor-suppressor function, simultaneously spreading malignant properties to neighboring cells, maintaining and protecting the oncogenic potential of the cancer cells. Moreover, treatments with specific drugs might induce the elimination of miRNAs with oncogenic potential to reduce cancer cells aggressiveness. The role of various compounds in EV-miRNA sorting will be discussed in Section 5.
3 Selective miRNA Packaging into EVs
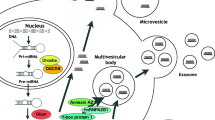
EV-mediated transfer of miRNAs is considered a novel genetic exchange mechanism between cells [25]. This idea has been described for Epstein-Barr virus-infected cells, in which secreted EVs transfer viral miRNAs into non-infected cells, leading to repression of virus-target genes [26]. A key question on secreted miRNAs concerns their stability in the circulation, despite the presence of ubiquitous ribonucleases [27]. Two possible theories suggest that secreted miRNAs could be stabilized by: I) their association with RNA-binding proteins, such as Argonaute 2 (AGO2), and II) protection by extracellular vesicle plasma membranes [28]. EV-miRNAs can post-transcriptionally modulate gene expression in recipient cells [29]. RNA polymerase II transcribes miRNAs as long primary miRNAs (pri-miRNAs), which are then processed by the nuclear RNAse Drosha into hairpin precursor miRNAs (pre-miRNAs). Pre-miRNAs are then transported into the cytoplasm, where the hairpin is cleaved, forming a double stranded mature miRNA. Dicer subsequently transfers the duplex to the AGO proteins, where one strand is integrated into the Ago protein containing RNA-induced silencing complex (RISC) [30, 31]. The guide strand of the duplex, usually starting with a 5’-Uracil, is preferentially loaded into Ago to regulate expression of target mRNAs. The passenger strand of the duplex starts with a 5’-Cytosine and is usually degraded. However, expression profiling shows that in some tissues and exosomes, both strands can be equally abundant [32]. It was demonstrated that loading of EV-miRNA can be independent of RISC, and can be mediated by other types of proteins. Villaroya-Beltri et al. identified short sequence motifs over-represented in miRNAs (EXO-motifs) through which heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) binds miRNAs and regulates their loading into exosomes (Fig. 1:1). Moreover, the data showed that directed mutagenesis of EXO-motifs inhibited miRNA cargo into exosomes [33]. Recently, the RNA binding protein Synaptotagmin-binding cytoplasmic RNA-interacting protein (SYNCRIP) has been identified as a component of the hepatocyte exosomal miRNA sorting machinery (Fig. 1:2). SYNCRIP directly binds to specific miRNAs enriched in exosomes, sharing a common extra-seed sequence (hEXO motif). SYNCRIP knockdown impairs sorting of miRNAs in exosomes, indicating a role for the hEXO motif in regulating miRNA localization [34].
miRNA formation and exosomal miRNA sorting mechanisms. Pri-miRNAs are processed by DROSHA into pre-miRNAs and exported into the cytoplasm through exportin 5. The DICER complex makes the last modification to create mature miRNAs. miRNAs are sorted inside the exosomes through 5 different methods: 1) Recognition of Exo-Domain by hnRNPA2B1: 2) hEXO motif by SYNCRIP; 3) 3’-UTR U-A recognition; 4) nsMase 2 protein; 5) AGO2 protein
MiRNA packaging occurs non-randomly, and specific miRNA populations are preferentially sorted into EVs [35]. Gibbings et al. described the existence of a selective sorting mechanism of miRNAs into exosomes, mediated by miRNA effector complexes coupled to multivesicular bodies [36]. Koppers-Lalic et al. showed that selective miRNA loading into exosomes is related to the adenylated and uridylated miRNA 3’-UTR isoforms [9] (Fig. 1:3). Kosaka et al. demonstrated that the overexpression of neural sphingomyelinase 2 (nsMase 2) leads to an increase in miRNA inside the exosomes and accordingly, its inhibition led to a decrease in EV-miRNA levels (Fig. 1:4). Understanding the molecular mechanism of this “on-demand system” should also shed light on novel approaches for cancer therapy [37]. Furthermore, AGO2, as discussed above, plays a key role in miRNA maturation and consequently in mRNA repression or degradation. A role for AGO2 was also described in miRNA sorting into exosomes (Fig. 1:5). The ability of AGO2 to drive miR-451, miR-150 and miR-142-3p in HEK293T derived-exosomes has been reported [38]. Recently, it was also demonstrated that the cytosolic proteins YB-1 and NSUN2 are possible mediators of the process for sorting particular mRNAs, recognizing specific motifs present in mRNAs enriched in exosomes [39].
Another key question is how secreted miRNAs, packaged in EVs, exert their biological functions in recipient cells. Various studies demonstrate that miRNAs delivered into target cells act as functional molecules to exert gene silencing through the same mechanism as endogenous miRNAs [40,41,42,43]. Alexander et al. suggested that individual miRNAs in EVs are transferred between cells in a functionally relevant manner. In order to demonstrate that miRNAs shuttled by EVs repressed gene expression in recipient cells by targeting a specific 3′-UTR sequence, the authors perform 3′-UTR luciferase reporter assays with binding-site mutant 3′-UTRs or using miRNA-mutant mimics. These analyses demonstrate that gene repression failed when EVs-miRNAs were unable to bind directly to the 3′-UTR [44].
Melo et al. also demonstrated that cancer exosomes mediate transcriptome alterations in target cells via RISC-associated miRNAs. The authors showed that breast cancer exosomal miRNAs are associated with the RISC Loading Complex (RLC) that induces a cell-independent capacity to process precursor microRNAs (pre-miRNAs) into mature miRNAs. Pre-miRNAs, along with Dicer, AGO2, and TRBP, are present in exosomes of cancer cells. In this manner, cancer exosomes mediate silencing of mRNAs to reprogram the target cell transcriptome [42]. Table 1 summarizes the EV-miRNAs with oncogenic or onco-suppressor functions, that play a role in tumor progression.
4 MiRNAs: Dual Role in Tumor Progression
4.1 Role of EVs As Transporters of miRNAs with Oncogenic Function
Valadi et al. described for the first time that miRNAs could be transferred between cells via exosomes [25]. Since 2008, several research groups observed that mature miRNAs were present in plasma and serum as cell-free circulating miRNAs or encapsulated in exosomes [59, 60]. Several studies have reported that EVs of different cellular origin contain a unique expression profile of mRNAs and miRNAs, reflecting the nature and even the state of producer cells [20, 61, 62]. MiRNA expression is frequently deregulated in tumors; a single miRNA can exploit both tumor-suppressive and oncogenic functions in different cellular contexts and its target genes can be selective for each cancer [46, 61].
4.1.1 EV-miRNAs and Immune System Inhibition
EVs transfer functional miRNAs that can modulate gene expression and impact the transcriptome of recipient cells [43, 45]. Several reports indicate that EVs are also efficient carriers of genetic information, including miRNAs, with a key role in immune modulation [63]. For example, miR-21 is involved in tumor-mediated immunosuppression [47]. In nasopharyngeal cancer-derived EVs, exosomal miR-21 induces interleukin (IL) 10 and B-cells that suppress CD8+ T-cell activity [64,65,66]. MiR-21, contained in exosomes released by melanoma cells [48, 67], promoted invasion and distant metastasis through the generation of myeloid-derived suppressor cells (MDSCs), a cell population characterized by immune regulatory activities [49, 50].
Moreover, miR-21 targets myeloid differentiation factor 88 (MyD88) and interleukin-1 receptor associated kinase 1 (IRAK1), two important regulatory checkpoints in the Toll-like receptor (TLR) signaling pathway, contributing to host immune system evasion. Exosomal miR-21 can also bind TLRs, such as murine TLR7 and human TLR8 in immune cells, inducing a TLR-mediated pre-metastatic inflammatory response that, in turn, leads to tumor growth and metastasis [51].
Other important miRNAs that are involved in immune response suppression are miR-9 and miR-222. MiR-9 is overexpressed in many cancers, where it exerts biological functions inhibiting the transcription of the MHC class I (MHCI) gene to prevent the recognition of tumor cells by the immune system [52]. It was also demonstrated that miR-9, identified as a pro-metastatic miRNA, is upregulated in exosomes of different breast cancer cell lines and is able to affect the properties of other cells, such as breast fibroblasts, enhancing the switch to a cancer associated fibroblast (CAF) phenotype, thus contributing to tumor growth [68]. miR-222 is also involved in immune system inhibition through targeting intracellular cell adhesion molecule 1 (ICAM-1) expressed on tumor cell surfaces. ICAM-1 binds to the lymphocyte function-associated antigen (LFA-1) inducing the optimal activation of cytotoxic T cells, which results in tumor cell lysis. MiR-222 was shown to down regulate the expression of ICAM-1, thus inhibiting the T cell lysis [69]. These reports indicate that exosomal miR-21, miR-9 and miR-222 could be considered as potential targets to inhibit the interaction between the immune system and tumor cells.
4.1.2 EV-miRNAs and Cancer Metastasis
EV-miRNAs can affect target cells of the tumor microenvironment, and thereby are involved in hypoxia, angiogenesis, and EMT to promote cancer metastasis.
Felicetti et al. demonstrated that melanoma cells are able to release exosomes enriched in miR-222, promoting the activation of several pathways involved in cell growth, apoptosis and angiogenesis induction. [54]. Under hypoxic conditions, multiple myeloma cells increase exosomal miR-135b release to promote an angiogenic response. As a result of the ability of miR-135b to bind the 3’-UTR of factor-inhibiting hypoxia-inducible factor-1 (FIH-1), endothelial cells receiving exosomal miR-135b had significantly increased HIF-1 alpha levels. This mechanism induced a hypoxic response and accelerated the angiogenic process [19].
Recently, it was reported that miR-126, a well-described miRNA with angiogenic properties, was actively sorted into chronic myelogenous leukemia- (CML) derived exosomes. Once released, CML-derived exosomal miR-126 is shuttled into endothelial cells (HUVECs), keeping its biological function in target cells. Increased levels of miR-126 in HUVECs, in turn, lead to a decrease of two targets involved in cancer progression, CXCL12 and VCAM1, negatively modulating CML cell motility and adhesion. This evidence supported the hypothesis that EV-miRNAs had an important role in tumor-endothelial cross-talk in the bone marrow microenvironment, potentially affecting disease progression [70]. Moreover, miR-105, classified as a miRNA with oncogenic potential and contained in metastatic breast cancer-derived exosomes, targeted the tight junction protein ZO-1 in endothelial cells and affected vascular permeability. In target cells, this miRNA destroyed tight junctions and the integrity of the endothelial barrier, inducing metastasis. Conversely, in highly metastatic tumors, miR-105 inhibition lead to a reduction in tumor invasiveness and restoration of vascular barrier functions [71]. Tominaga et al. described a new mechanism of brain metastasis mediated by EVs that triggers the destruction of the blood-brain barrier (BBB). Moreover, it has been reported that miR-181c contained in extracellular vesicles, promoted the destruction of the BBB through the abnormal localization of actin via the downregulation of its target gene, PDPK1. PDPK1 degradation by miR-181c leads to the downregulation of phosphorylated cofilin, which modulates actin dynamics. In vivo experiments demonstrate that systemic injection of brain metastatic cancer cell-derived EVs promoted brain metastasis of breast cancer cell lines [72].
Le et al. demonstrated that extracellular vesicles containing miR-200 promote breast cancer cell metastasis. MiR-200 could be transferred from metastatic to non-metastatic breast cancer cells, via extracellular vesicles, altering gene expression and promoting mesenchymal-to-epithelial transition [53]. Similarly, the transfer of exosomal miR-10b from metastatic breast cancer cells induced invasive properties in non-malignant cells [56]. Taken together, these findings indicated that tumor cells are able to release EVs containing miRNAs with oncogenic potential, to promote their metastatic behavior. For this reason, EVs are attractive candidates for clinical application as therapeutic targets in many cancers.
4.2 EVs Eliminate miRNAs with Tumor Suppressor Roles
Cancer cells actively promote their tumorigenic behavior by loading EVs with specific miRNAs and releasing them into the tumor microenvironment [55]. Experimental evidence suggests that EVs might dispose tumor-suppressor miRNAs that counteract metastatic progression. The possible intrinsic advantage for cancer cells is to eliminate miRNAs with tumor-suppressor function via EVs, to maintain and promote the intracellular tumorigenic potential.
Recently, several studies suggest that miRNAs with tumor-suppressor function, selectively packaged in EVs, contribute to coordinate increased tumorigenic potential, and activation of the metastatic cascade in different cancer models. Ohshima et al. demonstrated that the Let-7 miRNA family was downregulated in many solid cancers and secreted via EVs [73]. The Let-7 miRNAs function as tumor suppressor genes [74], targeting oncogenes, such as RAS and high mobility group A2 (HMGA2) [75]. Metastatic gastric cancer cells secreted the tumor suppressive Let-7 miRNA into the extracellular space via EVs, reducing the intracellular anti-tumorigenic capacity to maintain their tumorigenic and invasive behavior [73]. Similarly, Kanlikilicer et al. demonstrated that ovarian cancer cells discarded the EV-miRNA miR-6126, which acts as a tumor suppressor by directly targeting integrin β1, a key regulator of cancer cell metastases, thereby promoting their metastatic potential. The authors further demonstrate that the treatment of endothelial cells with a miR-6126 mimic, significantly reduced tube formation, as well as the invasion and migration capacity of ovarian cancer cells in vitro. Accordingly, high levels of miR-6126 in endothelial cells were associated with a longer survival of ovarian cancer patients [76]. Further supporting the notion that cancer cells eliminate miRNAs with tumor-suppressor function in order to promote invasion and metastasis, it was found that metastatic bladder carcinoma cells eliminate high levels of EV-miRNAs with tumor-suppressor roles, including miR-23b, miR-224 and miR-921, thereby abrogating their functions in inhibiting angiogenesis and lung metastasis [77].
It was also demonstrated that EVs can induce drug resistance. In particular, miR-145 and miR-34a were consistently secreted as passengers in EVs released by 5 fluorouracil (5FU)-resistant DLD-1/5FU cells compared to DLD-1 cells, after 5FU exposure. The intracellular level of miR-145 and miR-34a in cells sensitive to 5FU, was significantly increased after drug exposure [57]. Conversely, in cells resistant to 5FU, cellular miR-145 and miR-34a expression was markedly decreased and their EVs secretion was increased. This mechanism maintains low intracellular levels of miR-145 and miR-34a, contributing to drug resistance [58].
Overall, these data demonstrate that cancer cells promote their oncogenic potential through the selective elimination of miRNAs with tumor suppressor function, via EVs. Deregulation of EV-miRNAs could be indicative of tumor progression, suggesting that EVs-miRNAs could be used as biomarkers to diagnose early stage tumors and to monitor disease progression.
5 Involvement of External Stimuli in Selective EV-miRNAs Sorting: Focus on In Vitro and Pre-Clinical Studies
Recent scientific reports suggest that treatment of parental cells with various natural and chemical compounds can modulate selective miRNA sorting into exosomes. In this context, treatment of cells with different compounds may induce the elimination of miRNAs, thereby affecting the aggressiveness of cancer cells.
The natural and chemical compounds discussed in this section, with described effects on EV-miRNA sorting, are reported in Table 2.
Recently, it was demonstrated that treatment of CML cells with curcumin induced selective packaging of miR-21 into exosomes, leading to a decrease in miR-21 in CML parental cells both in vitro and in vivo. This, in turn, lead to an upregulation of PTEN, a well-known tumor suppressor gene and a decrease in CML cell growth, suggesting curcumin as a potential adjuvant agent in CML therapy [78]. Moreover, it has been reported that exosomes released by CML cells treated with curcumin were actively internalized into endothelial cells (HUVECs), where exosomal miR-21 performed its biological functions. Once internalized, exosomes derived from curcumin-treated CML cells attenuated the promotion of an angiogenic phenotype in HUVECs, mediated by untreated CML cell derived exosomes, also modulating the endothelial barrier organization. In particular, the endothelial barrier modulation was mediated by delivery of CML-derived exosomal miR-21 into HUVECs, targeting RHOB and pro-angiogenic proteins such as MARCKS [79].
A number of preclinical studies have demonstrated anticancer effects for natural compounds such as curcumin in various types of tumors [84]. Based on these promising preclinical results, several research groups have proceeded to test the antitumor effects of curcumin in clinical trials. Nevertheless, the poor bioavailability of this compound has been the major challenge for its clinical application. Despite the administration of curcumin at gram-level doses, plasma curcumin amount remain at low levels, insufficient to elicit any anticancer benefits of curcumin. This problem has been solved by the development of highly bioavailable forms of curcumin such as THERACURMIN®. It was demonstrated that with this compound, higher plasma curcumin levels can be achieved without increased toxicity in patients with pancreatic cancer [85]. Other natural elements such as sulforaphane (1-isothiocyanate-4-methylsulfinylbutane) contained in vegetables, regulate the expression of miRNA in breast cancer cells. Treatment with sulforaphane was found to increase exosomal miR-140 levels and decrease exosomal miR-29a and miR-21 levels in CD49f +/CD24- and ALDH1+ MCF10DCIS cells. In addition, sulforaphane decreased the expression of the cancer stem cell marker, ALDH1, and the formation of tumor spheres in these cells. These results indicate that sulforaphane can inhibit cancer-stem-like cells by modulating miRNA expression [86].
Currently, several clinical trials are evaluating natural compounds that may be useful for supplementation in different treatments and cancer management. One of these compounds is DHA (docosahexaenoic acid), whose anticancer properties have been demonstrated in vivo and in vitro. DHA is cytotoxic to tumor cells, but with little or no effects on normal cells. It was reported that DHA increases exosome secretion from breast cancer cell lines. In addition, the levels of exosomal miRNAs from DHA-treated tumor cells were altered. Specifically, let-7a, miR-21, miR-23b, miR-27b, and miR-320b levels were increased in exosomes from breast cancer cell lines, compared to normal breast cells. MiRNAs carried by the DHA-treated breast cancer cell exosomes are readily transferred to endothelial cells, inhibiting endothelial tube formation and suppressing angiogenic activity [87].
Moreover, Giallombardo et al. showed that exosomal miRNAs are useful for a follow-up analysis. The authors studied EGFR-mutated non-small cell lung cancer (NSCLC) patients during osimertinib (AZD9291) treatment, a third-generation tyrosine kinase inhibitor. In preliminary experiments, osimertinib treatment lead to an upregulation of miRNAs (miR-221-3p and miR-222-3p) with oncogenic function in exosomes isolated from patient plasma. Interestingly, this upregulation correlated with a good clinical outcome during the follow-up analysis, suggesting a selective exosomal disposal of miRNAs with oncogenic function during osimertinib treatment [80]. Osimertinib is a new irreversible EGFR inhibitor, effective against both EGFR-TKI inhibitor-sensitizing mutations and T790 M acquired resistance to earlier generation EGFR-TKIs [82, 83]. EGFR-mutated patients and EGFR wild-type patients with mutations in other genes can be naturally resistant to TKIs or develop resistance following treatments, leading to tumor progression. Zhao et al., demonstrated strong synergistic effects between second and third generation EGFR-TKIs and tumor suppressor miRNAs, to restore the efficacy of TKIs [88]. As a result of their ability to be internalized by target cells, EVs could also be a promising drug carrier candidate. It was also demonstrated that the administration of miR-34a induces TKI re-sensitization of NSCLC cells [81]. The tumor suppressor miR-34a is able simultaneously to repress about 30 oncogenes, as well as genes involved in tumor immune evasion, such as PD-L1and DGKζ [89,90,91], making it a promising drug target. It was demonstrated that exosomes released from hepatoblastoma cells contained miR-34a [92], suggesting that exosomes containing miR-34a could become a valid therapeutic approach in combination with TKI treatments [88]. Cortez et al. indicated that miR-34 family was associated with PD-L1 expression regulation; they showed that miR-34a bound to 3’UTR of PD-L1. Recently, a synthetic miR-34 (MRX34) has been discovered and examined, MRX34 application in NSCLC mouse model with PD-L1 expression resulted in a decrease of tumoral PD-L1 expression at protein and mRNA levels. Moreover, the co-administration of MRX34 and radiotherapy in a mouse model elevated the CD8+ T cell count and reduced tumor infiltration by radiation-induced macrophages and T-reg cells. This study suggests that the application of miR-34a treatment with standard therapy might represent a novel approach in cancer treatment [89]. Unfortunately, although miR-34 is considered a key regulator of multiple oncogenes, the Phase 1 trial investigating MRX34 in solid tumors was recently halted due to severe adverse effects (www.businesswire.com).
The modulation of EV-miRNA sorting is also described in cells of the tumor microenvironment, including endothelial cells. Bovy et al. reported that exosomes released by endothelial cells contributed to the antitumor response during breast cancer neoadjuvant chemotherapy via miRNA modulation. They showed an up regulation of exosomal miR-503 isolated from HUVECs in the presence of epirubicin and paclitaxel, compared to control cells. After internalization in breast cancer cells, endothelial cell-derived exosomes led to a modulation of miR-503 that, in turn, altered their proliferative and invasive capacities [60].
The modulation of EV-miRNA sorting can be also mediated by physical treatments such as radiation. It was described that exosomes released by a human normal embryonic lung fibroblast cell line (MRC-5) during X-ray radiation, transferred radiation-induced bystander effects to non-irradiated cells. X-ray radiation upregulates miR-21 in irradiated cells (IR cells) and an increase in miR-21 sorting into exosomes. After diffusion in the extracellular medium, exosomes derived from IR cells were taken up by non-irradiated cells, shuttling exosomal miR-21 into non-irradiated cells [93]. Taken together, these data suggest that the complex mechanism of miRNAs sorting into exosomes could be modulated by different stimuli, including anticancer drug therapies.
6 EVs in Clinical Approaches
Several exosomal miRNAs and proteins have been described as diagnostic and prognostic biomarkers that might be exploited as a source of specific biomarkers, because they reflect the pathological condition of the disorder [94, 95]. Exosomal miRNAs remain attractive in the field of biomarker discovery for disease monitoring and prognosis in cancer patients [96, 97].
MiR-21 was demonstrated to be enriched in EVs collected from the serum of glioblastoma patients, and expressed at higher levels in the serum of patients than in those of normal controls [61], highlighting the potential use of miRNAs as an effective biomarker. Strategies to interfere with the loading or delivery of tumor-promoting EV-miRNAs or to replenish tumor-suppressive miRNAs via EV delivery are currently under investigation. Currently, the major hurdles that need to be overcome include limitations in the study design and the technical challenges that remain.
In 2015, a review by the International Society for Extracellular Vesicles (ISEV) discussed the application of extracellular vesicles based therapeutics in clinical trials [98]. The translation of EVs into clinical therapies needs the classification of EV-based therapeutics in agreement with current regulatory outlines. The significant progress made in the EV research field has led to improved and standardized protocols for EVs isolation and storage, as well as amended methods, techniques and criteria for quality analyses of EV-based therapeutics [99]. Clinical trials using EVs as theranostic nanoparticles have been reported already in the early 2000s. The impact of exosomes, considered “diamonds in the rough” [100], on clinical research is demonstrated by several ongoing clinical trials (https://clinicaltrials.gov/). Among the clinical trials with exosomes, 20 studies investigate exosomes involved in neoplastic diseases (Table 3), in which the exosomes are studied as biomarkers for diagnosis prognosis, drug resistance, as devices for drug delivery or for liquid biopsy approaches. In these trials, the exosomes are also proposed as a screening modality and as a device for clinical evaluation.
EV-mediated horizontal transfer of miRNAs opens an exciting perspective for clinical and therapeutic approaches. EV-miRNAs are thought to be the predominant source of circulating miRNAs isolated from plasma or serum. An emerging idea is that detecting miRNAs in the exosome fraction isolated from plasma or serum can offer a higher quality and more consistent readout than “crude” investigation of plasma or serum samples [101]. A recent study by Eichelser et al. analyzed circulating EV-miRNAs from breast cancer patients in 50 breast cancer cases, and 12 healthy controls with matched serum and exosomes. They demonstrated that the levels of miR-101, miR-372 and miR-373 were significantly higher in cancer cases when detecting these miRNAs in RNA isolated from exosomes, but not in serum RNA preparations. In breast cancer patients and healthy donor women the relative exosomal serum concentrations of miR-101, miR-372 and miR-373 were higher than their cell-free levels, indicating that these miRNAs may predominantly circulate in exosomes in the peripheral blood. The serum levels of cell-free miR-101 and miR-373 could significantly differentiate between breast cancer and benign breast disease, indicating their potential diagnostic value [102]. Nowadays, the clinical translation is still limited by the lack of appropriate, scalable, and both cost- and time-effective nanotechnologies for the purification and loading of EVs. Further studies are needed for the proper application of EVs in clinical approaches [103].
7 Conclusions
EVs have a pleiotropic role in tumor progression and are considered as key drivers of the pro-tumorigenic dialog between the tumor mass and its microenvironment, by facilitating short- and long-distance communication [104]. These vesicles transfer biomolecules to distant sites in order to regulate the function of target cells, affecting several biological processes and promoting the interaction between different cells of the tumor microenvironment [105]. Several studies showed the importance of communication between cancer cells and their surroundings through EVs [106]. The discovery that EVs contain miRNAs indicates that they could be carriers of miRNAs specific for the tumor and can be used as non-invasive novel biomarkers and function as diagnostic, prognostic and predictive indicators of cancer [107]. Understanding the mechanisms by which EV-miRNAs act as miRNAs with either oncogenic or tumor suppressor functions, could contribute to creating new systems that modulate the sorting of EV-miRNAs to limit their effects on cancer progression. Identification and modification of the contents of cancer EVs may be useful in developing new diagnostic, preventive and therapeutic approaches, with potentially less invasive procedures [61]. The remaining goals of EV-based biomarker analysis include the significant reduction of sample complexity, when compared to whole body fluids and the reduction of invasiveness in a liquid biopsy scenario [95, 108].
References
Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–32.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20.
Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–25.
Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–88.
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241–4.
Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450.
Kim D-K, Kang B, Kim OY, Choi D-S, Lee J, Kim SR, et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. 2013;2.
Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8(6):1649–58.
Yang F, Ning Z, Ma L, Liu W, Shao C, Shu Y, et al. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol Cancer. 2017;16(1):148.
Bronisz A, Godlewski J, Chiocca EA. Extracellular vesicles and MicroRNAs: their role in Tumorigenicity and therapy for brain Tumors. Cell Mol Neurobiol. 2016;36(3):361–76.
Bayraktar R, Van Roosbroeck K, Calin GA. Cell-to-cell communication: microRNAs as hormones. Mol Oncol. 2017.
Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa M, Ikeda S-I, Kato T, et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8:14470.
Lobb RJ, Lima LG, Moller A. Exosomes: key mediators of metastasis and pre-metastatic niche formation. Semin Cell Dev Biol. 2017;67:3–10.
Corrado C, Flugy AM, Taverna S, Raimondo S, Guggino G, Karmali R, et al. Carboxyamidotriazole-orotate inhibits the growth of imatinib-resistant chronic myeloid leukaemia cells and modulates exosomes-stimulated angiogenesis. PLoS One. 2012;7(8):e42310.
Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7.
Mineo M, Garfield SH, Taverna S, Flugy A, De Leo G, Alessandro R, et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 2012;15(1):33–45.
Taverna S, Flugy A, Saieva L, Kohn EC, Santoro A, Meraviglia S, et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012;130(9):2033–43.
Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124(25):3748–57.
Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33(5):441–54.
Filipazzi P, Burdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol. 2012;22(4):342–9.
Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35.
Taverna S, Pucci M, Giallombardo M, Di Bella MA, Santarpia M, Reclusa P, et al. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci Rep. 2017;7(1):3170.
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107(14):6328–33.
Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–33.
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108(12):5003–8.
Tran N. Cancer Exosomes as miRNA factories. Trends Cancer. 2016;2(7):329–31.
Winter J, Diederichs S. Argonaute proteins regulate microRNA stability: increased microRNA abundance by Argonaute proteins is due to microRNA stabilization. RNA Biol. 2011;8(6):1149–57.
Yu X, Odenthal M, Fries JWU. Exosomes as miRNA Carriers: Formation-Function-Future. Int J Mol Sci. 2016;17(12).
Meijer HA, Smith EM, Bushell M. Regulation of miRNA strand selection: follow the leader? Biochem Soc Trans. 2014;42(4):1135–40.
Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13.
Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R, et al. The RNA-binding protein SYNCRIP is a component of the Hepatocyte Exosomal machinery controlling MicroRNA sorting. Cell Rep. 2016;17(3):799–808.
Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinform. 2015;13(1):17–24.
Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11(9):1143–9.
Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288(15):10849–59.
Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357.
Kossinova OA, Gopanenko AV, Tamkovich SN, Krasheninina OA, Tupikin AE, Kiseleva E, et al. Cytosolic YB-1 and NSUN2 are the only proteins recognizing specific motifs present in mRNAs enriched in exosomes. Biochim Biophys Acta. 2017;1865(6):664–73.
Chen X, Liang H, Zhang J, Zen K, Zhang C-Y. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–32.
Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan MLG, Karlsson JM, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–66.
Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–21.
Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–15.
Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321.
Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32(3–4):623–42.
Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Vol. 36, Cellular and molecular neurobiology. United States; 2016. p. 301–12.
Cereghetti DM, Lee PP. Tumor-derived Exosomes contain microRNAs with immunological function: implications for a novel Immunosuppression mechanism. MicroRNA (Shariqah, United Arab Emirates). 2014;2(3):194–204.
Gajos-Michniewicz A, Duechler M, Czyz M. MiRNA in melanoma-derived exosomes. Cancer Lett. 2014;347(1):29–37.
McClure C, Brudecki L, Ferguson DA, Yao ZQ, Moorman JP, McCall CE, et al. MicroRNA 21 (miR-21) and miR-181b couple with NFI-A to generate myeloid-derived suppressor cells and promote immunosuppression in late sepsis. Infect Immun. 2014;82(9):3816–25.
Chevolet I, Speeckaert R, Schreuer M, Neyns B, Krysko O, Bachert C, et al. Clinical significance of plasmacytoid dendritic cells and myeloid-derived suppressor cells in melanoma. J Transl Med. 2015;13:9.
Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110–6.
Gao F, Zhao Z-L, Zhao W-T, Fan Q-R, Wang S-C, Li J, et al. miR-9 modulates the expression of interferon-regulated genes and MHC class I molecules in human nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2013;431(3):610–6.
Le MTN, Hamar P, Guo C, Basar E, Perdigao-Henriques R, Balaj L, et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest 2014;124(12):5109–5128.
Felicetti F, De Feo A, Coscia C, Puglisi R, Pedini F, Pasquini L, et al. Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma. J Transl Med. 2016;14:56.
Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014;74(20):5758–71.
Bertoli G, Cava C, Castiglioni I. MicroRNAs: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5(10):1122–43.
Akao Y, Nakagawa Y, Hirata I, Iio A, Itoh T, Kojima K, et al. Role of anti-oncomirs miR-143 and -145 in human colorectal tumors. Cancer Gene Ther. 2010;17(6):398–408.
Akao Y, Khoo F, Kumazaki M, Shinohara H, Miki K, Yamada N. Extracellular disposal of tumor-suppressor miRs-145 and -34a via microvesicles and 5-FU resistance of human colon cancer cells. Int J Mol Sci. 2014;15(1):1392–401.
Jia Y, Chen Y, Wang Q, Jayasinghe U, Luo X, Wei Q, et al. Exosome: emerging biomarker in breast cancer. Oncotarget. 2017;8(25):41717–33.
Bovy N, Blomme B, Freres P, Dederen S, Nivelles O, Lion M, et al. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget. 2015;6(12):10253–66.
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6.
Mayers JR, Audhya A. Vesicle formation within endosomes: an ESCRT marks the spot. Commun Integr Biol. 2012;5(1):50–6.
Fernandez-Messina L, Gutierrez-Vazquez C, Rivas-Garcia E, Sanchez-Madrid F, de la Fuente H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell. 2015;107(3):61–77.
Ma G-J, Gu R-M, Zhu M, Wen X, Li J-T, Zhang Y-Y, et al. Plasma post-operative miR-21 expression in the prognosis of gastric cancers. Asian Pac J Cancer Prev. 2013;14(12):7551–4.
Miao B-P, Zhang R-S, Li M, Fu Y-T, Zhao M, Liu Z-G, et al. Nasopharyngeal cancer-derived microRNA-21 promotes immune suppressive B cells. Cell Mol Immunol. 2015;12(6):750–6.
Wang P, Zhuang L, Zhang J, Fan J, Luo J, Chen H, et al. The serum miR-21 level serves as a predictor for the chemosensitivity of advanced pancreatic cancer, and miR-21 expression confers chemoresistance by targeting FasL. Mol Oncol. 2013;7(3):334–45.
Xiao D, Ohlendorf J, Chen Y, Taylor DD, Rai SN, Waigel S, et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One. 2012;7(10):e46874.
Baroni S, Romero-Cordoba S, Plantamura I, Dugo M, D’Ippolito E, Cataldo A, et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016;7(7):e2312.
Ueda R, Kohanbash G, Sasaki K, Fujita M, Zhu X, Kastenhuber ER, et al. Dicer-regulated microRNAs 222 and 339 promote resistance of cancer cells to cytotoxic T-lymphocytes by down-regulation of ICAM-1. Proc Natl Acad Sci U S A. 2009;106(26):10746–51.
Taverna S, Amodeo V, Saieva L, Russo A, Giallombardo M, De Leo G, et al. Exosomal shuttling of miR-126 in endothelial cells modulates adhesive and migratory abilities of chronic myelogenous leukemia cells. Mol Cancer. 2014;13:169.
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–15.
Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716.
Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5(10):e13247.
Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18(10):505–16.
Boyerinas B, Park S-M, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocr Relat Cancer. 2010;17(1):F19–36.
Kanlikilicer P, Rashed MH, Bayraktar R, Mitra R, Ivan C, Aslan B, et al. Ubiquitous release of Exosomal tumor suppressor miR-6126 from ovarian cancer cells. Cancer Res. 2016;76(24):7194–207.
Falcone G, Felsani A, D’Agnano I. Signaling by exosomal microRNAs in cancer. J Exp Clin Cancer Res. 2015;34:32.
Taverna S, Giallombardo M, Pucci M, Flugy A, Manno M, Raccosta S, et al. Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: a possible role for exosomal disposal of miR-21. Oncotarget. 2015;6(26):21918–33.
Taverna S, Fontana S, Monteleone F, Pucci M, Saieva L, De Caro V, et al. Curcumin modulates chronic myelogenous leukemia exosomes composition and affects angiogenic phenotype via exosomal miR-21. Oncotarget. 2016;7(21):30420–39.
Giallombardo M, Chacartegui Borras J, Castiglia M, Van Der Steen N, Mertens I, Pauwels P, et al. Exosomal miRNA Analysis in Non-small Cell Lung Cancer (NSCLC) Patients’ Plasma Through qPCR: A Feasible Liquid Biopsy Tool. J Vis Exp. 2016;(111).
Stahlhut C, Slack FJ. Combinatorial action of MicroRNAs let-7 and miR-34 effectively synergizes with Erlotinib to suppress non-small cell lung cancer cell proliferation. Cell Cycle. 2015;14(13):2171–80.
Janne PA, Yang JC-H, Kim D-W, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–99.
Yang JC-H, Wu Y-L, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-lung 3 and LUX-lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–51.
Li L, Leung PS. Use of herbal medicines and natural products: an alternative approach to overcoming the apoptotic resistance of pancreatic cancer. Int J Biochem Cell Biol. 2014;53:224–36.
Kanai M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J Gastroenterol. 2014;20(28):9384–91.
Sayeed MA, Bracci M, Lucarini G, Lazzarini R, Di Primio R, Santarelli L. Regulation of microRNA using promising dietary phytochemicals: possible preventive and treatment option of malignant mesothelioma. Biomed Pharmacother. 2017;94:1197–224.
Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding W-Q. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA). Mol Cancer. 2015;14:133.
Zhao J, Guerrero A, Kelnar K, Peltier HJ, Bader AG. Synergy between next generation EGFR tyrosine kinase inhibitors and miR-34a in the inhibition of non-small cell lung cancer. Lung Cancer. 2017;108:96–102.
Cortez MA, Ivan C, Valdecanas D, Wang X, Peltier HJ, Ye Y, et al. PDL1 Regulation by p53 via miR-34. J Natl Cancer Inst. 2016;108(1).
Shin J, Xie D, Zhong X-P. MicroRNA-34a enhances T cell activation by targeting diacylglycerol kinase zeta. PLoS One. 2013;8(10):e77983.
Wang X, Li J, Dong K, Lin F, Long M, Ouyang Y, et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal. 2015;27(3):443–52.
Jiao C, Jiao X, Zhu A, Ge J, Xu X. Exosomal miR-34s panel as potential novel diagnostic and prognostic biomarker in patients with hepatoblastoma. J Pediatr Surg. 2017;52(4):618–24.
Xu S, Wang J, Ding N, Hu W, Zhang X, Wang B, et al. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 2015;12(12):1355–63.
Rolfo C, Giallombardo M, Reclusa P, Sirera R, Peeters M. Exosomes in lung cancer liquid biopsies: Two sides of the same coin? Lung Cancer. 2017;104:134–135.
Taverna S, Giallombardo M, Gil-Bazo I, Carreca AP, Castiglia M, Chacartegui J, et al. Exosomes isolation and characterization in serum is feasible in non-small cell lung cancer patients: critical analysis of evidence and potential role in clinical practice. Oncotarget. 2016;7(19):28748–60.
Kosaka N, Yoshioka Y, Fujita Y, Ochiya T. Versatile roles of extracellular vesicles in cancer. J Clin Invest. 2016;126(4):1163–72.
Reclusa P, Taverna S, Pucci M, Durendez E, Calabuig S, Manca P, et al. Exosomes as diagnostic and predictive biomarkers in lung cancer. J Thorac Dis. 2017;9(Suppl 13):S1373-S1382.
Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087.
Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120(10):1632–48.
Cordonnier M, Chanteloup G, Isambert N, Seigneuric R, Fumoleau P, Garrido C, et al. Exosomes in cancer theranostic: diamonds in the rough. Cell Adhes Migr. 2017;11(2):151–63.
Sempere LF, Keto J, Fabbri M. Exosomal MicroRNAs in Breast Cancer towards Diagnostic and Therapeutic Applications. Cancers (Basel). 2017;9(7).
Eichelser C, Stuckrath I, Muller V, Milde-Langosch K, Wikman H, Pantel K, et al. Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget. 2014;5(20):9650–63.
Tominaga N, Yoshioka Y, Ochiya T. A novel platform for cancer therapy using extracellular vesicles. Adv Drug Deliv Rev. 2015;95:50–5.
Hyenne V, Lefebvre O, Goetz JG. Going live with tumor exosomes and microvesicles. Cell Adhes Migr. 2017;11(2):173–86.
Fontana S, Saieva L, Taverna S, Alessandro R. Contribution of proteomics to understanding the role of tumor-derived exosomes in cancer progression: state of the art and new perspectives. Proteomics. 2013;13(10–11):1581–94.
Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123(Pt 10):1603–11.
Nedaeinia R, Manian M, Jazayeri MH, Ranjbar M, Salehi R, Sharifi M, et al. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2017;24(2):48–56.
Rolfo C, Castiglia M, Hong D, Alessandro R, Mertens I, Baggerman G, et al. Liquid biopsies in lung cancer: the new ambrosia of researchers. Biochim Biophys Acta. 2014;1846(2):539–46.
Acknowledgements
We would like to thank Axelle Staes for English language editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
Elena Durendez has a predoctoral fellowship by Asociacion Española Contra el Cancer (AECC, Valencia, Spain).
Conflict of Interest
LR gets research support from EXOSOME DX. All other authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Pucci, M., Reclusa Asiáin, P., Duréndez Sáez, E. et al. Extracellular Vesicles As miRNA Nano-Shuttles: Dual Role in Tumor Progression. Targ Oncol 13, 175–187 (2018). https://doi.org/10.1007/s11523-018-0551-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-018-0551-8