Abstract
The upper airway (UA) is in general thicker and narrower in obstructive sleep apnea (OSA) population than in normal. Additionally, the UA changes during sleep are much more in the OSA population. The UA changes can alter the tracheal breathing sound (TBS) characteristics. Therefore, we hypothesize the TBS changes from wakefulness to sleep are significantly correlated to the OSA severity; thus, they may represent the physiological characteristics of the UA. To investigate our hypothesis, we recorded TBS of 18 mild-OSA (AHI < 15) and 22 moderate/severe-OSA (AHI > 15) during daytime (wakefulness) and then during sleep. The power spectral density (PSD) of the TBS was calculated and compared within the two OSA groups and between wakefulness and sleep. The average PSD of the mild-OSA group in the low-frequency range (< 280 Hz) was found to be decreased significantly from wakefulness to sleep (p-value < 10−4). On the other hand, the average PSD of the moderate/severe-OSA group in the high-frequency range (> 900 Hz) increased marginally significantly from wakefulness to sleep (p-value < 9 × 10−3). Our findings show that the changes in spectral characteristics of TBS from wakefulness to sleep correlate with the severity of OSA and can represent physiological variations of UA. Therefore, TBS analysis has the potentials to assist with diagnosis and clinical management decisions in OSA patients based on their OSA severity stratification; thus, obviating the need for more expensive and time-consuming sleep studies.

Tracheal breathing sound (TBS) changes from wakefulness to sleep and their correlation with Obstructive sleep apnea (OSA) were investigated in individuals with different levels of OSA severity. We also assessed the classification power of the spectral characteristics of these TBS for screening purposes. Consequently, we analyzed and compared spectral characteristics of TBS recorded during wakefulness (a combination of mouth and nasal TBS) to those during sleep for mild and moderate/severe OSA groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The upper airway (UA) is a collapsible structure; it dynamically changes from wakefulness to sleep, and also between the sleep stages [1]. The patency of the UA is suggested to be dependent on the equilibrium between the dilating forces generated by the UA dilator muscles and the pressure exerted by the heterogeneous surrounding soft tissue [2]. The complete or partial collapse of the UA during sleep could lead to apnea, a cessation of airflow for ≥10 s, or hypopnea, a reduction of the peak airflow by ≥30% from pre-event baseline if it lasts ≥ 10 s, and is associated with a ≥3% oxygen desaturation [3].
Obstructive sleep apnea (OSA) is characterized by repetitive episodes of apnea and/or hypopnea during sleep [4]. OSA is a relatively common disorder that can affect the health of all age groups [5]. Between 9 and 38% of the general adult population suffer from OSA [5]. As OSA is still underdiagnosed, these values are believed to underestimate the actual numbers [6]. Untreated OSA is associated with many deficits including excessive daytime sleepiness, increased risk of motor vehicle accidents, memory impairment, and stroke [2, 7]. Untreated moderate/severe OSA is also associated with increased morbidity and mortality [7].
Currently, diagnosis of OSA is based on the full-night polysomnography (PSG) assessment, as the gold standard, and to some extent by home sleep study (HSS) systems. PSG measures the apnea-hypopnea index (AHI), which reports the average number of apneic events per hour of sleep [8]. However, full PSG studies are time-consuming, laborious, expensive, and usually, have a long waiting time. HSS helps with cost-effectiveness and waiting-time of PSG, yet it remains as an overnight test that still requires considerable resources for reliable outcomes [9]. Therefore, developing an alternative technology to overcome these difficulties is momentous.
Tracheal breathing sounds (TBS) are a measure of tracheal wall vibration set into motion by the passage of turbulent airflow from the UA including trachea and pharynx [10]. Structural and physiological properties of the UA affect the resonance frequency of the UA that is detected by TBS analysis [11, 12]. It is known that physiological properties of the UA, its patency, and its resistance change dynamically, but with different degrees with respect to the sleep/wakefulness status (different sleep stages/wakefulness) and OSA severity [13,14,15,16,17,18]. Accordingly, our team and a few others around the world are using TBS as a quick, inexpensive, and reliable technology with comparable outcomes with that of PSG or HSS for OSA screening [19,20,21,22,23]. However, the changes of TBS from wakefulness to sleep in relationship to the severity of OSA and whether those changes have a classification power to identify the moderate and severe OSA from others have not been investigated adequately that is the goal of this study.
In a study by Yadollahi et al. [23], a fully automatic acoustic technology was introduced to estimate AHI during sleep using pulse oximetry and TBS. The detected apnea-hypopnea events were highly correlated to those detected using PSG. Although that study reduced the number of required signals to only two, however, it was still a full overnight test. In a study by Hajipour et al. [22], contrarily, a set of TBS characteristics were used to separate OSA individuals from non-OSA individuals during wakefulness. The results showed an average area under the receiver operated characteristic curve (AUC) of 89.9 ± 1.7%. Although the selected features in that study were correlated to AHI, it was not investigated whether those features were robust enough to reflect the changes of the UA during sleep in relation to OSA severity that is one goal of this study.
On the other hand, according to tube law, maximum airflow (Vmax) in collapsible tubes is determined by
where A is the cross-section area of the tube, ρ is the gas density, and (∆A/∆P) is the pharyngeal airway compliance; a measure of distensibility of the tube. According to Eq. 1, Vmax has a direct relationship to the cross-section area of the tube while it is inverse for the tube’s compliance [24]. By this analogy, the changes in the cross-section area and compliance/collapsibility of the UA are important factors in the pathophysiology of OSA [25] and are potential factors to modify the TBS of OSA compared with that of non-OSA individuals. If TBS analysis can reveal these anatomical/physiological characteristics of the UA, it will be an excellent non-invasive, quick, and cost-effective alternative tool for OSA management, and it can lead to more appropriate therapeutic decisions [26].
In our previous and relevant study [27], we recorded TBS of 30 OSA participants during sleep and in two maneuvers of mouth and nasal breathing during wakefulness. Next, we compared the spectral and higher-order statistical characteristics of their TBS during stage 2 of sleep and then during wakefulness. The results showed a significant difference between the TBS characteristics of these two OSA groups during either wakefulness or sleep. However, in that study, we did not investigate the pattern of changes from wakefulness to sleep in each of the two groups, nor investigated whether those changes have any classification power for identifying the severity of OSA. We hypothesize the changes in acoustic properties of TBS from wakefulness to sleep are highly correlated with the severity of OSA; thus, they are useful in OSA screening and classification, and may reveal the changes in physiological characteristics of the UA (including narrowing, thickness, and resistance) due to OSA in a detailed but straightforward manner. Therefore, in this study using a larger database, our primary goal was to investigate TBS changes from wakefulness to sleep in individuals with different levels of OSA severity and to explore whether these changes are correlated with their OSA severity. We also assessed the classification power of these acoustic features to separate the two OSA groups for screening purposes. To achieve our goal, we analyzed and compared spectral characteristics of TBS recorded during wakefulness (a combination of mouth and nasal TBS) to those during sleep. We discuss the physiological interpretation of our findings and describe their ability to show the UA characteristic changes regarding OSA.
2 Methods
2.1 Participants
Sixty individuals referred for PSG assessment at Sleep Disorder Center of Misericordia Health Center (Winnipeg, Canada) participated in this study. The Biomedical Research Ethics Board of the University of Manitoba approved the study. All participants signed an informed consent before data collection. We excluded data of 20 participants from the study due to frequent noises (including vocal and blanket, audible alarms, and air conditioner noises) or the need of patients for a titration that the sleep technician disconnected our acoustic device.
2.2 Sound recording procedure
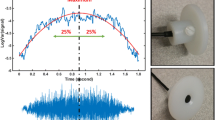
The TBS during both wakefulness and sleep were collected by a miniature microphone (Sony ECM-77B) inserted into a small chamber, allowing 2 mm cone-shaped space with skin. The chamber was mounted over the suprasternal notch of the trachea with double-sided adhesive tape. To ensure the microphone would not be misplaced during the night, we used a soft neckband, which was sealed softly around the patient’s neck to sustain microphone and chamber in site (Fig. 1). The sounds were band-pass filtered in the range of (0.05–5000 Hz), amplified by a Biopac (DA100C) Amplifier, and digitized at 10240 Hz sampling rate.
TBS during wakefulness was recorded before PSG assessment. We recorded 5 cycles of normal TBS through the nose with mouth closed, followed by 5 cycles of normal TBS through the mouth with a nose clip in place. We choose to record 5 breaths to be a representative of steady-state pattern of breathing without acceleration/deceleration of respiratory rate. After wakefulness recording, participants were prepared for PSG assessment. TBS during sleep were recorded simultaneously with the PSG assessment; they were real-time with the snoring sounds and respiratory events as appearing in the PSG. TBS segments for analysis were extracted from stable sleep periods in stage 2, void of snoring sounds or artifacts and in the supine position (determined using the PSG score sheet). Using the AHI outcome of the PSG assessment, we grouped the participants into mild-OSA (AHI < 15, N = 18) and moderate/severe-OSA (AHI > 15, N = 22) groups. The threshold of AHI = 15 has been traditionally used in many studies to identify OSA patients who might have increased cardiovascular or mortality risks and are in need of treatment [7]. Therefore, this threshold could potentially require more focus and earlier assessment for treatment. Anthropometric information of the 40 individuals, whose data were analyzed in this study, is presented in Table 1.
2.3 Pre-processing and signal analysis
In this study, we did not record the respiratory flow of participants; however, to ensure the respiratory phases, all recording procedures during wakefulness started at the inspiration and marked by the voice of the experimenter. Using that auditory marker, the inspiratory/expiratory phases during wakefulness were separated manually. For the TBS of sleep, however, we used our semi-manual technique, elaborated in [28], to identify the inspiratory/expiratory phases of breathing. In this study, we aimed to compare TBS during wakefulness with those during sleep. As there are no snoring sounds during wakefulness, to have a fair comparison between wakefulness and sleep, we examined all the recorded TBS data by audio and visual means in the time-and-frequency domain to exclude TBS with snoring sounds and noisy signals (including artifacts, vocal noises, and swallowing).
The majority of the moderate/severe-OSA participants of this study snored most of the time in the supine position. Since snoring usually occurs in the inspiration, because the UA collapse typically occurs at the end of expiration [14], the majority of the inspiratory phases of moderate/severe-OSA group have been eliminated. Therefore, we decided to remove the inspiratory phases of breathing for all participants to have a fair comparison between mild and moderate/severe OSA individuals. Furthermore, since there is no apneic event during wakefulness for even the moderate/severe OSA individuals [24], we also excluded sleep data with respiratory apneic events. Consequently, from the recorded sounds during sleep, we selected 5 normal (free of any apneic events including flow limitation), noise- and snore-free expiratory sounds in supine position and sleep stage 2 for further analysis. Data in stage 2 of sleep was selected because that was the most common (i.e., the highest number of individuals’ data) sleep stage in our dataset. We were also interested only in the supine position to match with position of data collection during wakefulness. From data recorded during wakefulness, we also selected 5 noise-free expiratory sounds for comparison with those extracted from data during sleep.
In this study, similar to our previous research [22], each selected sound was first passed through a 5th-order Butterworth band-pass filter in the range of (75–2500 Hz) to eliminate the effect of low- and high-frequency noises (including ambient noises, the highest amplitude component of the heartbeats (which is less than 75 Hz [29]), fundamental frequency of the power line (60 Hz in Canada), and muscle sounds) while keeping the primary frequency component of the sound. Next, each filtered sound signal was normalized by its variance envelope (a smoothed version of itself using the moving average method with 64 sample sequence ~ 6 ms) [30] to remove its extra fluctuations, and then by its standard deviation to compensate for plausible different flow rates between breathing cycles. Then, the logarithm of the variance of each TBS was calculated to acoustically estimate its respiratory flow. Afterward, the 50% duration around the maximum of the estimated flow signal was considered the stationary portion of that sound signal [30]. Finally, using Welch’s method, the power spectrum density (PSD) of the stationary portion of sound signals was estimated in segments of 20 ms with 50% overlapping windows to successive segments. Figure 2 outlines the abovementioned pre-processing.
As mentioned in Section 2.2, during wakefulness, we recorded TBS in two maneuvers of nose and mouth breathing. During sleep, however, one could be breathing through either nose or mouth, but without a video recording, we could not identify nose or mouth breathing. Thus, to have a fair comparison of wakefulness and sleep data, for each participant, we selected 3 breaths from mouth breathing and 2 breaths from nasal breathing of the wakefulness. Next, we considered the average of the estimated PSD of these signals and the average of the estimated PSD of the five TBS recorded during sleep as the representative data of wakefulness and sleep of each individual, respectively.
For feature extraction with the purpose of investigating TBS variations from wakefulness to sleep, within each OSA groups, we averaged the PSD signals during wakefulness and sleep and calculated their standard error (SE). We also assessed the difference between the average PSD (PSDavg) of TBS during wakefulness and sleep in two groups of mild (mild-Difference) and moderate/severe OSA (m/s-Difference). The regions with no overlap between the mild-Difference and the m/s-Difference were considered characteristic regions for extracting features for further statistical investigations. These regions reflect the frequency bands that the spectral characteristics of TBS change the most from wakefulness to sleep and are introduced in the Results section. Similar to our previous studies during wakefulness [22, 31], we considered the mean of these areas as characteristic features to be selected for classification. TBS features representing changes from wakefulness to sleep were the mean of the mild-Difference and m/s-Difference in the ranges mentioned above. TBS features during wakefulness and sleep were the mean of the PSDavg in the pre-mentioned regions for the two OSA groups during wakefulness and sleep, respectively. To be clearer, Fig. 3 shows the mild-Difference and m/s-Difference as well as the PSDavg during wakefulness and sleep for both mild and moderate/severe OSA groups. TBS features were extracted from the regions between the solid lines and the region between the dotted lines.
Average power spectra (PSDavg) of the combination of mouth and nasal tracheal breathing sounds (TBS) during wakefulness and sleep with their standard error intervals (shadows). a Averaged among participants of mild-OSA group during wakefulness (green) and sleep (black), b averaged among participants of moderate/severe-OSA group during wakefulness (green) and sleep (black), c difference of the PSDavg during wakefulness and sleep for mild-OSA (blue) and moderate/severe-OSA (red) groups. The areas between solid lines and dotted lines show the regions where the features were extracted
In addition to studying changes of TBS characteristics from wakefulness to sleep, we also studied and compared the classification ability of the TBS features reflecting changes from wakefulness to sleep (Fig. 3c) with those extracted during only wakefulness or only sleep (Fig. 3a and b). To perform classification, we used 10 ensembles of 2-class linear kernel support vector machine (SVM) classifiers, obtained from bootstrap samples of our data. Bootstrapping consists of repeatedly drawing samples of the same size, with replacement, from our original dataset. We trained our SVM classifier with the bootstrap samples. The remaining data (samples that were not selected in the bootstrapping procedure) were considered testing data and used to evaluate the performance of the SVM classifiers. We repeated this procedure 10 times and reported the average classification results.
2.4 Statistical analysis
Our hypotheses of this study were the spectral characteristics of TBS change from wakefulness to sleep, and these variations are different in OSA and non-OSA populations. As we have measured the TBS characteristics over two time-points (wakefulness and sleep), also our participants have been assigned in two groups (mild and moderate/severe OSA), we applied a multivariate two way mixed analysis of covariance (mixed ANCOVA) multifactorial statistical test to compare the within-groups’ changes (from wakefulness to sleep) and the between-groups’ changes (between mild and moderate/severe OSA groups), while considering the neck circumference (NC) as covariate. We included the NC as covariate in ANCOVA model to ensure that our TBS features are not just measuring the differences in NC between the two OSA groups. A p-value of 0.05 was considered significant. Next, we used simple effect post hoc analysis with p-value of 0.0064 to determine the effect of sleep/wakefulness status on OSA severity, and vice versa. The reason for choosing this p-value is explained in Results Section. Pearson’s correlation was used to determine the correlations among the TBS features and AHI. Independent unpaired t-test with p-value of 0.05 was also used to compare the anthropometric information and the differences of PSDavgof wakefulness and sleep in mild-OSA and moderate/severe-OSA groups.
3 Results
Table 1 shows the average and standard deviations of the anthropometric information of the mild and moderate/severe OSA groups, as well as their basic statistical comparisons. There was no significant difference between the two groups in terms of age, height, and sex. The participants with moderate/severe-OSA had a significantly higher weight, AHI, body mass index (BMI), and NC values than did those of mild-OSA individuals.
To predict OSA and its pathology by breathing sounds analysis, in this study, we focused on acoustical changes from wake to sleep. In general, in our studies, we aim to have a reliable acoustic OSA prediction by having breathing sounds of a short period of time. From the sleep data, as mentioned in Methods, we decided to select stage 2 of sleep data. On average, our participants spent 106.36 min before reaching stage 2 of sleep in supine position; this average time was 114.1 and 98.6 min for mild-OSA and moderate/severe-OSA participants, respectively. During these times, the participants slept either on other postural positions or at stage 1 of sleep. Stage 1 data was not selected because the majority of our participants snored and/or had several episodes of hypopneas. The average time to reach REM or stage 3 and 4 of sleep was longer than that of stage 2. It is worth to mention that not all of our participants reached REM or deep sleep stages.
Figure 3a and b shows the PSDavg and SE intervals during wakefulness and sleep for mild and moderate/severe OSA participants, respectively. These figures depict clear differences in low- and high-frequency ranges between PSDavg of TBS recorded during wakefulness and sleep and within two OSA groups. Figure 3c demonstrates the mild-Difference and m/s-Difference, in addition to their SE intervals. As Fig. 3c shows, among the regions with no overlap between the two OSA groups, at lower frequencies (150–280 Hz), the mild-Difference were significantly higher than m/s-Difference, (p-value < 0.03, Table 2). Contrarily, at higher frequencies (950–1150 Hz), the m/s-Difference values were significantly higher compared with those of mild-Difference (p-value < 0.04, Table 2).
Based on the aforementioned observations, we considered the PSDavg in the low-frequency range of (150–280 Hz) (F1) and the PSDavg in the high-frequency range of (950–1150 Hz) (F2) as the two potential features representing the most changes from wakefulness to sleep for further analysis. Since we compared the changes of F1 and F2 from wakefulness to sleep and also between the two OSA groups, we had 8 different statistical comparisons. Therefore, to satisfy the statistical significance of 95% for the overall post hoc tests, the significance level of the p-value of each post hoc test was considered 1 − (1 − 0.05)1/8 ≅ 0.0064 [32].
The mixed ANCOVA multifactorial test result showed a highly significant main effect of sleep/wakefulness status (being asleep or awake) on the TBS features, (p-value < 10−4, Table 3). This test also showed a significant interaction between the sleep/wakefulness status and the OSA severity level of participants (p-value < 2 × 10−2, Table 4).
Our results on the investigation of F1 depict both a highly significant main effect of sleep/wakefulness status (p-value < 10−4, Table 3) and a significant interaction effect of sleep/wakefulness status and OSA severity level on this feature (p-value < 8 ×10−3,Table 4). The simple effect post-hoc analysis showed a strongly significant decrease in F1 from wakefulness to sleep within the mild group (p-value < 3 × 10−5, Fig. 3a, Tables 2 and 5). A decrease was also observed in moderate/severe-OSA groups, but it was not significant (p- value = 0.2, Fig. 3b, Tables 2 and 5).
Our results on the investigation of F2 depict a significant main effect of sleep/wakefulness status (p-value < 10−3, Table 3), but no significant interaction effect of sleep/wakefulness status and OSA severity level was observed (p-value = 0.6, Table 4). The simple effect post-hoc analysis showed a marginally significant increase in this feature from wakefulness to sleep in moderate/severe OSA group (p-value < 9 ×10−3, Fig. 3b, Tables 2 and 5). Nevertheless, no significant change was observed for the mild group (p-value = 0.2, Fig. 3a, Tables 2 and 5).
Table 6 shows the Pearson correlation coefficients between the AHI and the spectral TBS features. AHI was correlated with F1 during wakefulness (− 0.42, p-value < 0.01), with F2 during sleep (0.36, p-value < 0.05) and with the change of F2 from wakefulness to sleep (− 0.39, p-value < 0.05).
Table 7 reports the percentage of the participants that their F1 and F2 decreased or increased from wakefulness to sleep within the two OSA groups and for the total participants of this study. Overall, F1 decreased from wakefulness to sleep in 77.5% of participants, and F2 increased from wakefulness to sleep in 67.5% of participants.
Table 8 reports the classification results of the SVM classifiers using TBS features’ changes from wakefulness to sleep, TBS features during wakefulness, and TBS features during sleep. The SVM classifier using the F1 and F2 changes from wakefulness to sleep (i.e., F1wakefulness-F1sleep, and F2wakefulness-F2sleep) resulted in 87.5±4.5% accuracy with the sensitivity (specificity) of 87.5 ±6.9% (87.5 ±13%). On the other hand, the SVM classifier using F1 and F2 extracted from wakefulness resulted in 78.8±4.2% accuracy with the sensitivity (specificity) of 85.7 ±1.7% (70.8 ±6.3%), and using F1 and F2 extracted from sleep data resulted in 70.1 ±6.5% accuracy with the sensitivity (specificity) of 75.7 ±16.6% (63.3 ±8.5%).
4 Discussion
TBS are affected by structural and physiological properties of the UA and contain rich information of the airway structure [11]. We hypothesize the TBS changes from wakefulness to sleep are highly correlated to the severity of OSA; thus, they may reveal UA structural and physiological characteristics and their variations due to OSA. Thus, they may be used as a screening/diagnostic tool to identify the severity of OSA. While there have been many investigations to examine TBS concerning OSA including our team’s previous studies [22, 23, 31, 33, 34], to the best of our knowledge, this study is the first to explore the potential role of TBS analysis in assessing UA changes from wakefulness to sleep with respect to OSA and its severity.
The findings of our TBS investigations manifested a significant main effect of sleep/wakefulness status on the TBS spectral characteristics and a significant interaction between the sleep/wakefulness conditions and the OSA severity level of participants. These results imply that the TBS spectral features change significantly, when one sleeps, and these changes are clearly different within mild and moderate/severe OSA groups. In addition, our results have shown that these spectral features are correlated to the OSA severity level of the participants (Table 6).
One of this study’s objectives was to investigate whether TBS characteristics have the potential to reveal the physiological and structural changes of UA regarding OSA and its severity. Imaging studies during wakefulness have reported individuals with OSA compared with non-OSA individuals have thicker velum, thicker pharyngeal wall, a reduced pharyngeal cross-sectional area, and a narrower pharyngeal lumen [15, 16]. According to the Hagen-Poiseuille’s law, a narrower UA implies more resistance to airflow. Higher resistance absorbs more energy, indicating lower average PSD at low frequencies (F1) for the generated TBS of the moderate/severe-OSA group compared with that of the mild-OSA group during wakefulness. This phenomenon can be seen clearly by comparing the wakefulness PSDavg of mild and moderate/severe OSA groups in Fig. 3a and b. The predominant energy absorbency at low frequencies is related to the higher net parallel impedance of the UA at lower frequencies. According to the cascade T circuits modeling of UA proposed in the study by Harper et al. [35], the net impedance of the UA is due to the UA wall tissues’ capacitance effect in parallel to the UA resistance. These findings might also conclude a thicker UA for moderate/severe OSA group compared with the mild-OSA group during wakefulness. This inference is based on findings of an acoustic study that shows the low-frequency sound absorbency of materials is directly related to the materials’ thickness [36]. Therefore, the lower the F1 of moderate/severe OSA group during wakefulness (Table 2), the higher their UA sound absorbency, and therefore the thicker their UA.
On the other hand, studies that investigated UA during sleep have been shown that the sleep would lead to a decrease in the airway caliber and an increase in the UA resistance [12]. As we mentioned earlier, higher resistance absorbs more energy at lower frequencies. Therefore, we expect a reduction in the F1 of both mild and moderate/severe OSA groups from wakefulness to sleep, as seen in (Fig. 3a and b). As Fig. 3c shows, the intensity of change from wakefulness to sleep is different in the two OSA groups. In moderate/severe-OSA group, the changes of the low-frequency PSD from wakefulness to sleep were much less pronounced compared with that of the mild-OSA group. We speculate this might be related to the narrower UA of moderate/severe OSA groups compared with that of mild group during wakefulness [15, 16].
Moreover, it has been shown that the UA collapse during sleep does not happen in the entire pharynx, but it is non-homogeneous and may narrow regionally at multiple anatomic locations with various degrees [16]. Tortuosity and flow resistivity are reasons for a drop of high-frequency sound absorbency [36], indicating an increase in the energy. Therefore, we expect to see an increase in high-frequency power (F2) of moderate/severe participants from wakefulness to sleep, as seen in Fig. 3b. Another reason for observing a higher F2 during sleep and a marginally significant increase of F2 from wakefulness to sleep for moderate/severe OSA group compared with those of mild-OSA is the increased stiffness of their UA [37]. Stiffness effect is reflected at higher frequencies as it increases the wave velocity [38]. Interestingly, this higher stiffness of the UA of the moderate/severe-OSA group shows itself more during sleep than wakefulness (Fig. 3b); it is in keeping with the rational expectation that as the airway caliber decreases during sleep, its stiffness is increased [12].
It is worth mentioning that the findings of the present study are congruent with our previous study results using different datasets [22]. In that study, we used TBS data of many more participants than this current study; data were recorded only during wakefulness, and we considered 80% of data for training and 20% of data as blind testing for evaluation. Using the training data, we considered the non-overlapped regions between the averaged PSD of the mild and moderate/severe OSA participants as potential areas for feature extraction. To eliminate the bias, we repeated this procedure 10 times and selected the common non-overlapped regions between various training sets. The non-overlapped regions of that study were common with the selected regions of this current study. Thus, the findings of that study, congruent with results of this current study, indicate a lower average power at lower frequencies and higher average power at higher frequencies for moderate/severe OSA individuals during wakefulness compared with those of non-OSA.
TBS power spectra do have variability from person to person. We speculate PSD analysis of the TBS and how its pattern changes (low versus high frequencies) from wakefulness to sleep may be representative of the shape of the individual’s velopharyngeal narrowing. For example, in a study by Finkelstein et al. [14], it was shown that the majority (85%) of the OSA participants had a predominantly velopharyngeal narrowing, while that narrowing was observed only in 12.5% of the control group. Narrowing of the pharyngeal area results in higher compliance leading to more collapsibility in the OSA population. In [14], it was also found that the shape of the narrowed airway in OSA subjects was predominantly bottle-shaped due to the increase in dilator-muscle activities that work on maintaining airway patency during wakefulness. That finding suggests regional stiffening and more compliance in the UA of the OSA group. Accordingly, the UA of OSA individuals may show both more stiffness and compliance compared with non-OSA individuals. Higher compliance is represented by a decrease in F1 (low-frequencies decreased power) from wakefulness to sleep that was observed in 89.9% of mild and 68.2% of moderate/severe-OSA (Table 7, Fig. 3). Higher stiffness in the UA due to narrowing is represented by an increase in F2 (high-frequency increased power) from wakefulness to sleep that was observed in 66.7% of mild and 68.2% of moderate/severe OSA (Table 7, Fig. 3). To prove this directly though, we will need simultaneous recording of TBS with UA imaging and pressure measurements.
In the classification point of view, we used an ensemble of linear kernel SVM classifiers to classify the participants as either mild or moderate/severe OSA (Table 8). The classification results of these classifiers over F1wakefulness-F1sleep and F2wakefulness-F2sleep resulted in the average accuracy of 87.5 ±4.5%. This classification result was higher than the average accuracy associated to the SVM classifiers over F1 and F2 during only wakefulness (78.8 ±4.2%) and only sleep (70.1 ±6.5%). These findings indicate the superiority of investigating the TBS changes from wakefulness to sleep rather than only sleep or wakefulness for OSA screening and differentiating between the two OSA groups. According to the mentioned points, one reason for the better classification results of the features reflecting changes from wake to sleep is the increased stiffness and thickness of the UA of moderate/severe-OSA group from wakefulness to sleep compared with those of mild group; such increased stiffness and thickness manifest themselves in the acoustical properties of TBS more significantly. The selected features of this study are following the features of our previous study during wakefulness [22]; therefore, confirm the robustness of those characteristics for OSA screening during wakefulness. In addition, it should be note that the sleep data in our study requires only a few breaths during sleep. Therefore, even with a short period of sleep, our proposed TBS analysis during both wakefulness and sleep can be useful for differentiating the two OSA severity groups accurately and reliably; thus, reducing the need for the entire night sleep study.
The results of this study also showed a significantly higher weight, BMI, and NC for the moderate/severe-OSA group compared with those of the mild-OSA group (Table 1). These observations are in accordance with the studies that suggest OSA might be a partial indication of the effect of general processes like increase in BMI on the UA [15]. These findings support our team’s previous study that used the anthropometric information as characteristic features for OSA screening during wakefulness, and achieved the test accuracy of more than 76% [31].
Lastly, in this study, we used data of the subjects who were referred for PSG assessment in a hospital. Therefore, it may be thought that the findings presented here may not be reproducible at other hospitals/institutions or homes with different level of ambient noise compared with the sleep labs. However, as we used a firm preprocessing step to eliminate the effect of noises and artifacts, the different noise level would not be a limitation factor; hence, the result of this study could be generalizable in other sleep lab environments and home sleep studies.
4.1 Limitations of the study
The main limitation of this study is the lack of a direct measure of the UA collapsibility of the study participants. Therefore, we compared our results with the general finding of the imaging studies. Another limitation of this study was related to investigating the TBS in only stage 2 of sleep. It was because not all of our study participants had enough data in other sleep stages that were also in supine position. Furthermore, we only had high-quality sounds recorded for a few hours and not the entire night data. In future studies, we will investigate TBS in various sleep stages in relation to OSA severity. It would also be interesting to analyze the transition period of wakefulness to sleep (stage 1) as that may show the dynamic of the UA changes better that is a goal of our future studies. Another limitation is related to the identification of mouth-nose breathing during sleep. It would be beneficial to record the respiratory flow of individuals using a nasal cannula to help in automatic mouth-nose breathing identification. It is true that nasal cannula does not register mouth breathing and it may look like an apnea episode; however, it is possible to distinguish mouth breathing from apnea mouth-breathing by sound analysis. The other limitation of the study is the limited number of participants. With more participants, it would be desirable to investigate the potential of TBS analysis to differentiate severe and moderate groups of OSA.
5 Conclusion
In this study, we investigated the application of spectral characteristics of TBS to reveal the pathophysiology of the UA and their change due to OSA. We also studied the relationship of the changes of these characteristics in correlation with the severity of OSA. Our results show significant differences in spectral characteristics of TBS between the mild- and moderate/severe-OSA groups during wakefulness, sleep, and from wakefulness to sleep; congruently indicative of changes in UA thickness and regional collapsibility. Consequently, spectral characteristics of the high sampling rate TBS during wakefulness, sleep, and their changes from wakefulness to sleep have potential to reveal the pathophysiology of the UA in relation to OSA. The findings of this study are especially useful to find the TBS characteristics that indirectly and non-invasively reveal the structural changes of the UA in relation to OSA. They are also beneficial to enhance the current OSA diagnosis methods to stratify the severity of OSA patients in a non-laborious, non-time-consuming, and less expensive manner using a short period of sleep instead of full overnight sleep study. From sleep data, we only considered a few normal breathing sounds at stage 2 of sleep. Since stage 2 is among the first sleep stages to reach when one sleeps, the proposed technology does not have to be run overnight; it can be during any short nap during daytime as well. Running a short-time sleep study during daytime will reduce healthcare cost significantly by reducing the need to expensive full overnight PSG study. Moreover, having a short sleep study during daytime is much more convenient for people, which is particularly important for dementia population that are usually reluctant to sleep the night in an unfamiliar environment away from their spouse/caregiver. Overall, the proposed technology will assist the sleep clinicians in the appropriate therapeutic decisions and focusing on the investment of resources to optimize compliance to treatment, particularly in the moderate/severe-OSA group. Further studies in larger sample size are needed to assess the efficacy of adding our proposed TBS analysis in diagnostic and therapeutic tools of sleep study centers.
Abbreviations
- AHI:
-
Apnea/hypopnea index
- ANOVA:
-
analysis of variance
- BMI:
-
Body mass index
- HSS:
-
Home sleep study
- MRI:
-
Magnetic resonance imaging
- NC:
-
Neck circumference
- OSA:
-
Obstructive sleep apnea
- PSD:
-
Power spectrum density
- PSDavg :
-
Average power spectrum density
- PSG:
-
Polysomnography
- SE:
-
Standard error
- SVM:
-
Support vector machine
- TBS:
-
Tracheal breathing sounds
- UA:
-
Upper airway
References
Bilston LE, Gandevia SC (2013) Biomechanical properties of the human upper airway and their effect on its behavior during breathing and in obstructive sleep apnea. J Appl Physiol 116:314–324. https://doi.org/10.1152/japplphysiol.00539.2013
Malhotra A, White DP (2002) Obstructive sleep apnoea. Lancet 360:237–245. https://doi.org/10.1016/S0140-6736(02)09464-3
Berry RB, Brooks R, Gamaldo CE, Harding SM, et al (2018) The AASM manual for the scoring of sleep and associated events: rules, Terminology and Technical Specifications, Version 2.5
Park JG, Ramar K, Olson EJ (2011) Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc 86:549–555. https://doi.org/10.4065/mcp.2010.0810
Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, Hamilton GS, Dharmage SC (2017) Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 34:70–81. https://doi.org/10.1016/j.smrv.2016.07.002
Memtsoudis SG, Besculides MC, Mazumdar M (2013) A rude awakening — the perioperative sleep apnea epidemic. N Engl J Med 368:2352–2353. https://doi.org/10.1056/NEJMp1302941
Young T, Palta M, Dempsey J et al (2010) Burden of sleep apnea: rationale, design, and major findings of the Wisconsin sleep cohort study. WMJ Off Publ State Med Soc Wisconsin 108:246–249
Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong T, Loube DL, Owens J, Pancer JP, Wise M (2005) Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep 28:499–523. https://doi.org/10.1093/sleep/28.4.499
Deutsch PA, Simmons MS, Wallace JM (2006) Cost-effectiveness of split-night polysomnography and home studies in the evaluation of obstructive sleep apnea syndrome. J Clin Sleep Med 2:145–153
Priftis KN, Hadjileontiadis LJ, Everard ML (2018) Breath sounds from basic science to clinical practice. Springer International Publishing, Cham
Penzel T, Sabil A (2017) The use of tracheal sounds for the diagnosis of sleep apnoea. Breathe 13:e37–e45. https://doi.org/10.1183/20734735.008817
Ayappa I, Rapoport DM (2003) The upper airway in sleep: physiology of the pharynx. Sleep Med Rev 7:9–33. https://doi.org/10.1053/smrv.2002.0238
Lan Z, Itoi A, Takashima M, Oda M, Tomoda K (2006) Difference of pharyngeal morphology and mechanical property between OSAHS patients and normal subjects. Auris Nasus Larynx 33:433–439. https://doi.org/10.1016/j.anl.2006.03.009
Jordan AS, White DP, Lo Y et al (2009) Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 32:361–368. https://doi.org/10.1093/sleep/32.3.361
Finkelstein Y, Wolf L, Nachmani A, Lipowezky U, Rub M, Shemer S’, Berger G (2014) Velopharyngeal anatomy in patients with obstructive sleep apnea versus normal subjects. J Oral Maxillofac Surg 72:1350–1372. https://doi.org/10.1016/j.joms.2013.12.006
Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI (2003) Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 168:522–530. https://doi.org/10.1164/rccm.200208-866OC
Fogel RB, Trinder J, White DP, Malhotra A, Raneri J, Schory K, Kleverlaan D, Pierce RJ (2005) The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol 564:549–562. https://doi.org/10.1113/jphysiol.2005.083659
Wu Z, Chen W, Khoo MCK, Davidson Ward SL, Nayak KS (2016) Evaluation of upper airway collapsibility using real-time MRI. J Magn Reson Imaging 44:158–167. https://doi.org/10.1002/jmri.25133
Nakano H, Furukawa T, Tanigawa T (2019) Tracheal sound analysis using a deep neural network to detect sleep apnea
Beck R, Rosenhouse G, Mahagnah M, Chow RM, Cugell DW, Gavriely N (2005) Measurements and theory of normal tracheal breath sounds. Ann Biomed Eng 33:1344–1351. https://doi.org/10.1007/s10439-005-5564-7
Kulkas A, Huupponen E, Virkkala J, Tenhunen M, Saastamoinen A, Rauhala E, Himanen SL (2009) New tracheal sound feature for apnoea analysis. Med Biol Eng Comput 47:405–412. https://doi.org/10.1007/s11517-009-0446-z
Hajipour F, Jafari Jozani M, Elwali A, Moussavi Z (2019) Regularized logistic regression for obstructive sleep apnea screening during wakefulness using daytime tracheal breathing sounds and anthropometric information. Med Biol Eng Comput 57:2641–2655. https://doi.org/10.1007/s11517-019-02052-4
Yadollahi A, Giannouli E, Moussavi Z (2010) Sleep apnea monitoring and diagnosis based on pulse oximetery and tracheal sound signals. Med Biol Eng Comput 48:1087–1097. https://doi.org/10.1007/s11517-010-0674-2
Younes M (2008) Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol 105:1389–1405. https://doi.org/10.1152/japplphysiol.90408.2008
Ryan CM, Bradley TD (2005) Pathogenesis of obstructive sleep apnea. J Appl Physiol 99:2440–2450
Rama AN, Tekwani SH, Kushida CA (2002) Sites of obstruction in obstructive sleep apnea. Chest 122:1139–1147. https://doi.org/10.1378/chest.122.4.1139
Hajipour F, Moussavi Z (2019) Spectral and higher order statistical characteristics of expiratory tracheal breathing sounds during wakefulness and sleep in people with different levels of obstructive sleep apnea. J Med Biol Eng 39:244–250. https://doi.org/10.1007/s40846-018-0409-7
Huq S, Moussavi Z (2012) Acoustic breath-phase detection using tracheal breath sounds. Med Biol Eng Comput 50:297–308. https://doi.org/10.1007/s11517-012-0869-9
Arvin F, Doraisamy S, Safar Khorasani E (2011) Frequency shifting approach towards textual transcription of heartbeat sounds. Biol Proced Online 13:1–7. https://doi.org/10.1186/1480-9222-13-7
Yadollahi A, Moussavi ZMK (2007) Acoustical flow estimation: review and validation. IEEE Eng Med Biol Mag 26:56–61. https://doi.org/10.1109/MEMB.2007.289122
Elwali A, Moussavi Z (2017) Obstructive sleep apnea screening and airway structure characterization during wakefulness using tracheal breathing sounds. Ann Biomed Eng 45:839–850. https://doi.org/10.1007/s10439-016-1720-5
Simes RJ (1986) An improved Bonferroni procedure for multiple tests of significance. Biometrika 73:751–754. https://doi.org/10.1093/biomet/73.3.751
Kalkbrenner C, Eichenlaub M, Rüdiger S, Kropf-Sanchen C, Rottbauer W, Brucher R (2018) Apnea and heart rate detection from tracheal body sounds for the diagnosis of sleep-related breathing disorders. Med Biol Eng Comput 56:671–681. https://doi.org/10.1007/s11517-017-1706-y
Kulkas A, Rauhala E, Huupponen E, Virkkala J, Tenhunen M, Saastamoinen A, Himanen SL (2008) Detection of compressed tracheal sound patterns with large amplitude variation during sleep. Med Biol Eng Comput 46:315–321. https://doi.org/10.1007/s11517-008-0317-z
Harper P, Kraman SS, Pasterkamp H, Wodicka GR (2001) An acoustic model of the respiratory tract. IEEE Trans Biomed Eng 48:543–550. https://doi.org/10.1109/10.918593
Seddeq HS (2009) Factors influencing acoustic performance of sound absorptive materials. Aust J Basic Appl Sci 3:4610–4617
Veldi, Vasar, Vain et al (2000) Computerized endopharyngeal myotonometry (CEM): a new method to evaluate the tissue tone of the soft palate in patients with obstructive sleep apnoea syndrome. J Sleep Res 9:279–284. https://doi.org/10.1046/j.1365-2869.2000.00208.x
Jensen OE (2002) Flows through deformable airways. In: Centre for Mathematical Medicine School of Mathematical Sciences University of Nottingham, UK. pp 27217–222
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hajipour, F., Giannouli, E. & Moussavi, Z. Acoustic characterization of upper airway variations from wakefulness to sleep with respect to obstructive sleep apnea. Med Biol Eng Comput 58, 2375–2385 (2020). https://doi.org/10.1007/s11517-020-02234-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-020-02234-5