Abstract
This study examines the effect of shear on the microstructure and oil binding capacity of three wax oleogels. For crystallization under a cooling rate of 1.5 °C/min, shear decreased crystal size, but increased the box-counting fractal dimension (Db) and decreased the pore area fraction (AFp) of the RBX oleogel network, creating a tortuous network for loosely-bound oil to migrate through that increased the oil binding capacity compared to statically cooled RBX gels For SFX oleogels, crystal size decreased under shear, while for CLX, crystal size drastically increased, causing an increase in oil loss for both. Trends for pore area fraction and fractal dimension were not consistent for oleogels structured by different waxes, which may be due to inherent differences in crystal surface quality and available area for oil adsorption. Under both high and low cooling rates, shear increased the oil binding capacity of a commercial peanut butter stabilizer. The effect of shear under rapid cooling for wax oleogels was more difficult to discern due to confounding effects of shear and cooling rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, oleogels have attracted significant attention due to their potential use as trans fat replacements [1]. These unique systems contain a low concentration (<10 % w/w) of a low molecular weight gelator that crystallizes upon cooling to form a thermo reversible and three dimensional network that entraps large volumes of liquid oil. The macroscopic functionality of an oleogel can be altered by modifying this network [2]. The implication of such versatility is that the hardness, texture, melting profile, and oil binding capacity of oleogels can be tailored to meet requirements that are specific to certain applications. This is an extremely appealing attribute of oleogels [3].
There are several methods available for modifying gel microstructure, such as varying the cooling rate, application of shear, addition of surfactants, and changing the solvent type [4–6]. The first two are perhaps the most direct means given that they influence two critical crystallization parameters: nucleation rate and crystal growth rate.
Shear is known to increase heat and mass transfer and increase the frequency of molecular collisions [3, 7, 8]. In addition, shear can cause crystal breakage, changing the size of microcrystalline elements. Fragments may also act as new nuclei for future crystal growth. This increase in nuclei population mimics the increase in nucleation rate observed under high levels of supercooling or supersaturation. Of course, these conditions will change depending on the shear rate and the extent of shear application [7, 8]. Less obvious, however, and often a confounding factor in studies on shear is the fact that shear can strongly affect cooling rate via enhancement of heat transfer in heat transfer limited systems. This is obviously not a “shear” related effect, but for example, shearing a very viscous fluid will enhance heat transfer by convection and thus cause a more rapid change in temperature. Thus, the “shear” effect becomes a “cooling rate” effect and has nothing to do with momentum transfer. For this reason, studies on shear must include very accurate control of temperature and cooling rates.
Shear sensitivity is a particular concern for oleogels due to their large liquid volume fractions that may reach 99 % (w/w). Network damage due to excessive shear may provoke oil syneresis, which would be detrimental to the surrounding food matrix. A thixotropic system will be able to recover from this damage by reforming network connections. In comparison, a non-thixotropic system will not be able to reform its network, and therefore shear will severely degrade the gel’s mechanical properties and oil binding capacity.
Network damage can be mitigated if a lower shear rate is applied since a lower shear rate is less likely to rupture network connections compared to a higher shear rate, and instead may result in crystal aggregation or induce a specific crystal orientation [9]. Alternatively, shearing during nucleation or to pre-crystallization conditions will cause even less network damage since the network will not have formed yet. However, changes in the rate of nucleation and the alignment of nuclei caused by shear fields will influence the size, distribution, and population of crystals, altering the physical properties of the eventual gel [7].
In addition to these parameters, a gel’s macroscopic properties are also dependent on the nature of the interaction between microstructural elements. These interactions occur at junction zones that exist between intersecting crystal branches or between entangled crystal fibers [3, 2]. The former constitute permanent junction zones and the latter transient junction zones. A high proportion of permanent junction zones is associated with high mechanical strength, while the presence of transient junction zones provides elasticity to a network [2].
Thus, the morphology (crystal size, distribution, shape, and topology) and the interaction between crystals at junction zones can be altered by changing the rate of nucleation via cooling rate or the use of shear. Studying the effects of shear on the microstructure and physical properties of oleogels will allow the functionality of these gels to be tailored to specific applications and provide more insight on the compatibility between oleogels and industrial processing conditions which utilize shear and mixing processes. In particular, the effect of shear on a gel’s oil binding capacity should be a main focus of such work considering that the oil binding capacity dictates its functionality and applicability for different commercial uses.
Da Pieve et al. observed that monoglyceride oleogels cooled in the presence of shear (50–2000s−1) developed weaker networks with lower oil binding capacities compared to monoglyceride gels that were cooled under quiescent conditions [10]. They reported that shear leads to the formation of a weak gel containing randomly distributed unorganized crystal clusters that interact weakly through transient junction zones, explaining why the elastic and storage moduli for sheared gels were lower than those obtained for static gels.
Co et al. examined how the application of oscillatory laminar shear applied during different crystallization regimes could be used to control the microstructure and mechanical properties of 12-HSA oleogels [11]. They found that oscillatory shear applied under high cooling rates promoted the formation of spherulites, while fibers formed under slow cooling rates. Continuous shear caused severe microstructural damage and high levels of oil loss.
Wax oleogels constitute a newer class of oleogels with great promise. In particular, rice bran wax (RBX), sunflower wax (SFX), and candelilla wax (CLX) are plant based waxes that have attracted growing interest given that they are capable of gelling liquid oil using 1–2 % (w/w) wax. However, the effects that shear may have on their crystal networks and oil binding capacity remains unknown.
Chopin-Doroteo et al. investigated the effect to shear (600 s−1) applied to CLX oleogels during cooling until reaching nucleation temperatures (47 °C) or metastable conditions prior to nucleation (52 °C) [12]. For both conditions, they reported an increase in crystal size for 3 % CLX oleogels prepared with safflower oil compared to statically cooled gels. The yield stress and elastic modulus were higher for CLX gels that were sheared to metastable conditions (52 °C) compared to gels sheared until nucleation (47 °C), and both sheared gels exhibited a higher elastic modulus compared to statically cooled gels. They concluded that shearing to metastable conditions promoted the formation of larger crystal platelets, as well as meshing between crystals, resulting in a higher proportion of transient junction zones to permanent junction zones compared to statically cooled gels.
More recently, their group has elaborated on the formation and contribution of these flow induced structures to crystal morphology [13, 14]. Shearing to pre-nucleation metastable conditions (52 °C), particularly at shear rates below 300 s−1, establishes optimal flow conditions that promote molecular alignment of wax components, resulting in the formation of molecular structures that influence nucleation and crystallization kinetics and serve as precursors of the large crystal platelets observed within sheared CLX gels. Higher shear rates are not conducive to the molecular alignment required for the formation of these molecular structures, for which reason large crystal platelets were not observed at shear rates above 300 s−1. Finally, they confirmed that shearing to metastable conditions prevented crystal breakage and allowed for the development of junction zones, while shearing to nucleation (47 °C) damaged the network that began developing during crystallization.
Although this work signals progress, the effect of shear on the oil binding capacity as a consequence of microstructural changes for wax oleogels remains unclear, creating a large gap hindering the use of wax oleogels in commercial food applications. The ability to deliberately tailor the oil binding capacity of wax oleogels via controlled shear-induced microstructural changes will allow for the use of these gels in a wide range of applications.
With this in mind, the objective of this study is to determine the effect of shear on the oil binding capacity of three wax oleogels. Changes in microstructure due to shear including crystal length, fractal dimension, and pore area, are used to explain oil loss trends in order to determine the shear sensitivity and the contribution of entrapment and adsorption on oil binding capacity. The shear sensitivity and oil binding capacity of oleogels prepared with an industrial commercial stabilizer is also included as an industry benchmark.
Materials and Methods
Gel Preparation
Gels were prepared by depositing a known amount of solid wax or commercial stabilizer in a 15 mL glass vial containing peanut oil. The wax-oil mix was placed in a 90 °C oven for 30 min to allow for complete melting and dissolution of wax crystals. After melting, the molten gel was distributed among 1.5 mL plastic centrifuge vials (three vials per gel). Vials were cooled under quiescent conditions (23 °C) for 24 h before testing.
Wax concentrations included 0.5, 1, 2 and 5 % (w/w), and concentrations of the commercial stabilizer included 5, 6, 7 and 8 % (w/w). These concentrations were selected to include wax concentrations that were below, equivalent to, and above the critical concentration of each gelator, denoted as C*. The C* of the commercial stabilizer was 6.5 % (w/w), while that of RBX, SFX, and CLX were determined to be 1, 1 and 2 % (w/w), respectively. This values are in agreement with previous reports, and were determined using an inversion test, as described in our previous study [15]. Waxes were provided by Koster Keunan Inc., (Connecticut, USA) and GRINDSTED PS 105 K-A, a commercial stabilizer, was provided by DuPont Danisco. The stabilizer is added to peanut butter to prevent oil separation and is a blend of fully hydrogenated rapeseed, soybean, and cottonseed oils [16]. The major fatty acids present in the stabilizer are stearic acid (57 %), behenic acid (25 %), palmitic acid (11 %), and arachidic acid (5 %). 100 % pure Peanut oil was provided by Loblaws Inc. (Toronto, ON).
Differential Scanning Calorimetry
Peak melting and crystallization temperatures for wax gels were obtained using a TA Instruments Q2000 Differential Scanning Calorimeter. The samples for DSC measurements were prepared by placing a weighed amount of the molten gel (3–7 mg) in an aluminum pan. The pans were then hermetically sealed and stored at ambient temperature for at least 24 h prior to testing. Samples were allowed to equilibriate at 20 °C for 2 min, and then heated to 90 °C at a rate of 5 °C/min. This heating cycle was followed by a cooling cycle from 90 to 20 °C at the same cooling rate. The peak melting temperatures (Tm) and peak crystallization temperatures (Tc) were determined from the DSC curves using TA Instruments’ Universal Analysis Software supplied with the instrument. Results were obtained in triplicate.
Shearing at Different Cooling Rates
Samples were sheared using a concentric cylinder geometry equipped to an AR 2000 Rheometer (Q2000, TA Instruments, New Castle, DE). Molten wax-oil mixtures were poured into the hollow cylinder, which was pre-heated to 90 °C. The geometry was kept as this temperature for 10 min to allow the mix to equilibriate. Using the software provided by TA Instruments, the temperature of the geometry was reduced at a preset cooling rate of 1.5 °C/min or 5 °C/min. The cooling cycle terminated once the temperature of the geometry reached Tm-10 °C. During cooling, the samples were sheared at a shear rate of 50s−1. A wide plastic pipette, preheated to Tm-10 °C using a convective oven, was then used to draw the molten gels from the cylindrical container and distribute each gel among three 1.5 mL plastic centrifuge vesicles. Vesicles were cooled under quiescent conditions (23 °C) for 24 h before testing.
Application of Cooling Rate
Statically cooled samples were cooled at 1.5 °C/min or 5 °C/min. A cooling rate of 1.5 °C/min was achieved by cooling under quiescent conditions, while cooling at 5 °C/min was achieved using a series of water baths calibrated to different temperatures. 1.5 °C/min was selected as the slow cooling rate since it was achieved by cooling the gels under static conditions at room temperature. 5 °C/min was selected as a faster cooling rate since it was easily achieved using a series of water baths.
The cooling process for 5 °C/min gels was as follows. First, molten gels were distributed among 1.5 mL plastic centrifuge vials (three vials per gel) and placed in a 72 °C water bath for 15 min to equilibriate. The vials were then placed in a 64 °C water bath for 60 s, placed at room temperature (23 °C) for 60 s, placed in a 55 °C water bath for 60 s, placed at room temperature for 4 min, and then placed in a 10 °C fridge for 3 min until the gels reached a temperature of 23 °C, determined using a thermometer probe. Once gels reached this final temperature, they were kept under quiescent conditions for 24 h before further testing. Temperature was monitored using a digital thermometer. The variance in cooling rate was ± 0.5 °C/min
Brightfield Microscopy
Samples were prepared by depositing a drop of gel onto a heated glass microscope slide. The gel was pressed with a glass cover slip to ensure a sample thin enough for light microscopy. Slides were allowed to cool under quiescent conditions for 24 h before imaging. Imaging was performed using a Leica DM RXA2 microscope in brightfield mode (Leica Microsystems, Richmond Hill, Canada) using a CCD camera (Q Imaging Retiga 1300, Burnaby, BC, Canada). Images were acquired using Openlab 6.5.0 software (Improvision, Waltham, MA, USA) and a 40X objective lens (Leica, Germany). Images were obtained in triplicate.
Oil-Binding Capacity
The oil-binding capacity was measured by centrifuging (Eppendorf Centrifuge 5410, maximum rotary speed: 14,000 rpm, or relative centrifugal force: 21.8 × 103 g) a 1.5 mL plastic centrifuge vial containing a known mass of sample (approximately 1–1.5 g) for 30 min. Centrifuge vials were weighed before and after the addition of gels so that the actual sample mass could be obtained. After each centrifuge cycle, the supernatant (loose oil) was decanted, and the mass of the sample was recorded. This was repeated for four centrifuge cycles, with each cycle lasting 30 min. OL1 and OL4 denote oil loss after the first and final centrifuge cycle, respectively. These are representative of short-term oil loss (OL1) and long-term oil loss (OL4), and were calculated as the percentage of decanted oil after the first and final centrifuge cycle:
Microstructural Analysis
ImageJ (National Institute of Mental Health, Bethesda, ML, USA) was used to measure crystal length for 40X micrographs. The measuring tool in the software program was calibrated using the scale bar of each micrograph. A line was then drawn along each crystal, and the length of that line was recorded as the crystal length. Three micrographs were analyzed following this procedure for each gel.
Fractal Dimension and Pore Area
The box-counting fractal dimension of micrographs was determined using Benoit 1.3 (Trusoft International Inc., St. Petersburg, FL, USA). Micrographs were thresholded using Adobe Photoshop CS2 (Adobe Systems Inc., San Jose, USA) using the automatic threshold setting, and processed by Benoit 1.3 to give a fractal dimension. The fractal dimension reported here is an average taken from three micrographs. A detailed description of the mathematical process and algorithms used to determine the fractal dimension has been provided by Tang and Marangoni [17]
For each micrograph, solid crystalline mass is represented by white pixels, while empty pore space is indicated by black pixels. Dividing the number of white pixels by the total number of pixels provides the percentage of space occupied by white pixels, or solid mass. This is denoted as %Fill. The percentage of area occupied by pores, referred to as the pore area fraction, AFp, can be determined using the following relationship:
As the percentage of the black pixels in a micrograph increase, so does the pore area fraction, meaning that a larger portion of the gel network captured in a micrograph is occupied by pore space.
Statistical Analysis
GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) was used for curve-fitting and statistical analysis. Two-way analysis of variance (ANOVA) and linear correlations between the microstructural data and oil-binding parameters of gels were conducted with three replicates for each measurement for each gel. The level of significance for a correlation was chosen as p = 0.05. Graphical figures were exported from Graphpad Prism 5.0 as tiff files.
Results
The aim of this study was to assess the effect of shear on the network morphology and oil binding capacity of RBX, CLX, and SFX oleogels. Oil loss is reported after the first centrifuge cycle (OL1) and final centrifuge cycle (OL4). Wax type, wax concentration, and microstructural parameters including mean crystal length, fractal dimension (Db), and pore area fraction (AFp) were compared between sheared and statically cooled gels prepared at either 1.5 °C/min (i.e. slow cooling) or 5 °C/min (i.e. fast cooling). Only results pertaining to sheared gels are compared since the effect of cooling rate on gel microstructure and oil binding capacity has been recently reported (A.I. Blake, A.G. Marangoni, The Use of Cooling Rate to Engineer the Microstructure and Oil Binding Capacity of Wax Crystal Networks, Under Review)
A commercial stabilizer (CS) commonly added to peanut butter to prevent oil separation was included in this study. The C* of the CS is 6.5 % (w/w), which is considerably higher than the C* of RBX, SFX, and CLX. This difference in C* demonstrates the superior oil structuring ability of these waxes. Unfortunately, CS oleogels were too dense to image using optical light microscopy due to the high solid concentrations required for gelation. As a result, information about crystal length, Db, or AFp could not be obtained. Only the OL1 and OL4 values of CS oleogels can be compared to the OL1 and OL4 values of wax oleogels.
Oil Loss Results – Shear Under Slow Cooling Rates
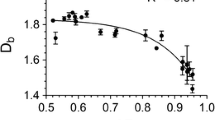
Shearing reduced OL1 and OL4 for all concentrations of RBX compared to statically cooled gels, as seen from the OL values listed in Table 1 (p < 0.001). For 1 and 2 % gels, sheared decreased AFp and increased Db (p < 0.05), as seen in Table 2. As reported in an earlier study, a high Db indicates that a network contains homogeneously distributed crystals [15]. A high Db or homogenous distribution of crystalline mass reduces the total pore area or pore area fraction, meaning that any free oil migrating through the network must traverse a highly tortuous pathway in order to escape the network. As a result, there is less oil loss compared to a network containing clustered crystals (characterized by a low Db) and a larger AFp, which allows greater volumes of oil to migrate through the network [15] (A.I. Blake, A.G. Marangoni, The Use of Cooling Rate to Engineer the Microstructure and Oil Binding Capacity of Wax Crystal Networks, Under Review).
There was no statistical difference between Db or AFp parameters for 0.5 % gels. Rather, the difference in oil loss for 0.5 % RBX gels may be a consequence of crystal orientation and aggregation, which would likely affect AFp and Db. The application of shear creates flow patterns that are parallel to the direction of shear. These flow patterns align nuclei and gelator molecules and may increase the frequency of collision between crystals, causing aggregation. As seen in Fig. 1, crystals present in sheared RBX gels appear thicker and darker, possibly due to shear-induced aggregation.
Optical light micrographs obtained for RBX gels at 20X. Gels cooled statically at 1.5 °C/min are shown in left column, and sheared gels cooled at the same cooling rate are shown in the right column. Increases in wax concentration follow the order of 0.5 % (top row), 1 % (second row), 2 % (third row), and 5 % (bottom row)
Finally, at equivalent wax concentrations, sheared gels contained shorter wax crystals, except for 0.5 % gels (p < 0.0001). This might be due to either increases in nucleation rates or breakage caused by shear. Mean crystal lengths for sheared and statically cooled gels are listed in Table 3.
For SFX gels, shear increased OL1 and OL4 compared to statically cooled gels (p < 0.001). This is surprising since sheared 1 % SFX gels had a higher Db than 1 % statically cooled gels (p < 0.001), and both 1 and 2 % SFX gels had lower AFp values compared to statically cooled gels (p < 0.05). Similarly to RBX gels, sheared SFX gels also contain thicker crystals, as seen in Fig. 2. This morphological change will reduce the amount of crystal surface area available for oil adsorption, which as discussed in a previous study (A.I. Blake, A.G. Marangoni, The Use of Cooling Rate to Engineer the Microstructure and Oil Binding Capacity of Wax Crystal Networks, Under Review) greatly influences the oil binding capacity of SFX gels.
Optical light micrographs obtained for SFX gels at 20X. Gels cooled statically at 1.5 °C/min are shown in left column, and sheared gels cooled at the same cooling rate are shown in the right column. Increases in wax concentration follow the order of 0.5 % (top row), 1 % (second row), 2 % (third row), and 5 % (bottom row)
Interestingly, a 0.5 % SFX gel could not form under static cooling, but formed when exposed to shear. Crystal aggregation and possibly reorientation due to shear may be responsible for this observation. It is possible that at such a low solid content, surface area alone is not capable of driving gelation. Instead, some degree of interconnectedness may be required to establish a network and induce gelation. Crystal aggregation or reorientation may provide this by forming more junction zones between microstructural elements.
Shear also increased OL1 and OL4 for CLX gels at equivalent wax concentrations (p < 0.001). However, under slow cooling conditions, shearing led to the development of large crystal platelets ranging between 20 and 30 um in diameter. This is in agreement with previous reports of increases in crystal size for CLX gels due to shear [12, 13]. Interestingly, there was no difference between Db or AFp values between sheared and static gels. The difference in oil loss between sheared and statically cooled CLX alludes to the influence of surface effects. A network comprised of large platelets would have less surface area. Therefore, less oil could adsorb onto the surface of wax crystals, thereby increasing the proportion of weakly bound oil. As a result, a system that is dependent on oil adsorption as an oil binding mechanism, such as CLX, is expected to exude more oil due to this change in surface area compared to a wax that is less dependent on oil adsorption, such as RBX [15] (A.I. Blake, A.G. Marangoni, The Use of Cooling Rate to Engineer the Microstructure and Oil Binding Capacity of Wax Crystal Networks, Under Review) (Fig. 3).
Optical light micrographs obtained for CLX gels at 20X. Gels cooled statically at 1.5 °C/min are shown in left column, and sheared gels cooled at the same cooling rate are shown in the right column. Increases in wax concentration follow the order of 1 % (top row), 2 % (second row), and 5 % (bottom row)
With the exception of 5 % gels, OL1 and OL4 were higher for sheared CS gels compared to statically cooled CS gels at equivalent gelator concentration (p < 0.01). This suggests that the CS is not shear sensitive. Considering that rapid cooling improved the oil binding capacity of CS gels, which presumably is related to a decrease in average crystal size due to supersaturation effects, it can be hypothesized that the oil binding capacity of CS oleogels can be improved by reducing crystal size using rapid cooling or shear (A.I. Blake, A.G. Marangoni, The Use of Cooling Rate to Engineer the Microstructure and Oil Binding Capacity of Wax Crystal Networks, Under Review). Whether this is related to an increase in surface area and oil adsorption remains unclear.
At equivalent wax concentration, OL1 and OL4 were higher for sheared SFX gels compared to sheared RBX gels (p < 0.001). Db and AFp values were not statistically different. However, 0.5 and 2 % SFX gels contained shorter crystals than RBX gels (p < 0.001). Shorter crystals yield a larger surface available for adsorption, which is why the expectation of this difference in crystal length is that SFX, which is strongly dependent on oil adsorption for oil binding, would retain more oil than RBX.
Instead, SFX retained less oil than RBX gels. As seen in Figs. 1 and 2, both RBX and SFX gels appear to have experienced an increase in crystal thickness when sheared. The fact that RBX responded positively to this change while SFX responded negatively implies that SFX is shear sensitive. This sensitivity may stem from changes in crystal surface area and oil adsorption, or from changes in the amount and type of interactions between microcrystalline elements at junction zones.
Sheared CLX gels retained less oil than sheared RBX gels at equivalent concentrations (p < 0.001). AFp was smaller for 1 % RBX gels compared to 1 % CLX gels (p < 0.01), and there was no statistical difference between Db values. It is unlikely that this difference in oil loss is due solely to an improvement in RBX’s oil binding capacity caused by shear-induced microstructural changes. The change in CLX crystal morphology is also responsible, considering the reduction in surface area and oil adsorption associated with the increase in platelet size.
OL1 and OL4 were higher for 2 and 5 % SFX gels compared to CLX gels (p < 0.001). This was not expected since RBX gels, with a similar microstructure to that of SFX gels, retained more oil than CLX gels. Presumably, SFX gels would then also retain more oil than CLX gels. However, Db was smaller for 2 and 5 % SFX gels (p < 0.05), and AFp was smaller for CLX gels (p < 0.05). Homogeneously distributed mass and a smaller pore area will constrict oil flow and reduce oil loss, explaining why sheared CLX gels retained more oil compared to sheared SFX gels.
It is also worth noting that at 1 % concentration, OL1 and OL4, Db, and AFp, were not statistically different between SFX and CLX gels despite drastic differences between their crystal morphology. This implies that Db and AFp are the fundamental factors that determine the oil binding capacity of a gel regardless of its morphology.
Under slow cooling, sheared RBX gels retained more oil compared to CS gels, as seen in Table 1. At all concentrations, OL1 and OL4 were higher for SFX gels than for CS gels at equivalent concentrations. For CLX gels, only 2 and 5 % gels had higher OL1 and OL4 values compared to CS gels. Even though shear improved the oil binding capacity of CS gels, wax oleogels remain more efficient gelators when sheared under slow cooling conditions (i.e. 1.5 °C/min) not only because they retain more oil than CS gels, but also because a lower concentration of wax gelator is required to achieve this higher level of oil binding. Thus, the CS is a less efficient gelator.
Oil Loss – Shear Under Fast Cooling Rates
As seen in Table 4, shear increased OL1, OL4, and mean crystal length for 2 % RBX gels compared to statically cooled 2 % RBX gels (p < 0.001).For 1 % gels, OL1 was higher for sheared gels, and OL4 was higher for static gels (p < 0.01). Shear had no effect on mean crystal length for 1 % RBX gels. For both concentrations, Db values were not statistically different, as seen in Table 5. However, shear decreased AFp for 1 % RBX gels (p < 0.001).
Shear increased OL4 for 2 and 5 % gels SFX gels. There was no difference in Db or AFp at these equivalent wax concentration. Sheared0.5 and 1 % SFX gels contained longer crystals compared to statically cooled SFX gels at equivalent wax concentrations (p < 0.01) (Table 6).
For CLX gels, shear reduced OL1 and OL4 for 1 % gels (p < 0.001). At this concentration, AFp between static and sheared gels. Shear increased crystal length for 1 and 2 % sheared CLX gels compared to statically cooled gels (p < 0.001). In addition, the AFp was higher for 2 % sheared gels compared to statically cooled gels, despite their similar OL values. For 5 % gels, crystal length decreased under the application of shear, perhaps due to breakage (p < 0.0001), but this did not cause any significant change in AFp. In addition, the large platelets observed in sheared CLX gels cooled at 1.5 °C/min were absent. There was no significant difference in Db between sheared and statically cooled gel at any equivalent wax concentration.
OL1 and OL4 increased for CS gels when sheared, but only when the wax concentration exceeded the C*. Again, these findings suggest that the oil binding capacity of CS gels can be improved by decreasing crystal size, assuming that is the microstructural consequence of shear.
Only sheared CLX gels consistently outperformed CS gels with respect to oil loss. RBX and SFX gels retained more oil than CS gels only when the wax concentration exceeded the C* (i.e. 1 % wax). Again, a greater concentration of CS is required to attain this level of oil binding, making it a less efficient and perhaps less economical choice of gelator compared to waxes.
The interpretation of these results is complicated given the confounding effects of shear and rapid cooling. Both factors affect the kinetics of nucleation and crystallization, making it difficult to separate their respective contributions to changes in gel microstructure and oil binding capacity. For instance, it is not clear if the similarity in mean crystal lengths between sheared and statically cooled gels containing equivalent wax concentrations is due to supersaturation and increases in nucleation rates caused by rapid cooling or by shear. The effect of cooling rate on nucleation and crystal growth may have dominated over the effects of shearing, particularly at a low shear rate of 50s−1, for which reason very few differences are observed between sheared and statically cooled gels containing equivalent wax concentrations.
Discussion
The effect of shear on the microstructure and oil binding capacity of wax oleogels varied with wax type. Under slow cooling conditions, RBX gels retained more oil when sheared, while SFX and CLX gels did not. For both RBX and SFX gels, shearing resulted in crystal aggregation. This was favorable for RBX gels, causing an increase in Db, decrease in AFp, and thus a decrease in oil loss.
For SFX gels, crystal aggregation increased oil loss, likely due to a decrease in crystal surface area. In a recent study examining the effect of cooling rate on the oil binding capacity of the same gels, we concluded that SFX’s oil binding is highly dependent on adsorption as an oil binding mechanism. A decrease in surface area is therefore expected to correspond with an increase in oil loss.
Another possible explanation is that crystal aggregation either increased or decreased the number of interactions between crystals (i.e., permanent and transient junction zones), which in turn will affect the macroscopic properties of the gel. A rheological comparison of the elastic and storage moduli obtained for sheared versus statically cooled SFX gels could confirm or disprove this hypothesis.
CLX gels did not experience crystal aggregation per se, but rather an increase in platelet size. This observation is in agreement with previous report by Chopin-Doroteo et al.[12]. Their explanation was that shear promoted molecular alignment that led to the formation of flow induced mesophase structures that augment nucleation and crystallization processes. This phenomenon may only be possible for chemically heterogeneous materials, since an array of major and minor components allows for a greater combination of crystallization pathways, thereby increasing the diversity of attainable morphologies.
This would explain why the morphology of RBX and SFX were not similar to the morphology of CLX gels when sheared at low cooling rates. It also points to the possibility of tuning the morphology of a gel network via the addition of minor components or impurities. The application of shear to these mixed systems may render unique microstructural arrangements with unique macroscopic behaviour.
At higher cooling rates, CLX gels did not display large platelets. It is likely that molecular alignment and the formation of flow induced structures was interrupted by an increase in the rate of nucleation due to faster cooling. In general, the combination of shear and rapid cooling create a highly complex and dynamic environment for crystal nucleation and growth that is difficult to interpret. In future studies, it might be beneficial to include the use of higher shear rates as they will affect the gel network more aggressively, which may ease the task of differentiating between network effects of shear and cooling rate.
Fortunately, the effect of shear rate as an isolated variable is easier to interpret. Shear will influence crystal nucleation and crystal aggregation. To address the first factor, the mean crystal length decreased when RBX and SFX gels were sheared under slow cooling. This is more likely due to an increase in nucleation rates rather than breakage since shearing did not continue throughout the entire crystallization process. Instead, shearing during nucleation likely increased the population of nuclei, requiring the reduced quantity of residual wax molecules available for crystal growth to distribute among more nuclei, resulting in a larger number of shorter crystals.
Subsequent changes in crystal growth rates due to this change in nucleation rate may have contributed to the formation of thicker fibers, seen in Figs. 1 and 2. It is more probable, however, that crystal aggregation is the culprit. Above a certain size, nuclei and growing crystals are easily caught in directional flow patterns caused by shear. At low shear rates such as 50s−1, crystals may collide with other crystals sharing their trajectory and experience little to no damage. At higher shear rates, such collisions may result in breakage due to the high velocity of the flow patterns and moving crystals, which are proportional to the velocity of the shear gradient.
It is these types of microstructural changes that complicate the interpretation of oil loss results. In a previous study examining the effect of cooling rate, we reported strong correlations between oil loss, Db, and AFp (A.I. Blake, A.G. Marangoni, The Use of Cooling Rate to Engineer the Microstructure and Oil Binding Capacity of Wax Crystal Networks, Under Review). More specifically, Db negatively correlated with oil loss and AFp, and oil loss positively correlated with AFp under both slow and rapid cooling. We demonstrated that the total pore area of a system can be manipulated to achieve a specific Db, irrespective of wax type or cooling rate, and concluded that both the pore area fraction and fractal dimension of s network can be used to tailor the gel’s oil binding capacity.
These trends were not consistently apparent for sheared gels. For instance, CLX gels retained less oil than RBX gels when sheared under slow cooling rate despite the fact that CLX gels had a higher Db and lower AFp. The explanation for these inconsistencies may be that shear alters the topology of wax crystals and causes either orientation or aggregation that is not completely captured by quantifying microstructural parameters such as Db and AFp.
Another important factor to consider is wax concentration. As seen in Tables 1 and 4, increasing the wax concentration reduced oil loss. However, significant differences in oil loss, AFp, and Db occurred more frequently for 1 and 2 % gels, and less commonly for 0.5 and 5 % gels. This may be due to a concentration-dependent shift in gel state. The addition of 5 % wax may impart too much “solid” character on the oleogel network, while the addition of very low concentrations, such as 0.5 % wax, may not impart enough solid character and instead produce a liquid-like system. There may exist a threshold solid concentration above which the solid volume fraction is so high that it dominates the physical behaviour of the system, causing a transition from a gel state to solid state.
A concentration of 5 % may exceed this threshold concentration, meaning that the oil binding capacity of a 5 % gel is not as sensitive to Db and AFp as a “true” gel. Similarly, at very low solid concentrations, a ‘weak’ gel state may develop with unique properties that do not completely align with the behaviour of a “true” gel. Unfortunately, a clear definition of what constitutes a “gel” does not exist, making it difficult to distinguishing the boundaries between a gel and a system containing either a very high or a very low amount of solids [15].
Gels sheared under high cooling rates demonstrated even less obedience to these trends, further demonstrating the complex relationship between the structure of a gel network and its oil binding capacity. A more general explanation proposed by Acevedo and Marangoni is that the oil binding capacity of a gel network is not just determined by crystal size, shape, Db, and solid content, but also by intermolecular interactions that are more difficult to detect, interpret, and quantify [7].
The threshold shear rate above which crystal aggregation or orientation effects are not observed is often referred to as the critical shear rate. It would be beneficial to include a wider range of shear rates in future studies to determine if the microstructure and oil binding capacity of wax oleogels can be controllably engineered as a function of shear rate. Such knowledge may also clarify the predictive power of Db and AFp as network parameters describing oil binding capacity.
In their study on the effect of shear on rheology of CLX oleogels, Chopin-Doroteo et al. determined that the critical shear rate was 300 s−1. Below this shear rate, CLX gels exhibited increasing G’ values and platelet morphology. At higher shear rates, the G’ decreased and platelets were not observed. Acevedo and Marangoni established that the crystal size, network permeability, and oil loss increased as the shear rate approached the critical shear rate of 240 s−1 [7]. In contrast, Maleky et al. found that cocoa butter crystal size decreased when sheared at higher shear rate of 340 s−1, and that this decrease in crystal size corresponded to an increase in the Young’s and storage modulus and a decrease in oil migration compared to statically cooled cocoa butter [18–20]. Clearly, the rate of shear is an important factor contributing to the network structure and macroscopic functionality of a gel.
Finally, shearing improved the oil binding capacity of the commercial stabilizer, particularly under low cooling rates. The fact that shearing or rapidly cooling the gel under static conditions, as seen in our previous report, are both capable of reducing oil loss, suggests that the oil binding capacity of CS gels can be improved by reducing crystal size. Further work is required to determine if this is due to surface area effects, or due to changes in the crystal packing and distribution (i.e. Db and AFp). However, the results obtained in this study indicate that under slow cooling rates, the CS provides better oil binding compared to SFX and CLX, albeit at a higher gelator concentration. At higher cooling rates, CS is not the economical or most efficient choice since very high concentrations are required to achieve a level of oil binding that is comparable to waxes.
Conclusions
This study examined the effect of shear under two different cooling regimes on the microstructure and oil binding capacity of wax oleogels. Under slow cooling at 1.5 °C/min, the application of shear at a rate of 50s−1 improved the oil binding capacity of RBX oleogels and decreased the oil binding capacity of SFX and CLX oleogels cooled. These changes in oil binding capacity were attributed to changes in crystal morphology.
Under a slow cooling rate, shear increased Db and decreased AFp for RBX oleogels, creating a tortuous network for loose oil to migrate through. In comparison, the morphological changes experienced by SFX and CLX oleogels reduced surface area and oil adsorption, increasing oil loss. These observations confirm SFX and CLX’s dependency on oil adsorption as oil binding mechanisms. The oil binding capacity of the CS oleogels improved with shear, likely due to changes in crystal size and changes in Db and AFp. Further work is required to confirm this theory.
Unfortunately, the interpretation of oil loss results obtained under rapid cooling are difficult to confidently interpret due to the confounding effects of shear and rapid cooling. Future studies including higher shearing rates may clarify this issue and provide greater insight into the potential of tailoring the microstructure and oil binding capacity of wax oleogels using shear rate as the independent variable. It is also possible that, under low shear rates, cooling rate is the dominating factor contributing to network morphology and formation.
From these findings, we can conclude that RBX is a more appropriate choice of gelator compared to other waxes if processing conditions will include shear, followed by the commercial stabilizer employed in this study. However, the difference in solid content between CS gels and wax gels required for this should be considered.
References
N.E. Hughes, A.G. Marangoni, A.J. Wright et al., Potential food applications of edible oil organogels. Trends Food Sci. Technol. 20, 470–480 (2009). doi:10.1016/j.tifs.2009.06.002
R. Wang, X.-Y. Liu, J. Xiong, J. Li, Real-time observation of fiber network formation in molecular organogel: supersaturation-dependent microstructure and its related rheological property. J. Phys. Chem. B 110, 7275–80 (2006). doi:10.1021/jp054531r
E. Co, A.G. Marangoni, Organogels: An alternative edible oil-structuring method. J. Am. Oil Chem. Soc. 89, 749–780 (2012). doi:10.1007/s11746-012-2049-3
M.A. Rogers, A.G. Marangoni, Non-Isothermal Nucleation and Crystalliztion of 12-Hydroxystearic Acid in Vegetable Oils. Cryst. Growth Des. 8, 8–13 (2008)
M.A. Rogers, A.G. Marangoni, Solvent-modulated nucleation and crystallization kinetics of 12-hydroxystearic acid: a nonisothermal approach. Langmuir 25, 8556–66 (2009). doi:10.1021/la8035665
M. Rogers, Novel structuring strategies for unsaturated fats – Meeting the zero-trans, zero-saturated fat challenge: A review. Food Res. Int. 42, 747–753 (2009). doi:10.1016/j.foodres.2009.02.024
N.C. Acevedo, J.M. Block, A.G. Marangoni, Critical laminar shear-temperature effects on the nano- and mesoscale structure of a model fat and its relationship to oil binding and rheological properties. Faraday Discuss. 158, 171 (2012). doi:10.1039/c2fd20008b
C. O’Sullivan, N. Acevedo, F. Peyronel, A.G. Marangoni, Fat Nanostructure. Edible Nanostructures A Bottom-Up Approach (2014)
G. Mazzanti, S.E. Guthrie, E.B. Sirota, et al., Orientation and Phase Transitions of Fat Crystals under Shear 2003. 1–5 (2003)
S. da Pieve, S. Calligaris, E. Co et al., Shear nanostructuring of monoglyceride organogels. Food Biophys. 5, 211–217 (2010). doi:10.1007/s11483-010-9162-3
E. Co, A.G. Marangoni, The formation of a 12-Hydroxystearic Acid/Vegetable oil organogel under shear and thermal fields. J. Am. Oil Chem. Soc. 90, 529–544 (2013). doi:10.1007/s11746-012-2196-6
M. Chopin-Doroteo, J.A. Morales-Rueda, E. Dibildox-Alvarado et al., The effect of shearing in the thermo-mechanical properties of candelilla wax and candelilla wax–tripalmitin organogels. Food Biophys. 6, 359–376 (2011). doi:10.1007/s11483-011-9212-5
F.M. Alvarez-Mitre, J.A. Morales-Rueda, E. Dibildox-Alvarado et al., Shearing as a variable to engineer the rheology of candelilla wax organogels. Food Res. Int. 49, 580–587 (2012). doi:10.1016/j.foodres.2012.08.025
F.M. Alvarez-Mitre, J.F. Toro-Vázquez, M. Moscosa-Santillán, Shear rate and cooling modeling for the study of candelilla wax organogels’ rheological properties. J. Food Eng. 119, 611–618 (2013). doi:10.1016/j.jfoodeng.2013.06.009
A.I. Blake, E.D. Co, A.G. Marangoni, Structure and physical properties of plant wax crystal networks and their relationship to oil binding capacity. J. Am. Oil Chem. Soc. 91, 885–903 (2014). doi:10.1007/s11746-014-2435-0
Danisco GRINDSTED® PS 105 K-A. http://www.ulprospector.com/en/na/Food/Detail/3882/111475/GRINDSTED-PS-105-K-A. Accessed 12 Jan 2015
D. Tang, A.G. Marangoni, Microstructure and fractal analysis of fat crystal networks. J. Am. Oil Chem. Soc. 83, 377–388 (2006). doi:10.1007/s11746-006-1216-9
F. Maleky, A.K. Smith, A. Marangoni, Laminar shear effects on crystalline alignments and nanostructure of a triacylglycerol crystal network. Cryst. Growth Des. 11, 2335–2345 (2011). doi:10.1021/cg200014w
F. Maleky, A. Marangoni, Thermal and mechanical properties of cocoa butter crystallized under an external laminar shear field. Cryst. Growth Des. 11, 2429–2437 (2011). doi:10.1021/cg200202u
F. Maleky, A. Marangoni, Nanoscale effects on oil migration through triacylglycerol polycrystalline colloidal networks. Soft Matter 7, 6012 (2011). doi:10.1039/c1sm05154g
Acknowledgments
The authors would like to acknowledge the financial support provided by the Natural Science and Engineering Research Council of Canada.
Conflict of Interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blake, A.I., Marangoni, A.G. The Effect of Shear on the Microstructure and Oil Binding Capacity of Wax Crystal Networks. Food Biophysics 10, 403–415 (2015). https://doi.org/10.1007/s11483-015-9398-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-015-9398-z