Abstract
In February 2015, 13 “spice-like” products, available on the German market, were analyzed. In total, eight different synthetic cannabinoids were identified by gas chromatography–mass spectrometry (GC–MS), namely 5-fluoro-AB-PINACA, AB-CHMINACA, AB-FUBINACA, 5-fluoro-PB-22, 5-fluoro-AMB, MDMB-CHMICA, EAM-2201 and STS-135. In most of the products (11/13), only one synthetic cannabinoid was identified, while two products contained two or three synthetic cannabinoids. For some of the compounds (5-fluoro-AB-PINACA, AB-CHMINACA, 5-fluoro-AMB) only insufficient physicochemical data are available in literature. To our knowledge MDMB-CHMICA (sometimes mistakenly referred to as MBMD-CHMINACA) was not described earlier in scientific literature, and, hence, an in-depth characterization of these compounds by nuclear magnetic resonance (NMR) spectroscopy, electron ionization–mass spectroscopy, electrospray ionization–tandem mass spectroscopy (ESI-MS/MS), infrared spectroscopy and ultraviolet spectroscopy was conducted for eight compounds. In addition, we developed an ESI-MS method for the direct quantification of synthetic cannabinoids in commercial smoking blends, without chromatographic separation. Quantification was achieved using methyl 3-(3-(1-naphthoyl)-1H-indol-1-yl)propionate as the internal standard with appropriate response factors. The total contents of synthetic cannabinoids in the investigated products ranged from 60 to 446 mg/g, while individual compounds ranged from 12 to 314 mg/g.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its first broad public perception in 2008 [1, 2], “spice/K2-like” herbal incenses, containing synthetic cannabinoids as psychoactive ingredients, have developed rapidly. For the past several years, this class of designer drugs showed the highest degree of diversification worldwide, leading to the highest numbers of newly appearing substances as listed by the annual reports of national and international monitoring institutions (European Monitoring Centre for Drugs and Drug Addiction [3] (EMCDDA) or United Nations Office on Drugs and Crime (UNODC) [4] ). Currently, most of the synthetic cannabinoids are not controlled by any international convention (exemption: JWH-018 and AM-2201 under schedule II by UNODC [5]). Hence, many compounds are individually regulated in many countries which leads to strong variations of detected and regulated substances from country to country. If national laws are installed to control these compounds, this new regulation is easily invalidated by new compounds already in place on other markets. In the beginning, the European market was the driving force in this vivid fluctuation of compounds, but gave way to the Asian Pacific region and the Russian federation, which is probably due to the fact that these markets are closer to the primary production sites probably in China and/or India. Especially changes in the perception and regulation of new psychotropic substances (NPSs) in Japan seem to have propelled the necessity to cope with new compounds by the fast adjusting Japanese regulations [6]. Especially a stepwise tightening of the legal framework resulted in the generic scheduling of substances showing “possible pharmacological activity”, which was estimated using a quantitative structure–activity relationship approach. As a result, since September 2013, a total of 881 substances are now regulated and restricted in Japan as “designated substances” [6]. However, this rapid evolution of new cannabimimetics in this region is now slowly leaking into other global markets in Europe or the USA. Some EU-member states, for example, the United Kingdom, Ireland or Luxembourg, have also adopted generic approaches to control similarly structured synthetic cannabinoids.
Looking at the specific situation for Germany, Fig. 1 shows the increase of NPSs over the last several years. By the end of 2013, 102 NPSs were listed by the EMCDDA [3]. Also, as shown in Fig. 1, the last update of the German regulation for narcotics from May 2015 lists a total of 52 substances [7].
Like in Japan, the current legal regulation has great impact on the national situation. In Germany, only the listed compounds are considered narcotics and their misuse is enforced by this law. However, in the past, the responsible authorities applied the German Medicines Act to control newly appearing substances, assuming that non-listed compounds with related structures show similar psychoactive properties. Two very recent judicial decisions, however, have strongly affected this common practice and created a kind of legal vacuum. First, the European Court of Justice has prohibited the application of the Medicines Act to NPSs because these compounds are not “pharmaceuticals” within the meaning of that law [8]. Secondly, as is common for other regulated narcotics, there is a differentiation concerning the amount of active substance in possession, the seriousness of the offense and the level of sanctions. While, for “traditional narcotics”, there is sufficient toxicological data to define the lowest effective dose and to calculate a small amount (for personal consumption) and a not-small amount (considered as dealing the drug); this was recently also applied for regulated synthetic cannabinoids [9]. By the judgment of the court, the not-small amount for JWH-018 and CP 47,497-C8-homologue were each set at 2 g, and JWH-073 and CP 47,497 were each set at 6 g. The variety of NPSs globally available and the recent national decisions have changed the rules in this field in Germany significantly and might pave the way for a new wave of NPSs and maybe even for the re-emerging of regulated compounds in smaller quantities. These changed circumstances motivated us to analyze a set of herbal products currently available in Germany and identify, characterize and quantitate newly occurring synthetic cannabinoids.
Materials and methods
Chemicals
All chemicals were of analytical reagent purity and purchased from Fluka (Buchs, Switzerland), Sigma-Aldrich (Steinheim, Germany) or Acros Organics (Geel, Belgium). Solvents were of high-performance liquid chromatography grade purity and used without further purification. Thin-layer chromatography (TLC) plates (Polygram SIL G/UV 40 × 80 mm) and silica gel (mesh 0.04–0.063) were obtained from Macherey–Nagel (Düren, Germany).
Nuclear magnetic resonance spectroscopy procedures
To achieve secure proof of the molecular structures the 1H and 13C nuclear magnetic resonance (NMR) spectra of all compounds were completely assigned by the use of two-dimensional (2D) experiments with H,H-correlation spectroscopy (COSY), H,C-heteronuclear single quantum correlation (HSQC) and H,C-heteronuclear multiple-bond correlation (HMBC) spectra. Iterative full bandshape analyses were performed for most of the aromatic second-order proton (1H) NMR multiplets. Initially, all NMR spectra of solutions were obtained in deuterated methanol (CD3OD). To facilitate comparisons with literature data by other authors, some NMR analyses were repeated using other appropriate solvents [deuterated chloroform (CDCl3), deuterated dimethyl sulfoxide (DMSO-d 6)]. 1H and 13C NMR spectra were recorded at 600 and 151 MHz, respectively, on a Bruker Avance II 600 spectrometer with a TCI cryoprobe (Bruker BioSpin, Rheinstetten, Germany). The solvents used were DMSO-d 6 (for 4), CDCl3 (for 9) and CD3OD (for all other compounds, including 4). Chemical shifts are reported in δ units (ppm) and were measured relative to tetramethylsilane (TMS; δ H = 0.00 ppm) and the solvents [δ C(DMSO-d 6) = 39.50, δ C(CD3OD) = 49.02, δ C(CDCl3) = 77.01 ppm]. Assignment techniques used were distortionless enhancement by polarization transfer (DEPT)-135, H,H-COSY, H,H-nuclear Overhauser effect spectroscopy (NEOSY), H,C-HSQC and H,C-HMBC, the latter being optimized for C,H-couplings of 7.5 Hz. Digital resolutions in the 2D NMR spectra were chosen small enough to permit the distinction of cross-peaks with similar chemical shifts (if possible). Iterative full bandshape analyses of 1H NMR spectra were performed with the program TopSpin 2.1, patch level 5 (Bruker).
Gas chromatography–mass spectrometry parameters
Two different systems were used. For high-resolution, accurate mass measurements, an Agilent 6890 gas chromatograph equipped with an analytical column (30 m × 0.25 mm i.d., film thickness 0.25 µm; ZB-5MS, Phenomenex, Aschaffenburg, Germany), using helium as carrier gas (1.0 mL/min; constant flow mode) and a temperature program of 100 °C (3 min)–10 °C/min–320 °C (10 min) was used. The gas chromatography (GC) instrument was coupled directly to a JMS-T100GC time-of-flight (TOF) mass spectrometer (GCAccuTOF, JEOL, Tokyo, Japan) in electron ionization (EI) mode at 70 eV. The source and transfer line temperatures were set at 200 and 310 °C, respectively. The detector voltage was set at 1850 V. The acquisition mass range was set from m/z 41 to m/z 600 with a spectrum recording interval of 0.4 s. The system was tuned with perfluorokerosene to achieve a resolution of 6000 (full width at half maximum) at m/z 292.9824. GC–EI-mass spectrometry (MS) afforded accurate masses to establish molecular formulae of molecular and fragment ions at ∆m < 3.0 mmu. JEOL MassCenter™ workstation software was used for data acquisition and data evaluation.
For chemical characterization/EI-MS-library identification of the synthetic cannabinoids at unit mass resolution, a GC–MS system consisting of an Agilent 6890 gas chromatograph coupled to an Agilent 5975B mass spectrometer (Agilent, Santa Clara, CA, USA) was used. The separation of the analytes was achieved on a DB-1MS capillary column (15 m × 0.32 mm; film thickness 0.25 µm; Agilent, Waldbronn, Germany). The following temperature program was used: 100 °C (1 min)–10 °C/min–320 °C (10 min). The following MS settings were applied: ionization energy 70 eV, ion source temperature 230 °C and interface temperature 290 °C. The compounds were identified by comparison of recorded mass spectra reference data from SWGDRUG Mass Spectral Library Version 2.2 and Cayman Spectral Library v09292014 [10, 11] using the MSD ChemStation Software (V. D.03.00.611, Agilent).
Electrospray-tandem mass spectrometry (ESI-MS/MS) parameters
Fractions or samples containing 1–8 (Fig. 2) were dissolved in methanol at concentrations of 5 µg/mL. These solutions were directly infused into the mass spectrometer using the integrated syringe pump of the 3200 QTrap instrument (syringe: 1000 µL, i.d. 2.3 mm; Hamilton, Reno, NV, USA) at a flow rate of 5 µL/min. The 3200 QTrap mass spectrometer (Applied Biosystem/SCIEX, Darmstadt, Germany) was equipped with an ESI interface (Turbo V). The instrument was operated in positive ion mode and in enhanced product ion (EPI) scan mode at a flow rate of 5 µL/min. Ionization and EPI conditions were: source voltage 5.5 kV, ambient temperature, curtain gas 10 psi, GS1 10 psi, GS2 0 psi, declustering potential 20 V, and collision energies between 18 and 39 V. Nitrogen was used as curtain and auxiliary gas.
Isolation and chemical characterization of synthetic cannabinoids
Commercially available herbal mixtures (13 packages; samples 1A–6C) were obtained from German-language Internet shops based in several European countries (Germany, Netherlands, Czech Republic, Belgium) The packages contained 2.5–4.1 g of fluffy, greenish-grey plant material. Package 5B contained 1.5 g of a brown hashish-like resin. The content of the packages were ground in a coffee mill. Depending on the available starting material, 1– 4-g aliquots were extracted consecutively with 20–30 mL of dichloromethane (DCM) by ultrasonication for 10 min (Elmasonic S120, 12.75 L, 37 kHz, effective ultrasound power 200 W; Hans Schmidbauer GmbH & Co. KG, Singen, Germany). The organic extract was filtered and concentrated in a vacuum. The residues were redissolved in 1 mL of DCM and further purified by silica gel chromatography (30 g SiO2, column size 15 × 2 cm). The first eluent was 150 mL of DCM/methanol (100:1, v/v) followed by DCM/methanol (20:1, v/v). Fractions of 5–10 mL were collected and screened by TLC under ultraviolet (UV) detection at 254 nm. Fractions containing pure synthetic cannabinoids were pooled and concentrated in vacuum and used for further spectroscopic characterization. Purity and authenticity of all isolated compounds were confirmed by NMR, GC–EI-MS and ESI-MS/MS.
Synthesis of the internal standard methyl 3-(3-(1-naphthoyl)-1H-indol-1-yl)propionate (9)
Indole (437 mg, 3.7 mmol) and potassium hydroxide (946 mg, 16.9 mmol) were dissolved in dry DMSO (10 mL) in an argon atmosphere. After stirring for 15 min, methyl 3-bromopropionate (0.7 mL, 5.5 mmol) was added drop-wise to the reaction mixture and stirred overnight. The reaction was stopped via addition of water, acidified to pH 2 with 1N HCl (25 mL) and extracted with ethyl acetate (4 × 30 mL). The combined organic phases were washed with brine (100 mL), dried over Na2SO4 and the solvent was removed in a vacuum to yield 3-(1H-indol-1-yl)propanoic acid as a crude oil. The crude material was used without purification, dissolved in 40 mL of methanol containing 1 mL of concentrated H2SO4 and stirred for 16 h. The reaction progress was monitored by TLC (R f = 0.69; DCM as the developing solvent). The reaction mixture was neutralized with aqueous NaHCO3 (4 mL), diluted with water (100 mL) and extracted with DCM (4 × 30 mL). The combined extracts were washed with brine (100 mL), dried over Na2SO4 and the solvent was removed in vacuo, yielding methyl 3-(1H-indol-1-yl)propionate as oil. The resulting product was purified by silica gel column with DCM.
In a 250-mL round bottom flask, AlCl3 (494 mg, 3.7 mmol) was suspended in 10 mL of dry DCM in an argon atmosphere and stirred for 15 min. 1-Naphthoyl chloride (0.55 mL, 3.7 mmol) was added drop-wise to the stirring suspension and held for 1 h. A solution of methyl 3-(1H-indol-1-yl)propionate in DCM (5 mL) was added drop-wise (observing the color of the solution changing from yellow to red). The reaction progress was checked by TLC (R f = 0.38, DCM). After stirring for 24 h at room temperature, the reaction mixture was poured into ice water (100 mL), extracted with DCM (4 × 30 mL), washed with brine, dried over Na2SO4 and concentrated. The crude reddish residue was purified by the silica gel column with DCM to give the internal standard (IS) methyl 3-(3-(1-naphthoyl)-1H-indol-1-yl)propionate (9; 824 mg) as a yellow oil.
EI-MS 70 eV, m/z [mass-to-charge ratio; in relative intensity (rel. int.)]: 357 (70), 340 (15), 326 (4), 296 (7), 283 (31), 270 (100), 254 (14), 241 (14), 230 (59), 213 (9), 189 (3), 170 (5), 155 (16), 144 (11), 127 (64), 115 (16), 101 (14), 87 (7), 77 (11), 59 (25), 51 (3), 45 (3)
Accurate mass EI-MS of M+ [theoretical/measured (theor./meas.)]: 357.13649/357.13856
ESI-EPI-MS/MS of protonated molecular ion [M + H]+ 358 m/z (rel. int.): 284 (2), 230 (17), 155 (100), 127 (3).
UV maxima nm (0.010 mg/mL in methanol): 245 and 313
Infrared (IR): ν (cm−1): 3124 (w), 3054 (w), 3011 (w), 2946 (w), 1729 (m), 1606 (m), 1519 (m), 1463 (w), 1382 (m), 1177 (s), 1052 (m), 884 (m), 744 (s), 668 (m), 642 (m), 595 (w)
For the NMR spectroscopic characterization and the comparison to published data [12], see the supplementary material and the section “NMR spectroscopy”.
Quantitation of synthetic cannabinoids by direct infusion ESI-MS/MS
The response factor (RF) for each compound was determined in relation to the IS by co-injecting solutions containing 2.5 µg of purified synthetic cannabinoid and 2.5 µg of IS in 1 % formic acid in methanol (v/v) or in a mixture of 90 % methanol, 10 % deionized water, 1 % formic acid and 0.1 % ammonium formate. These solutions were used for direct-infusion ESI-MS/MS analysis (multiple reaction monitoring mode, MRM) using the integrated syringe pump of the 3200 QTrap instrument. For each analyte and IS, two transitions per compound where selected (one used as a quantifier transition; MRM-QT). The RF for each compound versus IS was calculated based on the area for the analyte and the area of the IS for the corresponding quantifier MS/MS transition using Eq. 1.
The corresponding MRM transitions, calculated RFs and ESI–MS/MS parameters for the IS and each analyte are summarized in Table 1.
For quantification of the synthetic cannabinoids in the commercial products, each herbal product was extracted in a 1.5-mL Eppendorf tube. Approximately 10–25 mg of the homogenized ground plant material was weighed (0.1-mg accuracy) and 1.0 mg of IS dissolved in 100 µL of chloroform was added. This mixture was extracted with 0.5 mL of DCM and the organic supernatant was collected. The extraction was repeated three times. The fourth extract was concentrated and analyzed by ESI-MS, but was found to be free of synthetic cannabinoids and the IS, indicating exhaustive extraction. The combined organic phases were concentrated by a stream of nitrogen, and the residue was re-dissolved in 1 mL of chloroform and diluted 200-fold using 1 % formic acid in methanol (v/v) or a mixture of 90 % methanol, 10 % deionized water, 1 % formic acid and 0.1 % ammonium formate. These solutions were directly analyzed by direct infusion ESI-MS/MS and the concentrations of synthetic cannabinoids were calculated using the previously established RFs and Eq. 2.
All herbal mixtures were analyzed in duplicates each time starting from scratch.
Recovery rates of each synthetic cannabinoid in herbal samples
Leaves of Althaea officinalis (10 mg) were spiked with reference standard synthetic cannabinoids at three different levels of 100, 500 and 2000 µg using stock solutions in chloroform (STS-135, 5F-AB-PINACA or 5F-PB-22) and 500 µg of IS each. All samples underwent the sample workup protocol and were diluted 200-fold with 1 % formic acid (v/v in methanol) at the final stage and analyzed using the established direct infusion ESI-MS/MS method. The recovery was calculated as the rate of the detected value (added synthetic cannabinoid = 100 %).
Recovery rates of mixtures of synthetic cannabinoids in herbal samples
To cover possible interactions of coexisting compounds, synthetic cannabinoid-free leaves of A. officinalis (10 mg) were spiked with:
-
(a)
200 µg each of 5-fluoro-AMB (5), 5-fluoro-AB-PINACA (1), MDMB-CHMICA (6) and IS (9)
-
(b)
200 µg each of EAM-2201 (7), 5-fluoro-PB-22 (4) and IS (9)
-
(c)
200 µg each of 5-fluoro-AB-PINACA (1), 5-fluoro-PB-22 (4), STS-135 (8) and IS (9)
All samples underwent the extraction procedure and were diluted 200-fold with a mixture of 90 % methanol, 10 % water, 1 % formic acid and 0.1 % ammonium formate before direct infusion into ESI-MS/MS.
Evaluation of inter-day stability of the response factor
To test inter-day stability, mixtures of 2.5 µg of IS and 2.5 µg of each synthetic cannabinoid were stored at 4 °C for several weeks. They were analyzed by ESI-MS/MS. The RF was determined in comparison with the first injection (100 %) over the course of 4 weeks.
Physicochemical characterization of compounds 1–8
All isolated compounds were analyzed by spectrometric and spectroscopic methods for physicochemical characterization. The data included mass spectra by EI-MS, accurate mass data, product ion mass spectra obtained by ESI-EPI-MS/MS, UV maxima, and IR spectra.
Results and discussion
All analyzed samples were obtained in February 2015, shortly after the court decisions, which created a new legal framework situation in Germany. To obtain a broad spectrum of products available on the German market, it was decided to sample six different German language Internet shops for herbal smoking mixtures, covering several European countries (Germany, Netherlands, Czech Republic and Belgium). Two different products were obtained from each supplier. Altogether, 13 different brands/product packages were analyzed (12 plus one freebie: sample 6C).
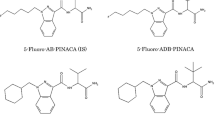
As a first screening approach, organic extracts were analyzed by GC–MS and major compound peaks were identified by available GC–MS databases [10, 11]. Figure 2 shows structures of all eight synthetic cannabinoids (1–8), which were identified by the EI–MS databases. A literature search revealed that some of these substances, to date, have not or have been only vaguely described and chemically poorly characterized.
STS-135 (8) was detected and chemically characterized earlier and is a known ingredient in herbal smoking blends on the German market [13]. 5-Fluoro-AB-PINACA (1) was recently detected in Japan in herbal preparations containing larger amounts of another active ingredient, diphenidine, a dissociative anesthesia drug [14], and has been characterized by EI- and ESI-MS methods. In addition, intensive metabolization data of 1 using human microsome preparations and ESI–MS/MS methods became available recently [15].
The coexistence of diphenidine was also reported for AB-CHMINACA (2) and 5-fluoro-AMB (5), and these compounds were analyzed and quantitated by HPLC–ESI-MS/MS in postmortem autopsy specimens associated in a fatal poisoning in Japan [16]. The occurrence of AB-CHMINACA (2), AB-FUBINACA (3), EAM-2201 (7) and 5-fluoro-PB-22 (4) has been documented; the first three (2, 3 and 7) were reported for the Asian countries, in particular, the Japanese market [17–21].The occurrence of 4 is more widely spread and has a reputation of being causal for acute health effects [21–23].
Little or no physicochemical data were available for 1 (5-fluoro-AB-PINACA), 2 (AB-CHMINACA), 5 (5-fluoro-AMB) and 6 (MDMB-CHMICA). In the course of our experiments we used 9 as an IS for quantification of synthetic cannabinoids in herbal blends. Hence, we did an in depth characterization, especially for the compounds 1, 2, 5, 6 and 9, using various instrumental analyses.
Basic physicochemical properties of compounds 1–8
5-Fluoro-AB-PINACA (1)
EI-MS 70 eV, m/z (rel. int.): 348 (M+, 1), 304 (82), 233 (100), 213 (10), 177 (7), 145 (37), 131 (18), 117 (5), 103 (10), 90 (7), 69 (7), 55 (8), 44 (18)
Accurate mass EI-MS of M+ (theor./meas.): 348.19615/348.19569
ESI-EPI-MS/MS m/z (rel. int.): 349 ([M + H]+, 3), 332 (30), 304 (100), 251 (2), 233 (50), 213 (18), 177 (1), 145 (1)
UV maximum nm (0.010 mg/mL in methanol): 302
IR: ν (cm−1): 3387 (w), 3331 (w), 3198 (w), 2962 (w), 2873 (w), 1649 (s), 1527 (s), 1491 (m), 1369 (w), 1313 (w), 1171 (m), 1005 (w), 750 (s), 565 (m).
AB-CHMINACA (2)
EI-MS 70 eV, m/z (rel. int.): 356 (M+, 1), 312 (82), 241 (100), 186 (3), 145 (55), 131 (15), 103 (18), 83 (5), 72 (3), 55 (35), 44 (22)
Accurate mass EI-MS of M+ (theor./meas.): 356.22123/356.22165
ESI-EPI-MS/MS m/z (rel. int.): 357 ([M + H]+, 7), 340 (64), 312 (100), 241 (28)
UV maximum nm (0.010 mg/mL in methanol): 303
IR: ν (cm−1): 3331 (w), 3193 (w), 2926 (m), 2852 (w), 1649 (s), 1527 (s), 1491 (m), 1368 (w), 1303 (w), 1175 (m), 749 (s), 603 (m), 568 (m).
AB-FUBINACA (3)
EI-MS 70 eV, m/z (rel. int.): 368 (M+, 1), 324 (74), 312 (4), 253 (90), 241 (5), 215 (3), 145 (18), 129 (2), 109 (100), 83 (17), 72 (3), 44 (18)
Accurate mass EI-MS of M+ (theor./meas.): 368.16485/368.16459
ESI-EPI-MS/MS m/z (rel. int.): 369 ([M + H]+,10), 352 (50), 324 (100), 253 (19)
UV maximum nm (0.010 mg/mL in methanol): 301
IR: ν (cm−1): 3366 (w), 3056 (w), 2961 (w), 2531 (w), 2397 (w), 2350 (w), 1638 (s), 1509 (m), 1481 (m), 1419 (w), 1356 (w), 1304 (w), 1228 (m), 1157 (w), 752 (s), 616 (w).
5-Fluoro-PB-22 (4)
EI-MS 70 eV, m/z (rel. int.): 376 (M+, 12), 232 (100), 212 (6), 144 (44), 129 (8), 116 (50), 102 (8), 89 (28), 63 (8)
Accurate mass EI-MS of M+ (theor./meas.): 376.15871/376.15653
ESI-EPI-MS/MS m/z (rel. int.): 377 ([M + H]+,10), 232 (100)
UV maxima nm (0.010 mg/mL in methanol): 216, 230, 294
IR: ν (cm−1): 3124 (w), 3039 (w), 2941 (w), 2856 (w), 1710 (s), 1529 (m), 1466 (m), 1380 (m), 1159 (m), 1084 (m), 962 (m), 849 (m), 812 (m), 750 (s), 613 (w), 569 (w).
5-Fluoro-AMB (5)
EI-MS 70 eV, m/z (rel. int.): 363 (M+, 12), 320 (2), 304 (70), 289 (2), 249 (24), 233 (100), 213 (22), 177 (7), 158 (3), 145 (57), 131 (24), 117 (7), 103 (22), 90 (17), 69 (15), 55 (16), 41 (28)
Accurate mass EI-MS of M+ (theor./meas.): 363.19582/363.19767
ESI-EPI-MS/MS m/z (rel. int.): 364 ([M + H]+,13), 346 (1), 332 (4), 318 (4), 304 (68), 286 (2), 251 (4), 233 (100), 213 (43), 185 (2), 177 (6), 145 (5)
UV maximum nm (0.010 mg/mL in methanol): 302
IR: ν (cm−1): 3415 (w), 2961 (w), 2873 (w), 1739 (m), 1667 (m), 1524 (s), 1490 (m), 1310 (m), 1203 (m), 1169 (m), 1002 (m), 751 (s), 537 (m).
MDMB-CHMICA (6)
EI-MS 70 eV, m/z (rel. int.): 384 (M+, 28), 328 (34), 296 (10), 268 (21), 245 (25), 240 (100), 157 (10), 144 (47), 129 (21), 116 (7), 102 (5), 57 (34), 55 (32)
Accurate mass EI-MS of M+ (theor./meas.): 384.24129/384.24297
ESI-EPI-MS/MS m/z (rel. int.): 385 ([M + H]+,10), 240 (100)
UV maxima nm (0.010 mg/mL in methanol): 216, 290
IR: ν (cm−1): 3325 (w), 3105 (w), 2926 (m), 2852 (w), 1738 (m), 1616 (s), 1536 (s), 1511 (s), 1464 (m), 1395 (m), 1217 (m), 1196 (m), 1157 (s), 1129 (m), 1014 (m), 776 (m), 742 (s), 618 (m), 549 (m).
EAM-2201 (7)
EI-MS 70 eV, m/z (rel. int.): 387 (M+, 100), 370 (65), 358 (40), 312 (54), 298 (14), 270 (28), 254 (29), 232 (47), 195 (21), 183 (16), 153 (31), 144 (42), 116 (21), 102 (17), 89 (9), 69 (10)
Accurate mass EI-MS of M+ (theor./meas.): 387.19984/387.20179
ESI-EPI-MS/MS m/z (rel. int.): 388 ([M + H]+,11), 232 (45), 183 (100), 155 (15), 144 (8)
UV maximum nm (0.010 mg/mL in methanol): 316
IR: ν (cm−1): 3378 (w), 2926 (m), 2855 (m), 1741 (m), 1623 (m), 1610 (m), 1520 (s), 1464 (m), 1393 (m), 1376 (m), 1169 (m), 1064 (w), 941 (w), 823 (s), 751 (s), 610 (w).
STS-135 (8) see supplemental Information file of Langer et al. [12]
EI-MS 70 eV, m/z (rel. int.): 382 (M+, 44), 365 (13), 325 (2), 307 (37), 264 (8), 232 (100), 212 (4), 173 (10), 144 (29), 116 (12), 91 (10), 79 (8), 67 (5), 41 (9)
Accurate mass EI-MS of M+ (theor./meas.): 382.24204/382.23989
ESI-EPI-MS/MS m/z (rel. int.): 383 ([M + H]+,22), 232 (16), 206 (5), 135 (100), 107 (7), 93 (5), 79 (2)
UV maximum nm (0.010 mg/mL in methanol): 302.
NMR spectroscopy
In accordance with our previous papers [2, 13], the atom numbering used is as follows: (a) 2, 3, … for the indole/indazole moiety, (b) 2′, 3′, … for the naphthalene moiety or other groups connected to 3-CO-/3-CONH-/3-COO–, (c) 2″, 3″, … for the alkyl groups bound to N-1 of the indole/indazole fragment.
NMR results for compounds 1, 5 and 6 have not been reported before. For compound 2, only unassigned 1H NMR data have been published in a patent [24]. The NMR data in the literature of the IS, 9, are not satisfactory, because neither the 1H nor the 13C NMR spectrum has been assigned and some erroneous information has been extracted from the 1H NMR spectrum [12]. In the present paper, a complete set of 1H and 13C assignments is given only for 6 (Table 2), because to our knowledge this compound was identified for the first time in two commercial products disclosed. The NMR data for compounds 1, 2, 3, 4, 5, 7 and 9 are given in the supplementary material.
The aromatic region of the 1H NMR spectrum of compound 4 in a CD3OD solution showed signals, which were close to first order. When the spectrum was repeated in DMSO-d 6 to compare it with the literature [22], its appearance was substantially different. The chemical shift difference of H-6′ and H-7′ was considerably smaller than for the CD3OD solution, which caused the change from a clean doublet of doublets (in CD3OD) to the 6-line X part of an ABX spin system (in DMSO-d 6). Moreover, changing the solvent induced distinct chemical shift changes of H-2 and H-7 (both deshielded in DMSO-d 6 by 0.15 and 0.12 ppm, respectively) and of H-4 (de-shielded in CD3OD by 0.08 ppm). The relevant spectrum sections of the two solutions are also given as figures in the supplementary material file. A comparison of the results of our 1H NMR analysis with those by the earlier authors [22] shows discrepancies with our assignments of H-5′ (δ = 7.95) as well as of H-7, H-6′ and H-7′ (δ = 7.71–7.67). This is most probably due to the incomplete and incorrect analysis of the multiplets in question by these authors. For example, the signal at δ = 7.95 was assigned by them to H-6′ (their labeling: H-3a). This is the X proton of the ABX system mentioned above. From the distance of the two outer intense lines of this multiplet the sum |J AX + J BX| is read off as 9.7 Hz, and, hence, it cannot correspond to a proton with two ortho neighbours (which should have a sum of ca. 16 Hz), i.e., not H-6′. Their incorrect proton assignments seem to have affected a number of 13C signal assignments, because the latter were deduced from heteronuclear chemical shift correlations. The NMR data of compound 3 have been described for a solution in DMSO-d 6 [20] and those of compound 7 for a solution in CDCl3 [26]. Our results for solutions of these compounds in CD3OD are listed in the supplementary material. The NMR data of compound 8 (in CDCl3) have been reported by us before [13].
Mass spectrometry
The majority of the synthetic cannabinoids identified (4/8; 1, 2, 3 and 5) belong to the group of 1H-indazole-3-carboxamides, containing a valine-amide/methyl ester (5) side chain. In addition, the newly found MDMB-CHMICA (6) is based on 1H-indole-3-carboxamide and the methyl ester of the tert-butyl homologue of valine. Based on the GC–EI-TOF-MS, elemental compositions of ions or ion losses could be assigned and were used to assign key fragment ions and helped to propose EI fragmentation schemes, which are also given in the supplementary material.
The EI mass spectra of the amides (1, 2, and 3) are characterized by showing only low-intensity (≤ 1 %) molecular ions (Fig. 3a; 2), while corresponding methyl esters (5 and 6) show significant molecular ion peaks of higher than 20 % intensity (Fig. 3b, 6). EI fragmentation was characterized by fragment ions originating from two α-cleavages of the valine-based side chains. For amides, typically, intense fragment ion peaks were observed by losses of 44 amu (CH2NO) and 115 amu (C5H11N2O; Fig. 3a), while 59 amu (C2H3O2) and 130 amu (C6H12NO2) were observed for the corresponding methyl ester (5, see the supplementary material). However, MDMB-CHMICA (6), which is a tert-butyl derivative of valine, showed a different behavior. Instead of the loss of the methyl ester functionality, the loss of the tert-butyl side chain (56 amu; C4H8) was favored (Fig. 3b) in addition to the α-cleavage of the total side chain [−144 amu (C7H14NO2)], which was true for all 1H-indole/indazole-3-carboxamide compounds in this study.
The fragmentation of the protonated molecular ions of the amide compounds (1, 2, and 3) in ESI-MS/MS was dominated by two consecutive neutral losses, −17 amu (NH3) and −28 (CO) of the amide group and an α-cleavage of the total valine side chain part of the molecule (Fig. 4a; 2), while the valine methyl ester derivative (5) showed, similar to the EI behavior of this compound, two α-cleavages, of the methyl ester group (–C2H4O2; −60 amu) and the total valine side chain (C6H13NO2; −131 amu). Again, strikingly different was the ESI-MS/MS of the tert-butyl valine homologue (6). Unlike the behavior of the related compound 5, no methyl ester fragmentation (–C2H4O2; −60 amu) was observed and only one major fragment, the loss of the complete side chain (C7H13NO2; −143 amu), was detected (Fig. 4b). Further information on EI-MS and ESI-MS/MS data and proposed fragmentation schemes for compounds 1–7 and 9 can be found in the supplementary material.
ESI-MS/MS quantification
Recently, direct analysis in real time mass spectrometry (DART-MS) was applied successfully to the analysis of synthetic cannabinoids in herbal mixtures [27]. The usefulness of this technique in forensic analysis of illegal drugs [like cocaine, tetrahydrocannabinol (THC), opioids, psilocin, etc.] was demonstrated before [28], and DART-MS is now a well established direct technique to screen complex biological matrices for synthetic cannabinoids, enabling a rapid and sensitive analysis overview without the need for sample extraction [29]. However, under these conditions, DART-MS is a preliminary qualitative screening method and cannot provide accurate quantitation data, because the illicit drugs are usually distributed very inhomogeneously in the herbal products [30].
We do not have access to DART-MS, however we were highly interested in speeding up quantitation of synthetic cannabinoids in herbal products. Hence, we developed a standard procedure that allowed sufficiently accurate quantification without the need of chromatographic separation. Our approach comprised homogenization of the whole sample, extraction and screening of the present synthetic cannabinoids by GC–MS. Quantification was achieved by utilizing an IS and a non-chromatographic direct infusion ESI-MS/MS method instead of a DART-MS ionization/analysis.
To take into account the different ionization efficiency/sensitivity of each compound, the individual RFs for each synthetic cannabinoid versus the IS were established in preceding experiments using direct infusion ESI-MS/MS of each compound/IS mixed together. The overall methodology was specifically adapted for the analysis of synthetic cannabinoids in such products and our available 3200 QTrap.
For instance, we made sure that the IS was added in approximately the same amount as those of the analytes expected, thus yielding final concentrations of approximately 5 µg/mL for the analytes and the IS. These solutions could be used right away for quantification without any further treatment.
Before the method was applied to analyze the commercial products, we tested the overall procedure using spiked A. officinalis leaves. A. officinalis is frequently used as plant material for herbal smoking mixtures [31, 32], and hence we used this plant material for model experiments to evaluate the overall procedure and the recovery rates. Three representative, but structurally different, synthetic cannabinoids were chosen for these model experiments: 8 (an adamantyl carboxamide derivative), 1 (an indazole dicarboxamide) and 4 (a quinolinyl carboxylate derivative). The recoveries for the quantification of each synthetic cannabinoid at three different concentration levels, covering the expected concentration in these products, ranged from 110 to 80 %, which demonstrated sufficient accuracy for the intended use/concentration range.
To reveal possible interactions of coexisting compounds that could affect the quantitation we conducted a second series of model experiments. A. officinalis leaves were spiked with the compounds that occurred in combination as identified by the GC–MS screening. The corresponding synthetic cannabinoids (at known concentrations) together with the IS were added and analyzed using the described extraction protocol. The calculated quantitative results were compared to the theoretical values and were in the range of 80–120 %, which proved to be sufficiently accurate for the intended use. However, it needs to be mentioned that if newer designer-drug mixtures grow more complex (3 or more synthetic cannabinoids plus IS), interactions of the individual compounds cannot be excluded and the combinations need to be tested for interaction to allow accurate quantification.
Using our instrumentation, single compound products could be analyzed straightforwardly with this methodology. Therefore, it turned out, if protonated/sodiated molecular ions coexisted for one compound in the ESI-process, this was counterproductive for accurate quantitation, because only MS/MS transitions of the protonated molecular ion are useful and the ratio might differ from injection to injection. Therefore, as a general approach, formic acid was used as a general modifier in the extraction procedure.
In addition, when combinations of several synthetic cannabinoids coexisted, a reliable quantification was difficult. To investigate the interaction of combinations of different synthetic cannabinoids, test mixtures were analyzed using several combinations of ESI–MS solvents and modifiers. Using formic acid and ammonium formate as modifiers, we were able to push the ratio towards a high excess of the protonated molecular ion species for all individual compounds in the mixture which, in turn, enabled the accurate quantification of herbal blends containing more than one synthetic cannabinoid.
The results and experiences of the model experiments were combined to a general sample preparation and analysis method, allowing the straightforward quantification of the synthetic cannabinoids in such products. This established protocol was facilitated by the fact that the concentration of synthetic cannabinoids is usually of the order of 50–300 mg/g [13, 33, 34], allowing the use of a single protocol for all samples analyzed, here, using 10 mg of each commercial product. This sample amount requires a final dilution by a factor of 100–200 to meet the dynamic range requirements of our instrumentation. However, this fact was very beneficial and helpful because matrix effects and matrix suppression of the extracted plant material were of no concern after dilution. These resulting solutions could be used right away for quantification by direct infusion ESI-MS/MS without any further treatment.
The individual RFs for each synthetic cannabinoid were used to calculate the actual amount of synthetic cannabinoids present in the products. The quantification results of the individual and total synthetic cannabinoid contents and cannabinoid receptor binding constants of each commercial product are summarized in Table 3. The total contents of synthetic cannabinoids found in the analyzed products ranged from 66 to 446 mg/g, reflecting the “normal” range frequently found for such products [13, 33, 34]. The calculation of the concentration of the synthetic cannabinoids in commercial products depends to a great extent on the RFs. We tested the variability of the RFs over a period of 4 weeks. Compared to the first determination (day 0 set to 100 %) the RFs were found to show less than ± 1 % deviation. This means that, if an RF for an IS/analyte is established for an existing instrumentation, it can be used for future analyses allowing a quick and simple quantification of this analyte in herbal smoking mixtures.
Conclusions
It is obvious that the synthetic cannabinoids on the German market are currently dominated by the 1H-indazole-derivatives (1, 2, 3 and 5), which seem to have conquered the European market and are replacing many of the existing compounds which were regulated over the past months. MDMB-CHMICA was found for the first time and its structural and physicochemical properties are described here in detail. Another characteristic finding was that, in many cases, shops (4/6) were selling differently labeled products/packages, but the synthetic cannabinoid contained in the products was identical. Interestingly, the product which was sent as a freebie contained a recently regulated compound (8). This seems to be a way for the suppliers to empty their warehouse of regulated NPSs and to stock new products that contain as of yet unregulated compounds. As a response to the very recent regulatory changes in Europe/Germany, where NPSs are not considered pharmaceuticals anymore [8], and the regulations on how to define the lowest effective doses for NPSs are being applied [9], these changes seem to be reflected by the common occurrence (4/8) of already-regulated compounds (here: 3, 4, 7 and 8). In addition, in vitro receptor binding data were available for only five out of the eight detected synthetic cannabinoids (Table 3), demonstrating an increased risk/tolerance of the clandestine producers to create new modifications of the existing core structures without estimating biological activities, not to mention possible hazardous side effects.
Here, we have also reported a method/protocol allowing a quick and reliable ESI-MS/MS quantification of diluted crude extracts from herbal products using an IS and appropriate RFs. This method may be a helpful alternative for the quantitative analysis, which will be necessary to monitor NPS content in connection with the new need to survey the regulations on “possession of small amounts or not-small amounts” of regulated synthetic cannabinoids in Germany [9]. The latter legal decision may lead to the re-occurring availability of already-regulated NPSs via local shops in Germany, generating the need for such simple and fast quantification procedures.
References
Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreiro N (2009) Spice and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom 44:832–837
Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, Beuerle T (2009) Spice: a never ending story? Forensic Sci Int 191:58–63
EMCDDA (2014) European drug report 2014: trends and developments, May 2014. http://www.emcdda.europa.eu/attachements.cfm/att_228272_EN_TDAT14001ENN.pdf. Accessed May 2015
UNODC (2014) World drug report 2014, June 2014. http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Accessed May 2015
UNODC (2015) Decision 58/10 and 58/11. https://www.unodc.org/unodc/en/commissions/CND/Resolutions_Decisions/Resolutions-Decisions_2010-2019.html. Accessed Sept 2015
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y (2014) Changes in the prevalence of new psychoactive substances before and after the introduction of the generic scheduling of synthetic cannabinoids in Japan. Drug Test Anal 6:832–839
28. BtMÄndV 2014, 13. December 2014. http://www.bgbl.de/banzxaver/bgbl/start.xav?start=%2F%2F*%5B%40attr_id%3D’bgbl104s0028.pdf’%5D#__bgbl__%2F%2F*%5B%40attr_id%3D%27bgbl114s1999.pdf%27%5D__1427399651317. Accessed May 2015
EU-Court (2014) Judgment of the court (Fourth Chamber), 10 July 2014. Medicinal products for human use. Joined cases C-358/13 and C-181/14. http://curia.europa.eu/juris/liste.jsf?language=de&num=C-358/13. Accessed May 2015
Bundesgerichtshof (2015) StR 302/13. 14. January 2015. http://juris.bundesgerichtshof.de/cgi-bin/rechtsprechung/document.py?Gericht=bgh&Art=en&nr=70354&pos=0&anz=1. Accessed May 2015
SWGDRUG (2014) SWGDRUG mass spectral library. http://www.swgdrug.org/ms.htm. Accessed Feb 2015
Cayman Chemical (2014) Cayman spectral library. https://www.caymanchem.com/app/template/SpectralLibrary.vm. Accessed Feb 2015
Lovett DP, Yanes EG, Herbelin TW, Knoerzer TA, Levisky JA (2013) Structure elucidation and identification of a common metabolite for naphthoylindole-based synthetic cannabinoids using LC–TOF and comparison to a synthetic reference standard. Forensic Sci Int 226:81–87
Langer N, Lindigkeit R, Schiebel HM, Ernst L, Beuerle T (2014) Identification and quantification of synthetic cannabinoids in ‘spice-like’ herbal mixtures: a snapshot of the German situation in the autumn of 2012. Drug Test Anal 6:59–71
Wurita A, Hasegawa K, Minakata K, Watanabe K, Suzuki O (2014) A large amount of new designer drug diphenidine coexisting with a synthetic cannabinoid 5-fluoro-AB-PINACA found in a dubious herbal product. Forensic Toxicol 32:331–337
Wohlfarth A, Castaneto MS, Zhu M, Pang S, Scheidweiler KB, Kronstrand R, Huestis MA (2015) Pentylindole/pentylindazole synthetic cannabinoids and their 5-fluoro analogs produce different primary metabolites: metabolite profiling for AB-PINACA and 5F-AB-PINACA. AAPS J 17:660–677
Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, Suzuki O (2015) Postmortem distribution of AB-CHMINACA, 5-fluoro-AMB, and diphenidine in body fluids and solid tissues in a fatal poisoning case: usefulness of adipose tissue for detection of the drugs in unchanged forms. Forensic Toxicol 33:45–53
Uchiyama N, Shimokawa Y, Kawamura M, Kikura-Hanajiri R, Hakamatsuka T (2014) Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products. Forensic Toxicol 32:266–281
Uchiyama N, Shimokawa Y, Kikura-Hanajiri R, Demizu Y, Goda Y, Hakamatsuka T (2015) A synthetic cannabinoid FDU-NNEI, two 2H-indazole isomers of synthetic cannabinoids AB-CHMINACA and NNEI indazole analog (MN-18), a phenethylamine derivative N-OH-EDMA, and a cathinone derivative dimethoxy-α-PHP, newly identified in illegal products. Forensic Toxicol 33:244–259
Takayama T, Suzuki M, Todoroki K, Inoue K, Min JZ, Kikura-Hanajiri R, Goda Y, Toyo'oka T (2014) UPLC/ESI–MS/MS-based determination of metabolism of several new illicit drugs, ADB-FUBINACA, AB-FUBINACA, AB-PINACA, QUPIC, 5F-QUPIC and α-PVT, by human liver microsome. Biomed Chromatogr 28:831–838
Uchiyama N, Matsuda S, Wakana D, Kikura-Hanajiri R, Goda Y (2013) New cannabimimetic indazole derivatives, N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA) and N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA) identified as designer drugs in illegal products. Forensic Toxicol 31:93–100
Chung H, Choi H, Heo S, Kim E, Lee J (2014) Synthetic cannabinoids abused in South Korea: drug identifications by the National Forensic Service from 2009 to June 2013. Forensic Toxicol 32:82–88
Shevyrin V, Melkozerov V, Nevero A, Eltsov O, Shafran Y (2013) Analytical characterization of some synthetic cannabinoids, derivatives of indole-3-carboxylic acid. Forensic Sci Int 232:1–10
Behonick G, Shanks KG, Firchau DJ, Mathur G, Lynch CF, Nashelsky M, Jaskierny DJ, Meroueh C (2014) Four postmortem case reports with quantitative detection of the synthetic cannabinoid, 5F-PB-22. J Anal Toxicol 38:559–562
Buchler IP, Hayes MJ, Hegde SG, Hockerman SL, Jones DE, Kortum SW, Rico JG, Tenbrink RE, Wu KK (2009) Indazole derivatives as CB1 receptor modulators and their preparation and use in treatment of diseases. Patent WO/2009/106980, Sept 2009
Banister SD, Stuart J, Kevin RC, Edington A, Longworth M, Wilkinson SM, Beinat C, Buchanan AS, Hibbs DE, Glass M, Connor M, McGregor IS, Kassiou M (2015) Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. Chem Neurosci 6:1445–1458
Uchiyama N, Matsuda S, Wakana D, Kikura-Hanajiri R, Goda Y (2013) URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci Int 227:21–32
Musah RA, Domin MA, Walling MA, Shepard JRE (2012) Rapid identification of synthetic cannabinoids in herbal samples via direct analysis in real time mass spectrometry. Rapid Commun Mass Spectrom 26:1109–1114
Grange AH, Sovocool G (2011) Detection of illicit drugs on surfaces using direct analysis in real time (DART) time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 25:1271–1281
Lesiak AD, Musah RA, Domin MA, Shepard JRE (2014) DART-MS as a preliminary screening method for “herbal incense”: chemical analysis of synthetic cannabinoids. J Forensic Sci 59:337–343
Moosmann B, Angerer V, Auwärter V (2015) Inhomogeneities in herbal mixtures: a serious risk for consumers. Forensic Toxicol 33:54–60
Seely KA, Prather PL, James LP, Moran JH (2011) Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol Interv 11:36–51
Ogata J, Uchiyama N, Kikura-Hanajiri R, Goda Y (2013) DNA sequence analyses of blended herbal products including synthetic cannabinoids as designer drugs. Forensic Sci Int 227:33–41
Simolka K, Lindigkeit R, Schiebel H-M, Papke U, Ernst L, Beuerle T (2012) Analysis of synthetic cannabinoids in “spice-like” herbal highs: snapshot of the German market in summer 2011. Anal Bioanal Chem 404:157–171
Ernst L, Krüger K, Lindigkeit R, Schiebel H-M, Beuerle T (2012) Synthetic cannabinoids in “spice-like” herbal blends: first appearance of JWH-307 and recurrence of JWH-018 on the German market. Forensic Sci Int 222:216–222
Acknowledgments
This work was supported by Zentrales Innovationsprogramm Mittelstand (ZIM) des Bundesministeriums für Wirtschaft und Energie (BMWI) FKZ: KF31565015K3. The authors express their gratitude to Lisa Sieverling, Judith Carla Schürmann and Kerstin Ollenborger for their helpful assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Langer, N., Lindigkeit, R., Schiebel, HM. et al. Identification and quantification of synthetic cannabinoids in “spice-like” herbal mixtures: update of the German situation for the spring of 2015. Forensic Toxicol 34, 94–107 (2016). https://doi.org/10.1007/s11419-015-0292-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-015-0292-7