Abstract
A new pyrazole-carboxamide type synthetic cannabinoid, AB-CHFUPYCA (1), was detected in illegal herbal products by our ongoing survey in Japan. The structure of 1 was identified by gas chromatography–mass spectrometry (GC–MS), liquid chromatography–mass spectrometry (LC–MS), liquid chromatography–high-resolution-mass spectrometry (LC–HR-MS) and nuclear magnetic resonance (NMR) analyses. Compound 1 showed a molecular weight of 400, and accurate mass measurement using LC–HR-MS revealed its molecular formula to be C22H29N4O2F. The MS and NMR spectrometric data revealed that the structure of 1 was N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-3-(4-fluorophenyl)-1H-pyrazole-5-carboxamide. Compound 1, which is a new type of synthetic cannabinoid, has a 3-(4-fluorophenyl)-1H-pyrazole group in place of a 1H-indazole group of AB-CHMINACA. To our knowledge, data on the chemistry and pharmacology of compound 1 have never been reported, and we therefore named compound 1 “AB-CHFUPYCA.”
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthetic cannabinoids have become a major group of new psychoactive substances (NPSs) both in Japan and worldwide. Since the first appearance of synthetic cannabinoids at the end of 2008, various types have continued to emerge on the illegal drug market [1, 2], as follows: cyclohexylphenols (e.g., cannabicyclohexanol) [3], indoles (e.g., JWH-018) [4], indazoles (e.g., APINACA) [5], benzimidazoles (e.g., FUBIMINA) [6], quinolinyl carboxylates (e.g., QUPIC: PB-22) [7] and carboxamide derivatives (e.g., AB-PINACA) [8], among others. Many types of synthetic cannabinoids are regulated in some countries in attempts to prevent the spread of their abuse. In Japan, a total of 858 synthetic cannabinoids were regulated as narcotics or designated substances as of April 2015. These synthetic cannabinoids contain 8 narcotics and 850 designated substances, including 759 substances that became controlled after the introduction of the generic scheduling for designating naphthoylindole-type synthetic cannabinoids [1].
Despite the various regulatory control measures, the appearance of new synthetic cannabinoids continues. In European countries, more than 30 new synthetic cannabinoids were reported as NPSs for the first time in 2014, monitored by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) [9]. Additionally, 2H-indazole isomers of synthetic cannabinoids were detected in illegal products purchased during the first half of the year in 2014 in Japan [10]. There is little pharmacological information about most of the detected synthetic cannabinoids. Several fatal cases resulting from carboxamide derivatives of synthetic cannabinoids such as 5-fluoro-AMB, AB-CHMINACA, 5-fluoro-ADB and MAB-CHMINACA were reported in 2015 in Japan [11–13]. It is thus of concern that further serious harm may be caused by synthetic cannabinoids, especially the carboxamide derivatives.
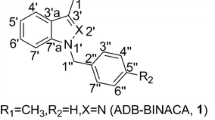
In this study, we describe the identification of a new pyrazole-carboxamide-type synthetic cannabinoid (1) in illegal products obtained in February 2015 (Fig. 1).
Materials and methods
Sample for analyses
The analyzed sample was obtained via the Internet in February 2015 as an herbal-type product being sold in Japan. The herbal-type product A contained approximately 3 g of mixed dried plants.
Chemicals and reagents
All common chemicals and solvents were of analytical reagent or high-performance liquid chromatography (HPLC) grade. As a solvent for the nuclear magnetic resonance (NMR) analysis, chloroform-d 3 (99.96 %) was purchased from the ISOTEC division of Sigma-Aldrich (St. Louis, MO, USA).
Preparation of sample solutions
For the qualitative analyses, 10 mg of each herbal-type product was crushed into powder and extracted with 1 ml of methanol under ultrasonication for 10 min. After centrifugation (3000 rpm, 5 min) of each extract, the supernatant solution was passed through a centrifugal filter (Ultrafree-MC, 0.45-µm filter unit; Millipore, Bedford, MA, USA) to serve as the sample solution for the analyses. If necessary, the solution was diluted with methanol to a suitable concentration before the instrumental analyses.
Analytical conditions
A sample solution was analyzed by ultra-performance liquid chromatography–electrospray ionization-mass spectrometry (UPLC–ESI-MS) and by gas chromatography–mass spectrometry (GC–MS) in the electron ionization (EI) mode according to our previous report [14]. The UPLC–ESI-MS analysis was performed on an ACQUITY UPLC system with a mass detector and a photodiode array (PDA) detector (Waters, Milford, MA, USA). The LC–MS analysis was carried out with a binary mobile phase consisting of solvent A (0.1 % formic acid in water) and solvent B (0.1 % formic acid in acetonitrile). The elution program was as follows: 5 % B to 20 % B (0–20 min) and up to 80 % B (20–30 min, 10-min hold) at a flow rate of 0.3 ml/min. GC–EI-MS was performed on an Agilent 6890 N GC system with a 5975 mass selective detector (Agilent Technologies, Santa Clara, CA, USA) using a capillary column (HP-1MS capillary, 30 m × 0.25 mm i.d., 0.25-μm film thickness; Agilent Technologies) with helium gas as a carrier at 0.7 ml/min. The conditions were: electron energy, 70 eV; injector temperature, 220 °C; injection, splitless mode for 1.0 min; oven temperature program, 80 °C (1-min hold) and an increase at a rate of 5 °C/min to 190 °C (15-min hold) followed by an increase at 10 °C/min up to 310 °C (15-min hold); transfer line temperature, 280 °C; scan range, m/z 40–550.
The obtained GC mass spectra were compared to those of an EI-MS library (Mass Spectra of Designer Drugs 2014; Wiley-VCH, Weinheim, Germany). We also used our in-house EI-MS library of designer drugs obtained by our ongoing survey of illegal products and commercially available reagents for the structural elucidation.
We measured the accurate mass numbers of the target compounds by liquid chromatography–quadrupole-time-of-flight-mass spectrometry (LC–QTOF-MS) in the ESI mode according to our previous report [7].
The nuclear magnetic resonance (NMR) spectra were obtained on ECA-800 and 600 spectrometers (JEOL, Tokyo, Japan). Assignments were made via 1H NMR, 13C NMR, heteronuclear multiple quantum coherence (HMQC), heteronuclear multiple-bond correlation (HMBC), 15N HMBC, HH correlation spectroscopy (HH-COSY) and nuclear Overhauser effect (NOE) spectra.
Isolation of compound 1
A 1.0-g sample of mixed dried plants (product A) was extracted with 100 ml of chloroform by ultrasonication for 10 min. Additionally, the extraction was repeated twice without sonication, and the supernatant fractions were combined and concentrated. The extract was placed on a preparative silica-gel thin-layer chromatography (TLC) plate (Silica Gel 60, 20 × 20 cm, 2 mm thick; Merck, Darmstadt, Germany), which was then developed using hexane/ethyl acetate (3:1, v/v). A portion of the silica gel containing a target compound in the TLC plate was detected under ultraviolet (UV) light (254 nm). It was then scraped from the plate and eluted with chloroform to obtain fraction 1, which was further purified by repeated preparative TLC with isopropyl ether/acetone/isopropanol (10:1:1, v/v). The extract was then recrystallized in acetone and washed with methanol, which gave compound 1 (7 mg) as a white needle.
Results and discussion
Identification of an unknown peak 1
An unknown peak 1 was detected in the LC–MS and GC–MS for product A (Fig. 2a, b, d). By LC–MS analysis, peak 1 showed the protonated molecular ion at m/z 401 ([M+H]+) (Fig. 2c). In the GC–MS analysis, peak 1 showed a molecular ion at m/z 400 (Fig. 2e). The fragment ions of peak 1 (m/z 95, 257, 285 and 356) were different from those of known synthetic cannabinoids such as indole or indazole derivatives (i.e., JWH-018 or AB-CHMINACA) [4, 10]. The accurate mass spectrum obtained by LC–QTOF-MS gave an ion peak at m/z 401.2344, suggesting that the protonated molecular formula of compound 1 was C22H30N4O2F (calcd. 401.2353).
Liquid chromatography–mass spectrometry and gas chromatography–mass spectrometry (GC–MS) analyses of product A. The liquid chromatography–ultraviolet-photodiode array (LC–UV-PDA) chromatogram (a), total ion chromatogram (TIC) (b), and electrospray ionization mass and ultraviolet spectra of peak 1 (c) are shown. TIC (d) and electron ionization mass spectra (e) of peak 1 obtained by GC–MS are also shown
The observed 1H and 13C NMR spectra (Table 1), HH-COSY, HMQC, HMBC and 15N HMBC correlations for compound 1 indicated the presence of three moieties, cyclohexylmethyl group (A), N-(1-amino-3-methyl-1-oxobutan-2-yl)-carboxamide group (B) and 4-fluorophenyl group (C), as shown in Table 1 and Fig. 3a, b. Groups A and B are the same parts as in AB-CHMINACA, but group C is not (Figs. 1, 3a, b; Table 1). Additionally, it was presumed that the remaining C3H1N2 unit was a pyrazole group or an imidazole group based on the one-dimensional (1D)- and 2D-NMR spectra (Figs. 1, 3a, b; Tables 1, 2).
First, we compared the 15N NMR and 13C NMR data of compound 1 with those of the known 1-methyl-1H-pyrazole and 1-methyl-1H-imidazole (Fig. 1; Tables 1, 2) [15, 16]. The chemical shifts of the corresponding nitrogens and carbons of compound 1 [N-1 (δN −167.8), N-2 (δN −71.1) and C-4 (δc 103.3)] were different from those of the 1H-imidazole moiety in 1-methyl-1H-imidazole [N-1 (δN −218.5), N-2 (δN −118.1) and C-4 (δc 130.2)], respectively. However, their chemical shifts of 1 were similar to those of 1-methyl-1H-pyrazole [N-1 (δN −178.4), N-2 (δN −71.6) and C-4 (δc 105.3)] (Tables 1, 2). These results strongly suggest that compound 1 has a pyrazole group.
In addition, we observed the HMBC correlations from H-4 to C-3, C-5 and the amide carbon (C-1″′), from H-1A to C-5, and from H-2″ and H-6″ to C-3 (Fig. 3a). The 15N HMBC correlations from H-4 to N-1 and N-2, and from H-1A to N-2, were also observed (Fig. 3b). These results revealed that the pyrazole group was connected at position 1 to the cyclohexylmethyl group at position 1A, at position 3 to the 4-fluorophenyl group at position 1″, and at position 5 to the N-(1-amino-3-methyl-1-oxobutan-2-yl)-carboxamide group at position 1″′ (Fig. 3a, b).
Finally, compound 1 was identified as N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-3-(4-fluorophenyl)-1H-pyrazole-5-carboxamide and named “AB-CHFUPYCA.” The fragment ions at m/z 95, 257, 285 and 356 of peak 1 in the GC–MS spectrum further confirmed the structure of compound 1 (Fig. 2e). Compound 1 is a new substance; its chemical and pharmaceutical data have not been reported previously.
Although it is not yet clear whether compound 1 has any pharmacological effects, it is possible that compound 1 has cannabimimetic activity, in light of its structure. Compound 1 was not a previously detected type of synthetic cannabinoid, such as indazole and indole derivatives (i.e., JWH-018, AB-CHMINACA and ADBICA [4, 7, 10]), but rather is a pyrazole derivative. A pyrazole derivative SR141716A (Rimonabant) is a selective cannabinoid (CB1) receptor antagonist (Ki = 1.98 nM), and it acts as an inverse agonist (IC50 = 48 nM) (Fig. 1) [17, 18]. SR141716A was developed as an appetite suppressant and anti-obesity drug. However, it has been withdrawn from the market because of concerns over its serious side effects, including suicidality and depression [19]. The other pyrazole derivatives, AM251 and AM281, which are analogs of SR141716A, are also selective CB1 receptor antagonists [Ki (nM) values = 7.5 and 12, respectively] [20, 21]. On the other hand, a pyrrole derivative of synthetic cannabinoid JWH-307, which was detected in illegal products [22], is known to have affinity for CB1 and CB2 receptors [Ki (nM) values = 7.7 and 7.1, respectively] [23]. Some detected types of synthetic cannabinoids were reported to have affinities for CB1 and/or CB2 receptors [23]. Nevertheless, the effects of compound 1 are unclear, and it is therefore necessary to elucidate the pharmacological functions of compound 1.
Conclusions
This report describes the identification of a new synthetic cannabinoid, AB-CHFUPYCA [N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-3-(4-fluorophenyl)-1H-pyrazole-5-carboxamide, 1], in illegal products. Compound 1 is a novel pyrazole-carboxamide derivative, and its pharmacological effects are unknown. The continuous provision of timely and objective information about NPSs and current trends is important to prevent the abuse of NPSs.
References
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y (2014) Changes in the prevalence of new psychoactive substances before and after the introduction of the generic scheduling of synthetic cannabinoids in Japan. Drug Test Anal 6:832–839
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y (2013) Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol 31:44–53
Uchiyama N, Kikura-Hanajiri R, Kawahara N, Haishima Y, Goda Y (2009) Identification of a cannabinoid analog as a new type of designer drug in a herbal product. Chem Pharm Bull 57:439–441
Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y (2009) Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicol 27:61–66
Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y (2012) Identification of two new-type synthetic cannabinoids, N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide (APICA) and N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide (APINACA), and detection of five synthetic cannabinoids, AM-1220, AM-2233, AM-1241, CB-13 (CRA-13) and AM-1248, as designer drugs in illegal products. Forensic Toxicol 30:114–125
Uchiyama N, Shimokawa Y, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2014) Two new synthetic cannabinoids, AM-2201 benzimidazole analog (FUBIMINA) and (4-methylpiperazin-1-yl)(1-pentyl-1H-indol-3-yl)methanone (MEPIRAPIM), and three phenethylamine derivatives, 25H-NBOMe 3,4,5-trimethoxybenzyl analog, 25B-NBOMe, and 2C-N-NBOMe, identified in illegal products. Forensic Toxicol 32:105–117
Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2013) Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol 31:223–240
Uchiyama N, Matsuda S, Wakana D, Kikura-Hanajiri R, Goda Y (2013) New cannabimimetic indazole derivatives, N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA) and N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA), identified as designer drugs in illegal products. Forensic Toxicol 31:93–100
EMCDDA (2015) New psychoactive substances in Europe. An update from the EU Early Warning System, March 2015. http://www.emcdda.europa.eu/attachements.cfm/att_235958_EN_TD0415135ENN.pdf. Accessed May 2015
Uchiyama N, Shimokawa Y, Kikura-Hanajiri R, Demizu Y, Goda Y, Hakamatsuka T (2015) A synthetic cannabinoid FDU-NNEI, two 2H-indazole isomers of synthetic cannabinoids AB-CHMINACA and NNEI indazole analog (MN-18), a phenethylamine derivative N-OH-EDMA, and a cathinone derivative dimethoxy-α-PHP, newly identified in illegal products. Forensic Toxicol. doi:10.1007/s11419-015-0268-7
Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, Suzuki O (2015) Postmortem distribution of AB-CHMINACA, 5-fluoro-AMB, and diphenidine in body fluids and solid tissues in a fatal poisoning case: usefulness of adipose tissue for detection of the drugs in unchanged forms. Forensic Toxicol 33:45–53
Hasegawa K, Wurita A, Minakata K, Gonmori K, Yamagishi I, Nozawa H, Watanabe K, Suzuki O (2015) Identification and quantitation of 5-fluoro-ADB, one of the most dangerous synthetic cannabinoids, in the stomach contents and solid tissues of a human cadaver and in some herbal products. Forensic Toxicol 33:112–121
Hasegawa K, Wurita A, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Watanabe K, Suzuki O (2015) Postmortem distribution of MAB-CHMINACA in body fluids and solid tissues of a human cadaver. Forensic Toxicol. doi:10.1007/s11419-015-0272-y
Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y (2013) URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci Int 227:21–32
Katritzky AR, Ramsden CA, Joule JA, Zhdankin VV (2010) Structure of five-membered rings with two or more heteroatoms. In: Handbook of heterocyclic chemistry, 3rd edn. Elsevier, Amsterdam, pp 139–207
Pinto DCGA, Santos CMM, Silva AMS (2007) Advanced NMR techniques for structural characterization of heterocyclic structures. In: Pinho e Melo TMVD (ed) Recent research developments in heterocyclic chemistry. Research Signpost, Kerala, pp 397–475
Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara P, Soubrié P, Brelière JC, Le Fur G (1994) SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–244
Rinaldi-Carmona M, Barth F, Héaulme M, Alonso R, Shire D, Congy C, Soubrié P, Brelière JC, Le Fur G (1995) Biochemical and pharmacological characterisation of SR 141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci 56:1941–1947
Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A (2007) Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370:1706–1713 (1671–1672 comment)
Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A (1999) Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem 42:769–776
Lan R, Gatley J, Lu Q, Fan P, Fernando SR, Volkow ND, Pertwee R, Makriyannis A (1999) Design and synthesis of the CB1 selective cannabinoid antagonist AM281: a potential human SPECT ligand. AAPS PharmSci 1(2):E4
Ernst L, Krüger K, Lindigkeit R, Schiebel HM, Beuerle T (2012) Synthetic cannabinoids in ‘‘spice-like’’ herbal blends: first appearance of JWH-307 and recurrence of JWH-018 on the German market. Forensic Sci Int 222:216–222
Huffman JW (2009) Cannabimimetic indoles, pyrroles, and indenes: structure-activity relationships and receptor interactions. In: Reggio PH (ed) The cannabinoid receptors. Humana Press, New York, pp 49–94
Acknowledgments
A portion of this work was supported by a Health and Labor Sciences Research Grant from the Ministry of Health, Labour, and Welfare, Japan.
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uchiyama, N., Asakawa, K., Kikura-Hanajiri, R. et al. A new pyrazole-carboxamide type synthetic cannabinoid AB-CHFUPYCA [N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-3-(4-fluorophenyl)-1H-pyrazole-5-carboxamide] identified in illegal products. Forensic Toxicol 33, 367–373 (2015). https://doi.org/10.1007/s11419-015-0283-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-015-0283-8