Abstract
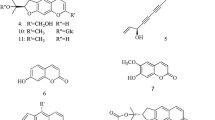
Orengedokuto is a Kampo formula that has been used for removing “heat” and “poison” to treat inflammation, hypertension, gastrointestinal disorders, and liver and cerebrovascular diseases. We report here our analysis of the anti-inflammatory effect of the component crude drugs of orengedokuto and their constituents using the inhibition of nitric oxide (NO) production in the murine macrophage-like cell line J774.1. An initial comparison of NO production inhibitory activities of the extracts of the component crude drugs and their combinations revealed that the activity could be attributed to Phellodendron Bark and Coptis Rhizome. Berberine (1), the major constituent of these crude drugs, showed potent activity (IC50 4.73 ± 1.46 μM). Quantitative analysis of 1 in the extracts of all combinations of component crude drugs revealed that the amount of 1 in each extract of the combination of Scutellaria Root with either Phellodendron Bark and/or Coptis Rhizome was lower than that in the corresponding mixtures of the extracts of the individual crude drugs and that 1 was present in the precipitates formed during the decoction process. To the contrary, the differences in the amounts of 1 were smaller in the extracts containing Gardenia Fruit. These results indicated that the constituents of Scutellaria Root precipitated with 1 and that the constituents of Gardenia Fruit dissolved the precipitates. To identify the constituents affecting the solubility of 1, we fractionated the hot-water extracts of Scutellaria Root based on solubility tests of 1 to give baicalin (2), wogonin (3) and oroxyloside (4), which formed precipitates with 1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orengedokuto (黄連解毒湯) is a Kampo formula consisting of Scutellaria Root (root of Scutellaria baicalensis), Phellodendron Bark (bark of Phellodendron amurense or P. chinense), Coptis Rhizome (rhizome of Coptis japonica, C. chinense, C. deltoidea, or C. teeta), and Gardenia Fruit (fruit of Gardenia jasminoides) [1]. It has been used to remove “heat” and “poison” in the treatment of inflammation, hypertension, gastrointestinal disorders, and liver and cerebrovascular diseases [2]. Although there have been many reports on the anti-inflammatory activities of the component crude drugs of orengedokuto and their constituents [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21], studies on the anti-inflammatory effects of orengedokuto itself in relation with the constituents that contribute to the activity are limited [22, 23].
In a previous study, we quantitatively evaluated the roles of the component crude drugs of orengedokuto on prostaglandin E2 (PGE2) production inhibitory activity in the lipopolysaccharide (LPS)-stimulated macrophage-like cell line J774.1 and found that Scutellaria Root was the source of the activity and that the combination of its flavonoid constituents, including baicalein, wogonin, 6-methoxywogonin, and oroxylin A, could quantitatively explain the activity of the orengedokuto extract [24]. In this study, we focused on the inhibition of nitric oxide (NO), another mediator of inflammation. We analyzed the roles of the component crude drugs of orengedokuto and their constituents using the inhibition of LPS-induced NO production in the same cell line and the same methods as those used in our previous study [24], and we determined the constituents responsible for the activity.
Results and discussion
The component crude drugs of orengedokuto, namely, Scutellaria Root (S), Phellodendron Bark (P), Coptis Rhizome (C) and Gardenia Fruit (G), were separately extracted in the manner that is used to prepare a Kampo decoction; after the removal of precipitates, the extracts were freeze-dried. All combinations of the four crude drugs tested in this study were also prepared in the same manner. For these combinations, the ratio of S:P:C:G = 3:1.5:1.5:2 was used since this is the ratio listed in the Japanese Pharmacopoeia [1]. Table 1 shows the amounts of the extracts obtained from 20 g of the crude drugs. Among the single crude drugs (entries 1–4), Scutellaria Root (entry 1) gave the largest amount of extract, followed by Gardenia Fruit (entry 4), Coptis Rhizome (entry 3), and Phellodendron Bark (entry 2).
It is possible that the constituents of the component crude drugs interact with each other during the decoction process and cause chemical changes in the extract. Therefore, in addition to the extracts prepared from mixtures of the crude drugs (combined extracts, Table 1, entries 5–15), we prepared mixtures of the extracts of the individual component crude drugs (blended extract) in the same ratio at which the crude drugs are found in orengedokuto and compared their activities. Figure 1 shows the inhibitory activities of the individual crude drugs, combined extracts and blended extracts at 10 μg/mL on LPS-induced NO production in the same cell line used in our previous study (J774.1 cells) [24]. None of the samples showed cytotoxicity at this concentration, indicating that NO production inhibition was not caused by a cytotoxic effect. Orengedokuto extract (SPCG; Table 1 entry 15) showed only a weak inhibitory activity (18.5%) at this concentration but showed significant activity at a higher concentration (100 μg/mL, 86.4%). Phellodendron Bark (P) and Coptis Rhizome (C) showed the most significant inhibitory activities among the individual crude drugs, and the extract of C showed more potent inhibitory activity than that of P. Among the combinations of the component crude drugs (SP−SPCG; Table 1, entries 5–15), the combination of P and C, each of which showed significant inhibitory activity, showed the strongest inhibitory activity among all of the combinations, indicating that the constituents contained in P and C are responsible for the activity.
Inhibitory activity of the extracts (10 μg/mL) on nitric oxide (NO) production. Cont Control, NMMA NG-monomethyl-l-arginine, 50 μM. S Scutellaria Root, P Phellodendron Bark, C Coptis Rhizome, G Gardenia Fruit. Asterisks and hash mark indicate significant differences at: *p < 0.05, **p < 0.01, ***p < 0.001 (vs. control); #p < 0.05. Gray bars indicate a single crude drug, Black bars indicate a blended extract, shaded bars with diagonal lines indicate combined extract. Data are presented as the mean ± standard error (SE)
Berberine (1), the major constituent of both P and C, has been reported to inhibit NO production in LPS-stimulated RAW264.7 macrophages [25]. Therefore, we first examined the activity of 1 and found that it showed significant and concentration-dependent inhibition, with an IC50 value of 4.73 μM. Our subsequent analysis of the amount of 1 in the extracts (Table 1) using the method described in the Japanese Pharmacopoeia [1] revealed that the extracts of P and C contained 382 ± 28 and 797 ± 149 mg of 1, respectively, which clearly explains the difference between the inhibitory activities of extracts of P and C. Among the combinations of crude drugs, the amounts of 1 in the PC (entry 8) and the CG (entry 10) combined extracts, both of which showed strong activity, were 690 ± 110 and 340 ± 24 mg, respectively. The amount of 1 in the PC extract may explain the difference between the activities of the combined and blended extracts of PC: the combined extract contained more berberine (690 mg) than the blended extract (589.5 mg, calculated from the amount of 1 in the P and C extracts). The amount of 1 in another combined extract with significant activity, PCG (entry 14), was 280 ± 15 mg. In contrast, the amounts of 1 in the other PC-containing combinations, SPC (entry 11) and SPCG (entry 15), both of which showed only weak activities, were 110 ± 11 and 110 ± 30 mg, respectively. From these results, it is evident that NO production inhibitory activities of the extracts corresponded well with the amounts of 1 in the extracts.
Figure 2 shows the inhibitory effect of the PC extract and the effect of 1 at an amount equivalent to that contained in the extract on the production of NO. The PC extract and the corresponding amount of berberine (1) showed comparable activities: the PC extract at 50 μg/mL showed 74.2% inhibition, and at the corresponding concentration (36.3 μM), 1 showed 72.6% inhibition. Figure 3 shows the inhibitory effects of the SPCG (orengedokuto) extract and the effect of 1 at an amount equivalent to that seen in the extract on the production of NO. Berberine (1) also showed comparable inhibition to the SPCG extract at each concentration. Thus, it would appear that the NO production inhibitory activity of the orengedokuto (SPCG) extract in this assay system is mainly attributable to 1.
Although the differences were not significant, the PC extract showed stronger inhibition than the corresponding amount of berberine (1), and the SPCG extract showed less activity. Fujii et al. recently reported the identification of the anti-inflammatory constituents in the methanol extracts of Phellodendron Bark and Coptis Rhizome by monitoring the suppression of NO production [26]. They divided the extracts into non-alkaloidal and alkaloidal fractions and showed that both fractions of the Phellodendron Bark extract and the alkaloidal fraction of the Coptis Rhizome extract suppressed NO production. They found that berberine (1) isolated from the alkaloidal fraction of both crude drugs, obakunone and limonin isolated from the non-alkaloidal fraction of Phellodendron Bark, and coptisine isolated from the alkaloidal fraction of Coptis Rhizome inhibited NO production. Coptisine [27, 28] and other berberine-type alkaloids may explain the stronger activity of the PC extract relative to the corresponding amount of 1. However, as we used water extracts, the contribution of limonoids to the activity may not be as large.
As described above, the SPC and SPCG extracts contained lower amounts of berberine and showed only weak activities. Combinations of S with P and/or C showed significantly lower activities than the combinations without S; i.e., the SP, SC and SPC combinations showed significantly weaker activities than the P, C, and PC extracts, respectively. Thus, although S did not inhibit NO production, it decreased the ability of P and C to inhibit NO production. The amount of 1 in the SPC combined extract was significantly lower (37%) than that in the corresponding blended extract (Table 1, entry 11). This result suggests that the constituents of the crude drugs were interacting during the extraction process and that the compounds affecting the extraction efficiency of 1 in the SPC combination seemed to be contained in S.
It has been reported that berberine (1) forms precipitates with the baicalin (2) and wogonoside (3) contained in Scutellaria Root [29, 30]. As we removed insoluble materials by centrifugation during the preparation of the extracts, a part of 1 may have been removed as precipitates. Therefore, we examined the amount of 1 in the precipitates. In the combinations of S with P and/or C (Table 1, entries 5, 6, 11, and 15), large amounts of 1 were found in the precipitates. The amount of 1 in the precipitates was especially large in the SPC extract (420 mg), which was 30-fold more than that calculated for PC (27 × 10/20 = 13.5 mg). However, when Gardenia Fruit (G) was added to this combination (i.e., SPCG), the amount of 1 in the precipitate decreased to 170 mg, which was only 17-fold greater than the predicted amount (27 × 7.5/20 = 10.1 mg). The same relationship was observed between SP and SPG (entries 5 and 12) and between SC and SCG (entries 6 and 13). Thus, it would appear berberine (1) interacts with the constituents of Scutellaria Root (S) to form precipitates, and the constituents of Gardenia Fruit (G) dissolve the precipitates.
Although it is known that 1 forms precipitates with baicalin (2) and wogonoside (3) present in S [29, 30], we searched for the constituents in the extract of S that form precipitates with 1. A hot-water extract of S was fractionated based on precipitate-formation tests, resulting in the isolation of three known flavone glucuronides, i.e., baicalin (2), wogonoside (3) and oroxyloside (4) (Fig. 4). Figure 5 shows the turbidity plot of the mixture of flavone glucuronides (2–4) with 1. Baicalin (2) and oroxyloside (4) showed similar turbidity changes and reached their highest turbidities at a molar ratio of 1:2 with 1. To the contrary, wogonoside (3), which has a substitution pattern on its A-ring that differs from that of 2 and 4, reached its maximum turbidity at a molar ratio of 2:1 with 1.
In our study we investigated the roles of the constituent crude drugs of the orengedokuto formula in the inhibition of NO production activity. The extracts of two of the component crude drugs, Phellodendron Bark and Coptis Rhizome, strongly inhibited NO production at the same concentration contained in the orengedokuto extract, and the active component in each of these materials was berberine (1), the major constituent of these crude drugs. We previously analyzed the inhibitory activity of orengedokuto on PGE2 production and found that this inhibitory activity could be attributed to a mixture of the flavonoids from Scutellaria Root [24, 31] and that these flavonoids showed a synergistic effect by acting on different steps in the biosynthesis of prostaglandin (PG) [32]. However, although Scutellaria Root and its constituents have been reported to inhibit NO production [5,6,7], and, in addition, we found in a previous study that the flavone constituents of Scutellaria Root inhibited NO production in our assay system [32], in the present study Scutellaria Root extract did not show significant inhibition of NO production compared to the inhibition of the Phellodendron Bark and Coptis Rhizome. In contrast, its flavonoid glucuronides, i.e., baicalin (2), wogonoside (3), and oroxyloside (4), reduced NO production inhibitory activity by forming precipitates with berberine (1). There have also been reports on the inhibition of NO production by Gardenia Fruit and its constituents [16, 19, 20]. However, the extract of Gardenia Fruit itself also did not show inhibitory activity at a concentration equivalent to that of the orengedokuto extract. To the contrary, the constituents of Gardenia Fruit dissolved the berberine–baicalin precipitates. The identification of the constituents of Gardenia Fruit necessary for this activity will be reported elsewhere.
A Kampo formula is a mixture of crude drugs that contains a variety of constituents. Although it is generally believed that the effects of a Kampo formula are the sum of the effects of its many constituents, detailed analyses of the constituents involved in the effects of Kampo formulae are limited. In the present study, our comparison of the NO production inhibitory activities of extracts of combinations of the component crude drugs together with the quantitative analysis of berberine (1) in the extracts enabled us to determine the roles of the constituents of the component crude drugs and their interactions within orengedokuto. This strategy will be effective for evaluating the effectiveness of Kampo formulae and contribute to the development of a scientific basis for Kampo medicines.
Materials and methods
General procedure
High-performance liquid chromatography analyses were performed on an LC-10A HPLC system (Shimadzu, Kyoto, Japan) equipped with a column compartment (CTO-10A), a degasser (DGU-12A), a pump (LC-10ADvp) and a detector (SPD-10A). 1H- and 13C-NMR spectra were recorded on a JEOL FT-NMR ECP-600 spectrometer (JEOL Ltd., Tokyo, Japan), and chemical shifts are expressed in δ (ppm) relative to tetramethylsilane (TMS) as an internal standard.
Materials
The crude drugs used for the preparation of extracts, Scutellaria Root (S; Lot. 004609007), Phellodendron Bark (P; Lot. 001310001), Coptis Rhizome (C; Lot. 001210002) and Gardenia Fruit (G; Lot. 001110001), were purchased from Tochimoto Co. (Osaka, Japan). Baicalin (Lot. STL2939) and baicalein (Lot. PKF2197) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Berberine chloride was purchased from Tokyo Chemical Industry Co. (Tokyo, Japan).
Preparation of the extracts
Extracts were prepared as described previously [24]. In brief, cut crude drug materials (single crude drug or a combination of multiple crude drugs [combined extract], 20 g) were boiled in distilled water (400 mL) in a decoction apparatus for 30 min. The extract was filtered through gauze and centrifuged at 1000 g at 25 °C for 10 min, and the supernatant was freeze–dried to provide the extract. The procedure was repeated to prepare a total of three batches, and the average weight and standard error were calculated. The ratio of the composition of the component crude drugs in orengedokuto is S:P:C:G = 3:1.5:1.5:2 [1].
The extracts of the component crude drugs prepared as above were mixed to prepare blended extracts corresponding to the combinations shown in entries 5–15 in Table 1 in the ratio of S:P:C:G = 4.7:1:1.3:2.2. The ratio was calculated as a product of the average yield of each extract and the composition ratio in orengedokuto of each crude drug, S:P:C:G = (6.34 × 3):(2.72 × 1.5):(3.44 × 1.5):(4.55 × 2).
Quantitative analysis of berberine
The quantitative analysis of berberine was carried out according to a published method [33]. Briefly, the analysis was carried out on a Capcell Pak C18 MG-type column (5 μm, 4.6 mm i.d. × 250 mm) (Lot. AKAD02624) (Shiseido Fine Chemical Co., Tokyo, Japan), with 50% CH3CN (1000 mL) containing KH2PO4 (3.4 g) and sodium lauryl sulfate (1.7 g) as the eluent at a flow rate of 0.9 mL/min. The column was maintained at 40 °C, and berberine was quantified by the absorbance at 345 nm.
Measurement of NO production in the J774.1 cell line
The NO produced was quantified by the Griess method [34]. Briefly, a murine macrophage-like cell line, J774.1, was grown in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies Japan Ltd., Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (Cat. no. S1560; Biowest Co., Nuaillé, France) and 1% penicillin/streptomycin/glutamine (Cat. no. 10378-016; Gibco Co., UK, Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C under a humidified 5% CO2 atmosphere. The cells were seeded into the wells of a 96-well culture plate (Falcon; Thomas Scientific, Swedesboro, NJ, USA) at a density of 5.0 × 105 cells/mL in 200 μL of medium, and the cells were allowed to adhere to the plate for 24 h, following which the medium was replaced with fresh medium containing LPS (Cat. no. L2637; Sigma-Aldrich Co., St. Louis, MO, USA; 5 μg/mL) and the test compound (each 100 μL). After further incubation for 24 h, the supernatant (50 μL) was transferred to a new plate and mixed with an equal volume of Griess reagent, which consists of 1% sulfanilamide (Cat. no. 191-04502, Wako Pure Chemical Industries, Ltd.) and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride (Cat. no. 147-04141, Wako Pure Chemical Industries, Ltd.) in 5% phosphoric acid solution. Absorbance at 550 nm was measured with a microplate reader (model no. MTP-810 lab; Corona Electric Co. Ltd., Hitachi City, Japan). The percentage inhibition was calculated as follows: % of control = [As/Ac] × 100, where Ac and As are the absorbances of the control (treated with LPS alone) and the cells treated with LPS and a sample, respectively. NG-monomethyl-l-arginine (NMMA; IC50 32 μM) (Cat. no. 345-07161; Dojindo Laboratories Co., Ltd., Osaka, Japan) was used as the positive control for NOS inhibition [35].
Cell viability
Cell viability was determined by the mitochondrial respiration-dependent 3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) reduction method [36]. Briefly, the cells in 50 μL of the medium described above were combined with 5 mg/mL MTT solution (Dojindo Laboratories Co., Ltd.) (5 μL) and incubated for 3 h at 37 °C under a humidified 5% CO2 atmosphere. The supernatant was then removed, the violet formazan crystals from the viable cells were dissolved in 50 μL of DMSO, and absorbance was measured at 595 nm.
Statistics
All data are presented as the mean ± standard error of three independent experiments. The differences between the activities of the LPS control and those of the samples were evaluated by a one-tailed Student’s t test using StatPlus 2009 for Mac OS (AnalystSoft Inc., Walnut, CA, USA). Multiple comparisons were conducted by one-way analysis of variance followed by Dunnett’s post hoc test.
Precipitation test with berberine
An aqueous solution (5 mL) of berberine chloride (1 mM) was mixed with an aqueous solution (5 mL) of a test sample at room temperature, and the turbidity of the resulting mixture was measured by a turbidimeter (model no. TR-55; Kasahara Chemical Instruments Co., Kuki-City, Japan). As a blank test, the turbidity of a mixture of test sample solution and water (5 mL) was measured and its turbidity subtracted from those of the test samples.
Isolation of precipitate-forming constituents from Scutellaria Root
Scutellaria Root (S; 1.5 kg) (Lot. P031010201) was extracted four times with hot water (4.5 L), 40 min each time. The combined extracts were applied to a column of Diaion HP-20 (ϕ 12 × 10 cm) and eluted with H2O (8 L) followed by MeOH (40 L) to obtain water (fresh dry weight [fr. DW] 492 g; turbidity [Tur.] 17.2) and MeOH [fr. DM 59.5 g; Tur. 0) eluates. A portion of the eluates (fr. DW 12.8 g) was dissolved in H2O (100 mL) and chromatographed on a column of Diaion CHP-20 P (ϕ 5 × 10 cm) with water-–eOH to obtain eight fractions: (1) fr. DW-1 [Tur. 0.02], H2O elute (1 L), 7.03 g; (2) fr. DW-2 [Tur. 19.1], H2O elute (0.5 L), 129 mg; (3) fr. DW-3 [Tur. 8.11], H2O:MeOH = 9:1 elute (0.1 L), 325 mg; (4) fr. DW-4 [Tur. 33.4], H2O:MeOH = 1:9 elute (2.5 L), 1.54 g; (5) fr. DW-5 [Tur. 41.0], H2O:MeOH = 1:4 elute (3.5 L), 1.02 g; (6) fr. DW-6 [Tur. 19.9], H2O:MeOH = 1:2 elute (1.8 L), 615 mg; (7) fr. DW-7 [Tur. 0.7], H2O:MeOH = 1:1 elute (2.5 L), 549.1 mg; (8) fr. DW-8 [Tur. − 6.3], MeOH elute (1.5 L), 656 mg.
Fr. DW-4 [Tur. 33.4] (1.54 g) was suspended in H2O (100 mL), chromatographed on CHP-20P column (ϕ 5 × 10 cm), and eluted with H2O−MeOH to give seven fractions: (1) fr. DW-4-1 [Tur. 0], H2O elute (0.5 L), 3.2 mg; (2) fr. DW-4-2 [Tur. 0], H2O:MeOH = 8:1 elute (0.5 L), 8.4 mg; (3) fr. DW-4-3 [Tur. 9.1], H2O:MeOH = 4:1 elute (0.5 L), 19.1 mg; (4) fr. DW-4-4 [Tur. 0], H2O:MeOH = 3:1 elute (0.5 L), 9.7 mg; (5) fr. DW-4-5 [baicalin (2)], H2O:MeOH = 2:1 elute (0.6 L), 225 mg; (6) fr. DW-4-6 [Tur. 29.5], H2O:MeOH = 2:1 elute (1.3 L) → H2O:MeOH = 1:1 elute (1.0 L), 1.19 g; (7) fr. DW-4-7 [Tur. 0], MeOH elute (1.0 L), 22.9 mg. A portion of fr. DW-4-6 (1.07 g) was fractionated on a CHP-20P column (ϕ 5 × 8 cm) with H2O –MeOH to obtain three fractions: (1) fr. DW-4-6-1 [Tur. 0], H2O elute (0.2 L), 7.7 mg; (2) fr. DW-4-6-2 [Tur. 0], H2O:MeOH = 4:1 elute (1.0 L), 10.6 mg; (3) fr. DW-4-6-3 [Tur. 29.5], MeOH elute (3.0 L), 503.3 mg]. Fr. DW-4-6-3 (398 mg) was chromatographed on CHP-20 P (ϕ 5 × 8 cm) with H2O–MeOH (1:0 → 0:1) to obtain seven fractions: (1) fr. DW-4-6-3-1 [Tur. 0], 5.0 mg; (2) fr. DW-4-6-3-2 [Tur. 0], 5.0 mg; (30 fr. DW-4-6-3-3 [Tur. 0], 3.5 mg; (4) fr. DW-4-6-3-4, 120.4 mg [baicalin (2)]; (5) fr. DW-4-6-3-5, 166.0 mg [baicalin (2)]; (6) fr. DW-4-6-3-6, 86.4 mg [oroxyloside (4)]; (7) fr. DW-4-6-3-7 [Tur. 0], 12.6 mg.
Fr. DW-5 (1.02 g) [Tur. 41.0] was suspended in H2O (200 mL) and its supernatant was applied to a CHP-20P column (ϕ 4.5 × 17 cm) with H2O–MeOH to obtain six fractions: (1) fr. DW-5-1 [Tur. 1.09], H2O elute, 32.5 mg; (2) fr. DW-5-2 [Tur. 6.45], H2O elute, 10.4 mg; (3) fr. DW-5-3 [Tur. 17.1], 20% MeOH elute, 11.5 mg; (4) fr. DW-5-4, 468 mg [baicalin (2)]; (5) fr. DW-5-5 [Tur. 12.2], 466 mg; (6) fr. DW-5-6 [Tur. 0], 8.6 mg.
Fr. DW-5-5 (466 mg) was suspended in H2O (10 mL), chromatographed on a column of MCI gel CHP-20 P (ϕ 4 × 24 cm) and eluted with CH3CN/MeOH/H2O → MeOH to obtain nine fractions: (1) fr. DW-5-5-1, 4.3 mg; (2) fr. DW-5-5-2, 1.7 mg; (3) fr. DW-5-5-3, 1.2 mg; (4) fr. DW-5-5-4, 4.3 mg; (5) fr. DW-5-5-5, 7.2 mg; (6) fr. DW-5-5-6, 1.9 mg; (7) fr. DW-5-5-7, 107.3 mg; (8) fr. DW-5-5-8, 159.5 mg; (9) fr. DW-5-5-9; 30.6 mg. Fr. DW-5-5-8 (159.5 mg) was applied to a PTLC ODS RP-18 developed with 50% MeOH to give wogonoside (3) (102 mg).
Baicalin (2) [37]
The identity was confirmed by direct comparison with a commercially available sample.
Wogonoside (3) [37]
Yellow powder. 1H-NMR (600 MHz, DMSO-d6, J = Hz): 8.10 (2H, d, J = 6.5, H-2′/-6′), 7.62 (3H, m, H-3′/-4′/-5′), 7.07 (1H, s, H-3), 6.69 (1H, s, H-6), 5.08 (1H, d, J = 7.1 Hz, H-1″), 3.91 (3H, s, OCH3), 3.5−3.0 (H-2″ ~ H-5″, overlapped). 13C-NMR (150 MHz, DMSO-d6, J = Hz): 182.9 (C-4), 172.0 (COOH), 164.1 (C-2), 157.2 (C-7), 156.5 (C-5), 149.6 (C-9), 132.8 (C-4′), 131.3 (C-1′), 129.8 (C-3′/-5′), 129.7 (C-8), 127.0 (C-2′/-6′), 105.8 (C-3), 105.7 (C-10), 101.0 (C-1″), 99.6 (C-6), 77.2 (C-5″), 74.4 (C-3″), 73.7 (C-2″), 72.5 (C-4″), 61.9 (OCH3).
Oroxyloside (4) [38]
Yellow powder. 1H-NMR (600 MHz, DMSO-d6, J = Hz): 8.07 (2H, d, J = 7.1, H-2′/-6′), 7.60 (3H, m, H-3′/-4′/-5′), 7.07 (1H, s, H-8), 7.02 (1H, s, H-3), 5.35 (1H, d, J = 6.0 Hz, H-1″), 3.78 (3H, s, OCH3), 3.5–3.0 (H-2″–H-5″, overlapped). 13C-NMR (150 MHz, DMSO-d6, J = Hz): 182.5 (C-4), 170.1 (COOH), 163.8 (C-2), 156.3 (C-7), 152.8 (C-5), 152.3 (C-9), 132.7 (C-6), 132.2 (C-4′), 130.6 (C-1′), 129.2 (C-3′/-5′), 126.4 (C-2′/-6′), 106.1 (C-10), 105.0 (C-3), 99.7 (C-1″), 94.3 (C-8), 76.1 (C-3″), 75.6 (C-5″), 73.0 (C-2″), 71.4 (C-4″), 60.6 (OCH3).
References
The Ministry of Health, Labour and Welfare of Japan (2016) The Japanese pharmacopoeia. 17th edn. The Ministry of Health, Labour and Welfare of Japan, Tokyo, pp 1752–1754
Cao YP, Gao C, Sun JH, Wang JZ, Zhou Q, Liu GQ (1996) Study on pharmacological effects of Huanglianjiedu Tang extract. J China Pharm Univ 27:605–608
Yoon SB, Lee YJ, Park SK, Kim HC, Bae H, Kim HM, Ko SG, Choi HY, Oh MS, Park W (2009) Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW264.7 macrophages. J Ethnopharmacol 125:286–290
Chen JJ, Huang CC, Chan HY, Li PY, Liang YC, Deng JS, Huang SS, Huang GJ (2017) Scutellaria baicalensis ameliorates acute lung injury by suppressing inflammation in vitro and in vivo. Am J Chin Med 45:137–157
Li J, Liu Y, Chen X, Ding X, Wu S, Xie W (2006) Anti-endotoxin effects of baicalin extracted from Scutellariae Radix. Yiyao Daobao 25:1237–1240
Chi YS, Cheon BS, Kim HP (2001) Effect of wogonin, a plant flavone from Scutellariae Radix, on the suppression of cyclooxygenase-2 and the induction of inducible nitric oxide synthase in lipopolysaccharide-treated RAW264.7 cells. Biochem Pharmacol 61:1195–1203
Chi YS, Kim HP (2005) Suppression of cyclooxygenase-2 expression of skin fibroblasts by wogonin, a plant flavone from Scutellariae Radix. Prostaglandins Leukot Essent Fatty Acids 72:59–66
Lin WH, Kuo HH, Ho LH, Tseng ML, Siao AC, Hung CT, Jeng KC, Hou CW (2015) Gardenia jasminoides extracts and gallic acid inhibit lipopolysaccharide-induced inflammation by suppression of JNK2/1 signaling pathways in BV-2 cells. Iran J Basic Med Sci 18:555–562
Mao YF, Li YQ, Zong L, You XM, Lin FQ, Jiang L (2010) Methanol extract of Phellodendri Cortex alleviates lipopolysaccharide-induced acute airway inflammation in mice. Immunopharmacol Immunotoxicol 32:110–115
Park YK, Chung YS, Kim YS, Kwon OY, Joh TH (2007) Inhibition of gene expression and production of iNOS and TNF-α in LPS-stimulated microglia by methanol extract of Phellondendri cortex. Int Immunopharmacol 7:955–962
Iizuka N, Miyamoto K, Hazama S, Yoshimura K, Okita K, Fukumoto T, Yamamoto S, Tangoku A, Oka M (2000) Anticachectic effects of Coptidis Rhizoma, an anti-inflammatory herb, on esophageal cancer cells that produce interleukin 6. Cancer Lett 158:35–41
Lee CH, Chen JC, Hsiang CY, Wu SL, Wu HC, Ho TY (2007) Berberine suppresses inflammatory agents-induced interleukin-1β and tumor necrosis factor-α productions via the inhibition of IκB-α degradation in human lung cells. Pharmacol Res 56:193–201
Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Asao S, Fujiwara H (1999) Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol 66:227–233
Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan T, Xu S, Peng J, Xie X, Huang H (2011) Berberine attenuates lipopolysaccharide-induced extracellular matrix accumulation and inflammation in rats masangial cells: involvement of NF-κB signaling pathway. Mol Cell Endocrinol 331:34–40
Kim HY, Koh EJ, Park J, Lee SM (2010) Gardenia jasminoides prevents galactosamine-induced acute hepatitis in rats. Yakhak Hoechi 54:403–409
Pan T, Shi X, Chen H, Chen R, Wu D, Lin Z, Zhang J, Pan J (2017) Geniposide suppresses interleukin-1β-induced inflammation and apoptosis in rat chondrocytes via the PI3K/Akt/NF-κB signaling pathway. Inflammation. https://doi.org/10.1007/s10753-017-0694-2
Xu GL, Li G, Ma HP, Zhong H, Liu F, Ao GZ (2009) Preventive effect of crocin in inflamed animals and in LPS-challenged RAW264.7 cell. J Agric Food Chem 57:8325–8330
Yang K, Min Z, Shi Y, Xiang L, Meng Y, Wu C, Huang W, Tang B (2009) Effects of total iridoid glycosides from Fructus Gardeniae on inflammatory reaction and neuronal apoptosis in rats with intracerebral hemorrhage. Zhongyao Xinyao Yu Linchuang Yaoli 20:8–10
Lim H, Park KR, Lee DU, Kim YS, Kim HP (2008) Effects of the constituents of Gardenia Fructus on prostaglandin and NO production. Biomol Therapeut 16:82–86
Koo HJ, Lim KH, Jung HJ, Park EH (2006) Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol 103:496–500
Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM, Kim SJ, Kim BC, Jun C, Lim CJ, Park EH (2004) Antiinflammatory effects of genipin, an active principle of gardenia. J Pharmacol 495:201–208
Zeng H, Dou S, Zhao J, Fan S, Yuan X, Zhu S, Li L, Zhong W, Liu R (2011) The inhibitory activities of the components of Huang-Lian-Jie-Du-Tang (HLJDT) on eicosanoid generation via lipoxygenase pathway. J Ethnopharmacol 135:561–568
Lu J, Wang JS, Kong LY (2011) Anti-inflammatory effects of Huang-Lian-Jie-Du decoction, its two fractions and four typical components. J Ethnopharmacol 134:911–918
Oshima N, Narukawa Y, Hada N, Kiuchi F (2013) Quantitative analysis of anti-inflammatory activity of orengedokuto: importance of combination of flavonoids in inhibition of PGE2 production in mouse macrophage-like cell line J774.1. J Nat Med 67:281–288
Kim KW, Ha KT, Park CS, Jin UH, Chang HW, Lee IS, Kim CH (2007) Polygonum cuspidatum, compared with baicalin and berberine, inhibits inducible nitric oxide synthase and cyclooxygenase-2 gene expressions in RAW-264.7 macrophage. Vascul Pharmacol 47:99–107
Fujii T, Okuyama T, Wakame K, Okumura T, Ikeya Y, Nishizawa M (2017) Identification of anti-inflammatory constituents in Phellodendri Cortex and Coptidis Rhizoma by monitoring the suppression of nitric oxide production. J Nat Med 71:745–756
Zhou K, Hu L, Liao W, Yin D, Rui F (2016) Coptisine prevent IL-β-induced expression of inflammatory mediators in chondrocytes. Inflammation 39:1558–1564
Wu J, Zhang H, Hu B, Yang L, Wang P, Wang F, Meng X (2016) Coptisine from Coptis chinensis inhibits production of inflammatory mediators in lipopolysaccharide-induced RAW 264.7 murine macrophage cells. Eur J Pharmacol 780:106–114
Wang JR, Tanaka T, Zhang H, Kouno I, Jiang ZH (2012) Formation and conformation of baicalin–berberine and wogonoside–berberine complexes. Chem Pharm Bull 60:706–711
Yi L, Xu X (2004) Study on the precipitation reaction between baicalin and berberine by HPLC. J Chromatogr B 810:165–168
Huang L, Fuchino H, Kawahara N, Narukawa Y, Hada N, Kiuchi F (2016) Application of a new method, orthogonal projection to latent structure (OPLS) combined with principal component analysis (PCA), to screening of prostaglandin E2 production inhibitory flavonoids in Scutellaria Root. J Nat Med 70:731–739
Shimizu T, Shibuya N, Narukawa Y, Oshima N, Hada N, Kiuchi F (2018) Synergistic effect of baicalein, wogonin and oroxylin A mixture: multistep inhibition of the NF-κB signalling pathway contributes to an anti-inflammatory effect of Scutellaria Root flavonoids. J Nat Med 72:181–191
The Ministry of Health, Labour and Welfare of Japan (2016) The Japanese pharmacopoeia. 17th edn. The Ministry of Health, Labour and Welfare of Japan, Tokyo, p 1747
Dirsch VM, Stuppner H, Vollmar AM (1998) The griess assay: suitable for a bio-guided fractionation of anti-inflammatory plants extract. Planta Med 64:423–426
Park KH, Park M, Choi SE, Jeong MS, Kwon JH, Oh MH, Choi HK, Seo SJ, Lee MW (2009) The Anti-oxidative and anti-inflammatory effects of caffeoyl derivatives from the roots of Aconitum Koreanum R. RAYMOND. Biol Pharm Bull 32:2029–2033
Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, Scudiero DA, Monks A, Boyd MR (1990) Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst 82:1113–1118
Wu S, Sun A, Liu R (2005) Separation and purification of baicalin and wogonoside from the Chinese medicinal plant Scutellaria baicalensis Geroge by high-speed counter-current chromatography. J Chromatogr A 1066:243–247
Abe K, Inoue O, Yumioka E (1990) Biliary excretion of metabolites of baicalin and baicalein in rats. Chem Pharm Bull 38:208–211
Acknowledgements
This work was supported in part by MEXT-Supported Program for the Strategic Research Foundation at Private Universities from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oshima, N., Shimizu, T., Narukawa, Y. et al. Quantitative analysis of the anti-inflammatory activity of orengedokuto II: berberine is responsible for the inhibition of NO production. J Nat Med 72, 706–714 (2018). https://doi.org/10.1007/s11418-018-1209-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1209-7