Abstract
Our previous work showed that geniposide affected glucose-stimulated insulin secretion (GSIS) via regulating glucose uptake and metabolism in pancreatic β cells; however, the molecular mechanisms remain largely unknown. Substantial evidence suggests that activation of 5′-AMP-activated protein kinase (AMPK) plays a central role in GSIS. Here, we aim to determine the role of AMPK on geniposide-regulated GSIS in rat pancreatic INS-1 cells. The results demonstrated that 6-[4-(2-piperidin-1-yletoxy)-phenyl]-3-pyridin-4-yl-pyrazolo[1,5-α] pyrimidine (Compound C; an AMPK inhibitor) significantly attenuated the effects of geniposide on glucose uptake, energy metabolism, and insulin secretion in INS-1 cells. We also observed that geniposide induced phosphorylation of acetyl-CoA carboxylase (ACC), a marker of AMPK activity, in a time-dependent manner in INS-1 cells; however, in the presence of Compound C, the influence of geniposide on ACC phosphorylation was obviously inhibited. Furthermore, the knockdown of AMPK protein with AMPK siRNA treatment decreased the effects of geniposide on glucose uptake, adenosine triphosphate production, and GSIS. All these data indicate that AMPK plays an essential role in geniposide-regulated GSIS in pancreatic β cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An impressive amount of evidence shows that type 2 diabetes (T2D) has reached epidemic proportions worldwide, and the International Diabetes Federation predicts that the number of cases of T2D will increase to 600 million by 2035 [1]. Although there are several medications currently available to treat T2D, there is a great need for more safe and efficacious treatment [2, 3].
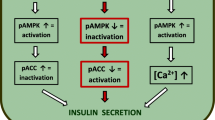
The metabolic disturbances in T2D include diminished insulin-stimulated glucose uptake by skeletal muscle and fat, increased hepatic glucose production, and impaired insulin secretion by pancreatic β cells [4]. Among those, glucose-stimulated insulin secretion (GSIS) from pancreatic β cells is one of the key players in regulating cellular adaptation demands to nutritional and metabolic variations [5]. Mounting evidence indicates that GSIS is regulated by the rate of glucose metabolism within β cells [6]. Unfortunately, it has been known for many decades that insulin response to a rise in blood glucose is markedly diminished in T2D [7, 8]. Therefore, improvement of GSIS in pancreatic β cells has been used as an efficient strategy to treat T2D [5, 9].
Geniposide, isolated from Gardenia jasminoides Ellis, which belongs to the iridoid family, has a wide range of therapeutic activities, including anti-oxidative and anti-inflammatory effects and treatment of hepatic disorders and diabetes [10–14]. We previously reported that geniposide was a novel agonist for glucagon-like peptide 1 receptor (GLP-1R), which showed neurophic and neuroprotective effects on neurons [15–18]. Additionally, in pancreatic β cells, we observed that with the activation of GLP-1R, geniposide could regulate GSIS, which enhanced GSIS in the presence of low and moderately high concentrations of glucose in pancreatic β INS-1 cells, but exerted an acute inhibitory effect on GSIS in the presence of high concentrations of glucose (>25 mM) [19–21]. Our further research confirmed that the effect of geniposide on GSIS was involved in its role on the dynamic equilibrium of energy metabolism through regulating the expression of pyruvate carboxylase, a key enzyme for the metabolism of glucose. Furthermore, geniposide significantly affected the uptake and metabolism of glucose in the presence of different concentrations of glucose in pancreatic INS-1 cells [20]. Recently, we observed that geniposide induced phosphorylation of 5′-AMP-activated protein kinase (AMPK) and expression of NAD-dependent deacetylase sirtuin-1 (SIRT1) in the presence of low concentrations of glucose; however, geniposide played a contrary role in the presence of high concentrations of glucose [22]. The molecular mechanisms need to be clarified.
Mounting evidence suggests that AMPK plays an essential role in GSIS in pancreatic β cells in acute treatment settings (hours) and chronic treatment settings (days), and pharmacological activation of AMPK has proven to be of benefit in the treatment of T2D [23–25]. In this study, we aim to investigate the role of AMPK on geniposide-regulated GSIS. The results indicated that both 5-aminoimidazole-4-carboxamide1-β-D-ribofuranoside (AICAR; an AMPK activator) and Compound C (an AMPK inhibitor) could affect GSIS and phosphorylation of acetyl-CoA carboxylase (ACC), a marker of AMPK activity. Furthermore, using RNA interference, we also investigated the role of AMPK on geniposide-regulated GSIS in pancreatic β INS-1 cells.
Materials and methods
Reagent
AICAR (an AMPK activator) and Compound C (an AMPK inhibitor) were obtained from Sigma (St. Louis, MO, USA), and AMPK siRNA (sc-270142) for AMPKα1 and specific antibodies for ACC phosphorylation were obtained from Santa Cruz Biotechnology Inc. (Texas, CA, USA). ACC1 antibody was purchased from Proteintech Group Inc. (Rosemont, IL, USA), and horseradish peroxidase-labeled GAPDH antibody was bought from KangChen Bio-Tech Inc. (Shanghai, China). Geniposide was bought from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Cell culture
The rat INS-1 pancreatic β cell line, purchased from the China Center for Type Culture Collection, was cultured at 37 °C in a humidified atmosphere containing 5 % CO2. The culture medium was RPMI medium 1640 containing 11 mM glucose and supplemented with 10 % FBS, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate and 50 μM mercaptoethanol. The culture medium was replaced every second day, and the cells were passaged once a week following trypsinization.
Insulin secretion assay
Detection of insulin content was performed as previously described [19, 20]. Briefly, INS-1 cells were seeded onto 6-well plates and cultured for 24 h. The cells were then washed twice with Krebs–Ringer bicarbonate buffer (KRBB; 129 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 5 mM NaHCO3, 0.1 % BSA, 10 mM HEPES, (pH 7.4) and then starved for 2 h in KRBB. The cells were incubated for 60 min in fresh KRBB containing geniposide and indicated concentrations of glucose in the presence or absence of AICAR and Compound C. The supernatants were collected to measure insulin content using a rat/mouse insulin ELISA kit according to the manufacturer’s instructions (Linco Research, Inc., St Charles, MO, USA).
Glucose uptake and metabolism
To determine the effect of AICAR and Compound C on glucose uptake and metabolism, INS-1 cells were seeded onto 6-well plates. After overnight incubation, the cells were washed once with KRBB and starved for 2 h in fresh KRBB. The buffer was then replaced with KRBB containing indicated concentrations of glucose, geniposide and/or AICAR and Compound C. After 60 min incubation, the buffer was collected for measurement of glucose concentration, which was used to calculate glucose uptake as reported previously [20]. The cell lysates were used to determine adenosine triphosphate (ATP) content. Glucose concentration in the buffer was measured using a glucose assay kit according to the manufacturer’s instructions (Bioversion, Mountain View, CA, USA). The content of ATP in cell lysates was measured using ATP bioluminescence assay kits according to the manufacturer’s instructions (Roche, Mannheim, Germany).
Small interfering RNA (siRNA) on AMPK
The experimental procedure of RNA interference on AMPK was carried out as previously described [11]. Generally, before transient transfection, INS-1 cells were placed into 6-well plates and incubation was continued overnight. The cells were transfected with AMPK siRNA via Lipofectamine 2000 Transfection Reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA), and continued to culture for 24 h. The interfering efficiency was checked by Western blot.
Western blot analysis
Cell lysates (10–20 μg) were resolved by 10 % SDS-PAGE and transferred to polyvinylidene difluoride membranes. After blocking, the membranes were probed with anti-phospho-ACC and anti-ACC1 antibodies (1:2000–3000) followed by incubation with anti-horseradish-conjugated GAPDH antibody (1:8000–10,000). Immunoreactive proteins were detected by chemiluminescence using ECL reagent (Amersham Pharmacia, Piscataway, NJ, USA), and immunoblot signals were analyzed with Quanty One software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are shown as mean ± SD from three independent experiments. Analysis of variance was carried out using OriginLab software. For comparison between groups, Student’s t test was performed. A one-way ANOVA followed by Tukey’s or Dunnett’s tests was used to compare all groups or selected groups to control. p < 0.05 was considered statistically significant.
Results
Effects of Compound C on geniposide-regulated GSIS
Our previous work suggested that, consistent with the effect on GSIS, geniposide played a controversial role on the phosphorylated level of AMPK in the presence of low (5 mM) and high (25 mM) concentrations of glucose in rat pancreatic INS-1 cells [22], suggesting that AMPK might be involved in the effect of geniposide on GSIS. In this study, we determined the influence of AICAR (an AMPK activator) and Compound C (an AMPK inhibitor) on GSIS regulated by geniposide in the presence of low (5 mM) and high (25 mM) glucose in INS-1 cells. As shown in Fig. 1, in the presence of low (5 mM) concentrations of glucose, geniposide obviously increased GSIS, and AICAR potentiated the effect of geniposide on GSIS by increasing insulin secretion (p < 0.01); pre-incubation with Compound C significantly attenuated the role of geniposide on GSIS (p < 0.01). However, in the presence of high glucose concentrations (25 mM), geniposide remarkably decreased GSIS (p < 0.01). Moreover, although AICAR could not potentiate further the effect of geniposide on GSIS, pre-incubation with Compound C significantly prevented the role of geniposide on GSIS (p < 0.05) in high (25 mM) glucose-incubated INS-1 cells.
The role of AICAR and Compound C on geniposide-regulated GSIS. After INS-1 cells were replaced into 6-well plates, and incubation continued overnight, the cells was washed once with PBS and starved for 2 h in KRBB. The medium was then replaced with fresh KRBB, and 10 µM geniposide (Gen), 20 µM Compound C or 1.0 mM AICAR was added, and incubation continued for 60 min. The supernatant was collected to determine the insulin content using commercial ELISA kits according to the manufacturer’s instructions. Data are expressed as mean ± SD (n = 3, two wells for each replicate)
Compound C regulated glucose uptake and metabolism in geniposide-treated INS-1 cells
To investigate the role of AMPK on glucose uptake and metabolism, we measured the effects of Compound C on glucose uptake and ATP production in geniposide-treated INS-1 cells. The results indicated that geniposide accelerated the uptake of glucose in the presence of low glucose concentrations (5 mM), but prevented it in the presence of high glucose concentrations (25 mM). Compound C (an AMPK inhibitor) significantly decreased the effect of geniposide on glucose uptake in INS-1 cells (p < 0.05) (Fig. 2a). In addition, Compound C apparently suppressed the role of geniposide on glucose metabolism by decreasing the ATP content in geniposide-treated INS-1 cells (p < 0.01) (Fig. 2b).
Compound C regulated glucose uptake (a) and metabolism (b) in geniposide-treated INS-1 cells. After INS-1 cells were replaced into 6-well plates, and incubation continued overnight, the cells were washed once with PBS and starved for 2 h in KRBB. Then, 10 µM geniposide (Gen) and/or 20 µM Compound C was added, and incubation continued for 60 min. The supernatant was collected to determine the glucose concentrations using a glucose assay kit according to manufacturer’s instructions. The cell lysates were used to determine ATP content using ATP bioluminescence assay kits according to the manufacturer’s instructions. Data are expressed as mean ± SD (n = 6)
Pre-treatment with AMPK siRNA abolished the effects of geniposide on glucose uptake, ATP production, and GSIS
To further identify the role of AMPK on geniposide-regulated GSIS, we used RNA interference to knockdown AMPK expression in INS-1 cells (the interfered efficiency of siRNA is shown in Fig. 3a), and then determine the influence of geniposide on GSIS, glucose uptake and ATP production. The results demonstrated that in the presence of low (5 mM) glucose, pre-treatment with AMPK siRNA significantly attenuated the potentiating effects of geniposide on GSIS (p < 0.05) (Fig. 3b), glucose uptake (statistical difference not significant) (Fig. 3c) and ATP production (statistical difference not significant) (Fig. 3d). In the presence of high (25 mM) glucose, pre-treatment with AMPK siRNA obviously decreased the inhibiting effects of geniposide on GSIS (p < 0.05) (Fig. 3b), glucose uptake (p < 0.05) (Fig. 3c) and ATP production (p < 0.01) (Fig. 3d) in INS-1 cells.
AMPK siRNA prevented the effects of geniposide on GSIS (b), glucose uptake (c) and ATP production (d) in the presence of low (5 mM) and high (25 mM) glucose in INS-1 cells. a After INS-1cells were treated with 0, 20, 30 or 50 nM AMPK siRNA for 24 h, the interfering efficiency was determined by Western blot. To explore the influence of AMPK siRNA on geniposide-regulated GSIS, glucose uptake and metabolism, the cells were pre-treated with 30 nM AMPK siRNA for 24 h, and were then washed once with PBS and starved for 2 h in KRBB. The medium was replaced with fresh KRBB, and 10 µM geniposide (Gen) and indicated concentrations of glucose were added, and incubation continued for 60 min. The supernatant was collected to determine the insulin and glucose concentrations using commercial kits according to manufacturer’s instructions. The cell lysates were used to determine ATP content using ATP bioluminescence assay kits according to the manufacturer’s instructions. Data are expressed as mean ± SD (n = 6)
Geniposide regulated ACC phosphorylation
Substantial evidence suggests that ACC phosphorylation is a marker of AMPK activity [26]. To provide more evidence for the role of AMPK on geniposide-regulated GSIS, we determined the influence of geniposide on the expression and phosphorylation of ACC protein with Western blot assay. The results showed that in the presence of low glucose (5 mM), geniposide induced ACC phosphorylation in a time-dependent manner, and reached the maximum after 60 min of incubation (Fig. 4a). However, in the presence of high glucose (25 mM), geniposide rapidly increased the ACC phosphorylation level, and reached the maximum after 15 min of incubation, but quickly returned to normal (Fig. 4b). Furthermore, at this time point, we determined the effect of Compound C on ACC phosphorylation; the results suggested that ACC phosphorylation induced by geniposide could be prohibited by Compound C (p < 0.01) (Fig. 4c).
Effects of Compound C on ACC phosphorylation induced by geniposide. After the cells were treated with 10 µM geniposide for indicated times in the presence of 5 mM (a) or 25 mM (b) glucose, the phosphorylated level of ACC was measured with Western blot. c The cells were treated with 20 µM Compound C for 30 min, and then incubated with 10 µM geniposide in the presence of 25 mM glucose for 15 min. The cell lysates were used to detect ACC phosphorylation. Data are shown as mean ± SD from three independent experiments. d The cells were incubated with indicated concentrations of glucose for 15 min and the phosphate level of ACC was detected by Western blot. Data are expressed as mean ± SD from three independent experiments
To further observe the phosphorylated state of ACC during geniposide-regulated GSIS, we checked the phosphorylated level of ACC after 60-min incubation in the presence of different concentrations of glucose. The data demonstrated that geniposide apparently enhanced ACC phosphorylation in the presence of low (5 mM) and moderate (11 mM) concentrations of glucose; however, after 60-min incubation, geniposide showed no significant effect on ACC phosphorylation in the presence of high glucose (25 mM) in INS-1 cells (Fig. 4d).
Treatment with AMPK siRNA attenuated the effect of geniposide on ACC phosphorylation
To clarify the role of AMPK on geniposide-regulated ACC phosphorylation, we determined the phosphorylated level of ACC in AMPK siRNA-treated INS-1 cells. The results showed that in the presence of 25 mM glucose, treatment with 10 µM geniposide for 15 min significantly increased ACC phosphorylation (p < 0.01); however, in AMPK siRNA-treated INS-1 cells, the effect of geniposide on ACC phosphorylation was obviously cancelled (p < 0.05) (Fig. 5).
AMPK siRNA attenuated the effect of geniposide on ACC phosphorylation. After the cells were treated with 30 nM AMPK siRNA or scrambled siRNA for 24 h, the cells were treated with 10 µM geniposide for 15 min in the presence of 25 mM glucose. The phosphorylated level of ACC was measured with Western blot. Data are shown as mean ± SD from three independent experiments
Discussion
Circumstantial evidence accumulated over many decades by several research groups suggested that AMPK played an essential role in the function of pancreatic β cells, including GSIS [23, 27]. To clarify the relationship between geniposide-regulated GSIS and AMPK activation, we determined the influence of geniposide on ACC phosphorylation, a marker of AMPK. As shown in Fig. 4, geniposide induced ACC phosphorylation in the presence of low glucose (5 mM) in INS-1 cells. This was similar to the role of geniposide on AMPK phosphorylation [22], and GSIS [19, 20], suggesting that activation of AMPK by geniposide in low glucose conditions is beneficial to GSIS. However, in the presence of high glucose (25 mM), inhibition of AMPK with Compound C decreased geniposide-regulated GSIS and ACC phosphorylation in INS-1 cells. This might be beneficial to lower excessive insulin secretion and contribute to improvement of glucose homeostasis in vivo or deleterious because of insufficient insulin secretion.
To further clarify the role of AMPK on geniposide-regulated GSIS, we used RNA interference to directly knockdown AMPK expression. In AMPK siRNA-treated INS-1 cells, the effects of geniposide on glucose uptake, metabolism, and GSIS were significantly abolished, and ACC phosphorylation was distinctly decreased. All these data indicate that geniposide-regulated GSIS is associated with AMPK activity.
Although the role of AMPK in insulin secretion of β cells has been widely studied, the results concerning the relationship between AMPK and GSIS are still controversial [27]. Many studies from different research groups show that AMPK negatively regulates β-cell insulin secretion in various β-cell lines and in primary rat islets [28–30]. This theory was supported by the fact that activation of AMPK by AICAR and thiazolidinediones decreased insulin secretion [27, 31]. In contrast, there is some evidence showing that activation of AMPK by AICAR failed to suppress GSIS in a study involving three different β-cell model systems including primary islets [32]. The cause for these discrepancies is unknown, but the effect of AMPK on GSIS may be influenced by glucose concentrations in the medium-, short- or long-term activation of AMPK, cell culture conditions, etc. [27, 33, 34].
AMPK is now widely considered as a therapeutic target for the treatment of T2D and metabolic disorders based on its effects on whole-body metabolism [23, 24, 35]. There is some evidence indicating that activation of AMPK has beneficial effects on glucose and lipid metabolism and, conversely, AMPK knockout inhibits gluconeogenesis in hepatocytes [23, 35, 36]. The data shown in this study revealed that geniposide-regulated AMPK phosphorylation in order to influence the uptake and metabolism of glucose would be beneficial in T2D patients to preserve β-cell function and glucose homeostasis.
References
Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414:782–787
Paneni F, Costantino S (2015) Mechanisms-based therapeutic strategies in type 2 diabetes. Cardiovasc Diagn Ther 5:340–342
Stumvoll M, Goldstein BJ, van Haeften TW (2005) Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365:1333–1346
DeFronzo RA (1997) Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med 50:191–197
Woerle HJ, Carneiro L, Derani A, Goke B, Schirra J (2012) The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes 61:2349–2358
Rutter GA, Pullen TJ, Hodson DJ, Martinez-Sanchez A (2015) Pancreatic beta-cell identity, glucose sensing and the control of insulin secretion. Biochem J 466:203–218
Gerdes JM, Christou-Savina S, Xiong Y, Moede T, Moruzzi N, Karlsson-Edlund P, Leibiger B, Leibiger IB, Ostenson CG, Beales PL, Berggren PO (2014) Ciliary dysfunction impairs beta-cell insulin secretion and promotes development of type 2 diabetes in rodents. Nat Commun 5:5308
Zou CY, Gong Y, Liang J (2016) Metabolic signaling of insulin secretion by pancreatic beta-cell and its derangement in type 2 diabetes. Eur Rev Med Pharmacol Sci 20:391
Ilkova H, Glaser B, Tunckale A, Bagriacik N, Cerasi E (1997) Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care 20:1353–1356
Chen JY, Wu H, Li H, Hu SL, Dai MM, Chen J (2015) Anti-inflammatory effects and pharmacokinetics study of geniposide on rats with adjuvant arthritis. Int Immunopharmacol 24:102–109
Guo L, Zheng X, Liu J, Yin Z (2016) Geniposide suppresses hepatic glucose production via AMPK in HepG2 cells. Biol Pharm Bull 39:484–491
Liu HT, He JL, Li WM, Yang Z, Wang YX, Yin J, Du YG, Yu C (2010) Geniposide inhibits interleukin-6 and interleukin-8 production in lipopolysaccharide-induced human umbilical vein endothelial cells by blocking p38 and ERK1/2 signaling pathways. Inflamm Res 59:451–461
Wu SY, Wang GF, Liu ZQ, Rao JJ, Lu L, Xu W, Wu SG, Zhang JJ (2009) Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol Sin 30:202–208
Kojima K, Shimada T, Nagareda Y, Watanabe M, Ishizaki J, Sai Y, Miyamoto K, Aburada M (2011) Preventive effect of geniposide on metabolic disease status in spontaneously obese type 2 diabetic mice and free fatty acid-treated HepG2 cells. Biol Pharm Bull 34:1613–1618
Liu J, Yin F, Zheng X, Jing J, Hu Y (2007) Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem Int 51:361–369
Liu J, Zheng X, Yin F, Hu Y, Guo L, Deng X, Chen G, Jiajia J, Zhang H (2006) Neurotrophic property of geniposide for inducing the neuronal differentiation of PC12 cells. Int J Dev Neurosci 24:419–424
Liu JH, Yin F, Guo LX, Deng XH, Hu YH (2009) Neuroprotection of geniposide against hydrogen peroxide induced PC12 cells injury: involvement of PI3 kinase signal pathway. Acta Pharmacol Sin 30:159–165
Zhang Y, Yin F, Liu J, Liu Z, Guo L, Xia Z, Zidichouski J (2015) Geniposide attenuates insulin-deficiency-induced acceleration of beta-amyloidosis in an APP/PS1 transgenic model of Alzheimer’s disease. Neurochem Int 89:7–16
Guo LX, Xia ZN, Gao X, Yin F, Liu JH (2012) Glucagon-like peptide 1 receptor plays a critical role in geniposide-regulated insulin secretion in INS-1 cells. Acta Pharmacol Sin 33:237–241
Liu J, Guo L, Yin F, Zhang Y, Liu Z, Wang Y (2013) Geniposide regulates glucose-stimulated insulin secretion possibly through controlling glucose metabolism in INS-1 cells. PLoS One 8:e78315
Guo LX, Liu JH, Yin F (2014) Regulation of insulin secretion by geniposide: possible involvement of phosphatidylinositol 3-phosphate kinase. Eur Rev Med Pharmacol Sci 18:1287–1294
Guo L, Liu CY, Yin F, Liu JH (2016) Possible role of AMPK/SIRT signaling on energy balance in geniposide-treated INS-1 cells. Med Chem 6:33–38
Coughlan KA, Valentine RJ, Ruderman NB, Saha AK (2014) AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 7:241–253
Viollet B, Lantier L, Devin-Leclerc J, Hebrard S, Amouyal C, Mounier R, Foretz M, Andreelli F (2009) Targeting the AMPK pathway for the treatment of Type 2 diabetes. Front Biosci (Landmark Ed) 14:3380–3400
Zhang BB, Zhou G, Li C (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9:407–416
Peterson JM, Aja S, Wei Z, Wong GW (2012) CTRP1 protein enhances fatty acid oxidation via AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) inhibition. J Biol Chem 287:1576–1587
Fu A, Eberhard CE, Screaton RA (2013) Role of AMPK in pancreatic beta cell function. Mol Cell Endocrinol 366:127–134
Salt IP, Johnson G, Ashcroft SJ, Hardie DG (1998) AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J 335(Pt 3):533–539
da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA (2003) Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J 371:761–774
Leclerc I, Rutter GA (2004) AMP-activated protein kinase: a new beta-cell glucose sensor?: regulation by amino acids and calcium ions. Diabetes 53(Suppl 3):S67–S74
Mantovani J, Roy R (2011) Re-evaluating the general(ized) roles of AMPK in cellular metabolism. FEBS Lett 585:967–972
Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ (2007) The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem 282:10341–10351
Dufer M, Noack K, Krippeit-Drews P, Drews G (2010) Activation of the AMP-activated protein kinase enhances glucose-stimulated insulin secretion in mouse beta-cells. Islets 2:156–163
Winder WW, Hardie DG (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277:E1–E10
Alves CR, Ferreira JC, de Siqueira-Filho MA, Carvalho CR, Lancha AH Jr, Gualano B (2012) Creatine-induced glucose uptake in type 2 diabetes: a role for AMPK-alpha? Amino Acids 43:1803–1807
Hutchinson DS, Summers RJ, Bengtsson T (2008) Regulation of AMP-activated protein kinase activity by G-protein coupled receptors: potential utility in treatment of diabetes and heart disease. Pharmacol Ther 119:291–310
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (81373459), Chongqing Science and Technology Committee (CSTC, 2015jcyjbx0064), Chongqing Science Found for Distinguished Young Scholars (2014jcyjjq10003), the Innovation of Science and Technology Leading Talent in Chongqing (2014kjcxljrc0018), and the Innovative Research Team Development Program at the University of Chongqing (CXTDX201601031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Hao, Y., Liu, C., Yin, F. et al. 5′-AMP-activated protein kinase plays an essential role in geniposide-regulated glucose-stimulated insulin secretion in rat pancreatic INS-1 β cells. J Nat Med 71, 123–130 (2017). https://doi.org/10.1007/s11418-016-1038-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-1038-5