Abstract
Purpose
To analyze the role of geniposide in the protein degradation of Txnip and to determine the impact of Txnip on geniposide-regulated GSIS in pancreatic INS-1 cells.
Methods
The content of Txnip protein was measured by western blot; insulin content and glucose uptake were determined by ELISA; and knockdown of Txnip was the method of RNA interference.
Results
Glucose induces a rapid increase in Txnip protein, and geniposide accelerates the degradation of Txnip via proteasome pathway in the presence of high glucose (25 mM) in INS-1 pancreatic β-cells. And MG132, a proteasomal inhibitor, potentiates glucose uptake, metabolism (ATP production) and glucose-stimulated insulin secretion (GSIS) in high-glucose (25 mM)-treated INS-1 cells, but geniposide significantly prevents these effects. Furthermore, the combination of geniposide and Txnip knockdown shows substantial synergistic effects to reduce glucose uptake, metabolism and GSIS in high-glucose (25 mM)-treated INS-1 cells.
Conclusions
Txnip protein played an essential role in glucose uptake, metabolism and GSIS, and geniposide could accelerate the degradation via proteasome pathway in high-glucose-treated pancreatic INS-1 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a growing public health issue, which was characterized by peripheral insulin resistance and progressive impairment of insulin secretion in pancreatic β-cells [1]. Usually, glucose-stimulated insulin secretion (GSIS) in pancreatic β-cells is beautifully regulated such that the precise amount of insulin is delivered to the bloodstream to regulate whole-body glucose metabolism [2, 3]. On the other hand, when insulin secretion from pancreatic β-cells can no longer keep up with the demand, blood glucose concentrations rise and diabetes ensues [4]. Elevated glucose levels have detrimental effects on various tissues including the pancreatic β-cells, which lead to dysfunction of β-cells, impairment of insulin gene expression, and even irreversible β-cell loss by apoptosis. Proposed mechanisms to explain the glucotoxicity on pancreatic β-cells include (but are not limited to) the generation of reoxidative stress, the production of inflammatory factors, and the induction of endoplasmic reticulum stress [5, 6]. All of these damaging pathways are relative to the dysfunction of pancreatic β-cells. However, the exact molecular mechanisms by which glucotoxicity lead to the dysfunction and failure of β-cells are still not fully understood, and there are no efficient strategies to cope with it.
It has been proposed that thioredoxin-interacting protein (Txnip), as a highly glucose-regulated proapoptotic factor, played a critical role on the dysfunction and apoptosis induced by high concentration of glucose in pancreatic β-cells [7, 8]. An impressive number of evidence indicated that Txnip participated in regulation of the redox state of a cell by interacting and inhibiting thioredoxin, which had been identified as one of the most dramatically inducible genes at glucose effects on isolated human pancreatic islets [9,10,11]. In addition, glucose-stimulated Txnip expression, which does not require glucose metabolism or new protein synthesis, is regulated by a carbohydrate response element that consists of two E-box elements in the human Txnip promoter [12]. Furthermore, it has been reported that exendin-4, a long-term effective glucagon-like peptide 1 (GLP-1) receptor agonist, 8-bromo-cAMP, and the cAMP promoting agent forskolin attenuated the effect of high glucose on the protein level of Txnip via stimulating proteasome-dependent Txnip degradation in INS-1 cells, and protected INS-1 cells from high glucose-induced apoptosis [13]. All these studies suggest that regulation of proteasome-dependent Txnip degradation may be a novel strategy to prevent the glucotoxicity in pancreatic β-cells.
Our previous work showed that geniposide, isolated from the fruit of Gardenia jasminoides Ellis, was a novel agonist for GLP-1 receptor, which regulated GSIS with the activation of GLP-1R in INS-1 cells [14, 15], and induced the release cAMP in PC12 cells [16]. But the molecular mechanisms of geniposide-regulated GSIS and the effects of geniposide on the influence of Txnip protein level remain largely unknown. At present, we therefore aim to analyze the role of geniposide in the protein degradation of Txnip and determine the impact of Txnip on geniposide-regulated GSIS in pancreatic INS-1 cells.
Materials and methods
Reagent
MG132, leupeptin, and cycloheximide (CHX) were obtained from Sigma (St. Louis, MO, USA), Txnip siRNA was obtained from Santa Cruz Biotechnology Inc. (Texas, CA, USA). Specific antibodies for Txnip were bought from Cell Signaling Technology (Danvers, MA, USA) and horseradish peroxidase (HRP)-labeled GAPDH antibody was bought from KangChen Bio-Tech Inc. (Shanghai, China).
Cell culture
Rat INS-1 pancreatic β-cell line, purchased from the China Center for Type Culture Collection, was cultured at 37 °C in a humidified atmosphere containing 5% CO2. The culture medium was RPMI medium 1640 containing 11 mM glucose and supplemented with 10% FBS, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate, and 50 μM mercaptoethanol. The culture medium was replaced every second day, and cells were passaged once a week following trypsinization.
Insulin secretion assay
Insulin content was measured as described before [14, 15]. Briefly, INS-1 cells were seeded onto 12-well plates and cultured for 24 h. Then, the cells were washed two times with Krebs-Ringer bicarbonate buffer (KRBB, 129 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 5 mM NaHCO3, 0.1% BSA, 10 mM HEPES, pH 7.4 and starved for 2 h in KRBB. The cells were incubated for 1 h in fresh KRBB containing 10 μM geniposide with or without 10 μM MG132 in the presence of 25 mM glucose. The supernatants were collected to measure insulin concentration using rat/mouse insulin ELISA kits (Linco Research, Inc., St Charles, MO, USA) according to the kit’s instructions.
Glucose uptake and metabolism
To determine the effect of geniposide and/or MG132 on the uptake and metabolism of glucose, INS-1 cells were placed into 6-well plates. After overnight incubation, the cells were washed two times with KRBB and starved for 2 h in fresh KRBB in the presence or absence of 10 μM geniposide. The buffer was then replaced with KRBB containing 25 mM glucose with or without 10 μM MG132. After 60 min of incubation, the buffer was collected to determine the content of glucose using a glucose assay kit according to the protocol supplied by the manufacturer (Biovison, Mountain, CA, USA), which was used to calculate glucose uptake as reported before. The cell lysates were used to determine the content of ATP using ATP bioluminescence assay kits according to the instruction from the supplier (Roche, Mannheim, Germany).
Small interfering RNA (siRNA) on Txnip
The experimental procedure of RNA interference on Txnip was performed as described before [17]. Generally, before transient transfection, INS-1 cells were placed into 6-well plates and continued to incubate overnight. The cells were transfected with Txnip siRNA via Lipofectamine 2000 Transfection Reagent according to the suggestions from the supplier (Invitrogen, Carlsbad, CA, USA) and continued to culture for 24 h. Interfering efficiency was checked by Western blot.
Preparation of cell lysates
After the cells were washed three times with ice-cold PBS, whole cell extracts were obtained by using modified RIPA buffer (Beyotime, Shanghai, China) in the presence of 1% protease/phosphatase inhibitor cocktail (Calbiochem, La Jolla, CA, USA). Protein concentrations were determined by a BCA protein assay kit (Beyotime, Shanghai, China), and all of the protein extracts were stored at −70 °C.
Western blot analysis
10–20 μg of whole cell lysates was separated with 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking, membranes were probed with an anti-Txnip (1:2000–3000, Cell signaling Technology, MA, USA) followed by incubation with anti-horseradish-conjugated secondary antibodies. Excess antibody was washed off with 20 mM Tris-buffered saline containing Tween 20 (TBST, 20 mM Tris, 150 mM NaCl and 0.1% Tween 20, pH 7.5). Immunoreactive proteins were detected by chemiluminescence using the ECL reagent (Amersham Pharmacia, Piscataway, NJ, USA), and immunoblot signals were analyzed by densitometry scanning with the software of Quantity One (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are shown as mean ± SD from three independent experiments. Analysis of variance was carried out using the software of Origin Lab. A one-way ANOVA followed by Tukey’s or Dunnett’s tests was used to compare all groups or selected group to control. And p < 0.05 was regarded as statistically significant.
Results
Geniposide reduces the protein level of Txnip in high glucose-treated INS-1 cells
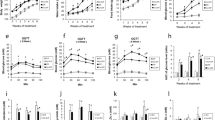
As shown in Fig. 1a, treatment with high glucose (25 mM) induced a rapid increase of Txnip protein in a time-dependent manner. Compared to the control, incubation with 25 mM glucose for 24 h increased the protein level of Txnip about threefold in INS-1 cells. To determine the effect of geniposide on Txnip protein, rat pancreatic INS-1 cells were addressed with indicated time points in the presence of high concentration of glucose (25 mM). The results showed that geniposide induced a significant decrease of Txnip protein (Fig. 1b), and treatment with 10 µM geniposide for 8 h could attenuate the protein level of Txnip about 70% (p < 0.01) in the presence of 25 mM glucose in INS-1 cells.
Geniposide reduces Txnip level induced by high glucose in INS-1 cells. a After INS-1 cells were incubated with 25 mM glucose for 0, 1, 2, 4, 8, and 24 h, the whole cell lysates were used to determine the protein level of Txnip by Western blot. Data are shown as mean ± SD from at least three independent experiments. *p < 0.05, **p < 0.01 versus control (treating time was 0 min). b After INS-1 cells were incubated with 10 µM geniposide for 0, 2, 4, 8, 12, and 24 h in the presence of 25 mM glucose, the whole cell lysates were used to determine the protein level of Txnip by Western blot. Data are shown as mean ± SD from at least three independent experiments. **p < 0.01 versus control (treating time was 0 min)
Possible pathways of geniposide-regulating Txnip degradation
It has been noted that activation of GLP-1 receptor could accelerate the degradation of Txnip protein through proteasome-dependent pathway [13], and our previous works demonstrated that geniposide could induce the release of cAMP with the activation of GLP-1 receptor [16]. To explore the mechanisms of geniposide attenuating the protein level of Txnip and probe the associated pathways, we detected the influence of MG132 (a proteasomal inhibitor) and leupeptin (a lysosomal inhibitor) on geniposide-regulating degradation of Txnip protein, data shown that pre-incubation with 10 µM MG132 could distinctively prevent geniposide-inducing degradation of Txnip protein (p < 0.01), but leupeptin had no significant role on the degradation of Txnip-induced geniposide in the presence of 25 mM glucose in INS-1 cells (Fig. 2a).
The possible pathway of geniposide-inducing Txnip degradation. a After the cells were pre-incubated with 10 µM MG132 or 1 µg/mL leupeptin for 30 min, 10 µM geniposide was added and continued to incubate for 8 h, and whole cell lysates were used to determine Txnip content by Western blot. Data are shown as mean ± SD from at least three independent experiments.*p < 0.05, **p < 0.01 versus control; # p < 0.05 versus the group of geniposide alone; &p < 0.01 versus the group of MG132 alone. b After INS-1 cells were incubated with 300 µM CHX with or without 10 µM geniposide for 4 h, whole cell lysates were used to determine Txnip content by Western blot. Data are shown as mean ± SD from at least three independent experiments.**p < 0.01 versus control; ##p < 0.05 versus the group of CHX alone
To make sure that the effect of geniposide on Txnip protein was through the pathway of proteasome-dependent degradation, we use cycloheximide (CHX) to inhibit the protein synthesis of Txnip and then determine the influence of geniposide on the protein level of Txnip in 25 mM glucose-treated INS-1 cells. As shown in Fig. 2b, CHX, a general protein synthesis inhibitor, effectively decreased Txnip protein expression content (p < 0.01), and in the presence of CHX, geniposide also substantially reduced the protein level of Txnip in high glucose (25 mM)-treated INS-1 cells (p < 0.05). All these data suggested that geniposide reducing protein level of Txnip induced by high glucose was highly associated with the degradation through the proteasome-dependent pathway in INS-1 cells.
Effects of MG132 on glucose uptake, metabolism, and GSIS
Our previous works showed that geniposide attenuated the release of insulin in the presence of elevated glucose [14, 18]. To explore the role of Txnip on geniposide-regulating GSIS, we determined the influence of MG132 on GSIS in high glucose (25 mM)-treated INS-1 cells, and the results indicated that geniposide significantly decreased the release of insulin (p < 0.05) and MG132 obviously increased the content of insulin in media (p < 0.01) in the presence of 25 mM glucose. Moreover, incubation with 10 µM MG132 noticeably prevented the inhibition of geniposide on GSIS in INS-1 cells (p < 0.01; Fig. 3a).
Effects of MG132 on geniposide-regulating glucose uptake, metabolism, and GSIS. a After INS-1 cells were replaced into 6-well plates and continued to incubate overnight, the cells were washed two times with KRBB and starved for 2 h in KRBB. After that, the medium was changed with fresh KRBB, and 10 µM geniposide (Gen) and/or 10 µM MG132 were added and continued to incubate for 1 h, and the supernatant was collected to determine the content of insulin using commercial ELISA kits according to the instructions from supplier. Data are expressed as mean ± SD (n = 3, two well for each replicate). *p < 0.05, ** p < 0.01 versus control, # p < 0.01 versus the group of geniposide alone. b The supernatant from same treatment was used to determine the concentrations of glucose using a glucose assay kit according to protocol supplied by the manufacturer. Data are expressed as mean ± SD (n = 3, two wells for each replicate). *p < 0.05, &p < 0.05 versus control, #p < 0.05 versus the group of geniposide alone. c The whole cell lysates from the same treatment were used to determine the content of ATP using ATP bioluminescence assay kits according to the manufacturer’s instructions. Data are expressed as mean ± SD (n = 6). **p < 0.01 versus control, # p < 0.05 versus the group of geniposide alone
To further illuminate the effects of MG132 on geniposide-regulating GSIS, we determined the influence of MG132 on glucose uptake and metabolism, and the results demonstrated that MG132 prominently attenuated the role of geniposide on the uptake and metabolism of glucose in high glucose-treated INS-1 cells (Fig. 3b, c).
Effects of Txnip siRNA on geniposide-regulating glucose uptake, metabolism, and GSIS
To further clarify the role of Txnip on geniposide-regulating GSIS, we used siRNA to knock down the expression of Txnip and determine the effects of geniposide on GSIS, glucose uptake, and metabolism. To knock down the expression of Txnip protein, INS-1 cells were incubated with 0, 10, 30, and 50 nM Txnip siRNA for 24 h. Data indicated that treatment with 30 nM Txnip siRNA for 24 h decreased the protein level of Txnip over 70% (Fig. 4a).
Txnip siRNA prevents the effects of geniposide on GSIS (B), glucose uptake (C), and ATP production (D) in the presence of high (25 mM) glucose in INS-1 cells. a After INS-1 cells were treated with 0, 10, 30, or 50 nM Txnip siRNA for 24 h, the interfering efficiency was determined by Western blot. Data are shown as mean ± SD from at least three independent experiments. *p < 0.05, **p < 0.01 versus control. To explore the influence of Txnip siRNA (si txnip) on geniposide-regulating GSIS, glucose uptake, and metabolism, the cells were pre-treated with 30 nM Txnip siRNA for 24 h, and then, the cells were washed two times with KRBB and starved for 2 h in KRBB. After that, the medium was changed with fresh KRBB, and 10 µM geniposide (Gen) and indicated concentrations of glucose were added and continued to incubate for 1 h, and the supernatant was collected to determine the concentrations of insulin and glucose using the commercial kits according to protocol supplied by the manufacturer. And the cell lysates were used to determine the content of ATP using ATP bioluminescence assay kits according to the manufacturer’s instructions. Data are expressed as mean ± SD (n = 6). *p < 0.05, **p < 0.01 versus control, # p < 0.05 versus the group of geniposide alone
We continued to determine the influence of Txnip knockdown on glucose uptake, metabolism, and GSIS in geniposide-treated INS-1 cells. The results demonstrated that in the presence of high glucose (25 mM), both Txnip siRNA and geniposide significantly attenuated the release of insulin (p < 0.05; Fig. 4b), glucose uptake (p < 0.05; Fig. 4c), and ATP production (p < 0.05; Fig. 4d). Furthermore, the combination of geniposide and Txnip siRNA treatment showed substantial synergistic effects on GSIS (Fig. 4b), glucose uptake (Fig. 4c), and glucose metabolism (Fig. 4d).
Discussion
In pancreatic β-cells, chronic exposure to high glucose is expected to increase the production of reactive oxygen species (ROS), such as superoxide anion (O2 −) and H2O2, through the pathways of oxidative phosphorylation, glucose auto-oxidation, the Schiff reaction during glycation, and hexosamine metabolism [19]. For many years, ROS has been exclusive thought of as the unfortunate by products of respiratory energy production in mitochondria and believed to be deleterious to biological systems [20, 21]. Thioredoxin (Trx) is emerging as an important antioxidant in the β-cell defense against oxidative stress, which interacts with thioredoxin reductase and thioredoxin peroxidase to reduce oxidized proteins and scavenge free radicals. However, the role of Trx in pancreatic β-cells was inhibited by thioredoxin-interacting protein (Txnip), also known as vitamin D3 upregulated protein-1 (VDUP-1) and thioredoxin binding protein (TBP-2), by binding to its redox active cysteine residue [22]. An impressive number of evidence suggested that Txnip was robustly induced by glucose in islets and β-cell lines, and thereby could modulate the cellular redox homeostasis and promote oxidative stress [12]. In addition, overexpression of Txnip has been shown to render fibroblasts and cardiomyocytes more susceptible to apoptosis [23, 24]. Strikingly, islets derived from Txnip-deficient mice are fully protected from glucose-induced β-cell apoptosis [25]. In the present study, we observed that geniposide significantly decreased the level of Txnip protein in high glucose-treated INS-1 cells. We further identified that geniposide decreasing the protein level of Txnip induced by high concentration of glucose was involved in its role on the proteasome degradation, but not through the lysosomal pathway, because pre-incubation with MG132, a proteasomal inhibitor, prevented the effect of geniposide on the level of Txnip protein, but leupeptin, an inhibitor of lysosomal pathway, had no significant effect on that.
Metabolically, Txnip knockout animals exhibit phenotypes of familial combined hyperlipidemia that are consistent with enhanced glucose uptake [26, 27]. Given that glucose availability affects reactive oxygen species production in mitochondria, the diverse effects of Txnip on thioredoxin functions and on glucose uptake suggest a unifying mechanism for maintaining homeostasis. Our previous works indicated that, together with attenuating GSIS, geniposide also affected the uptake and metabolism of glucose, and the dynamic equilibrium of energy metabolism through regulating the expression of pyruvate carboxylase, a key enzyme in the metabolism of glucose in the presence of high concentration of glucose in pancreatic INS-1 cells [15]. Here, we confirmed that, accompanied with its inhibition on glucose uptake, metabolism (ATP production), and GSIS, geniposide also accelerated the degradation of Txnip via the proteasome pathway. MG132, a proteasomal inhibitor, reversed these effects induced by geniposide in the presence of high concentration of glucose (25 mM) in INS-1 cells, and geniposide could reverse these effects obviously, suggesting that geniposide-inducing proteasome degradation of Txnip might be helpful to maintain glucose homeostasis and decrease glucotoxicity in pancreatic β-cells.
On the other hand, independent of its thioredoxin-binding property, overexpression of Txnip repressed cellular glucose uptake, whereas Txnip knockdown increases glucose uptake in peripheral tissues in both an insulin-dependent and an insulin-independent manner [28]. Here, we showed that Txnip knockdown decreased glucose uptake, ATP production, and GSIS in the presence of high glucose (25 mM) in rat pancreatic INS-1 cells, and geniposide significantly potentiated these effects, hinted that Txnip was highly correlated with GSIS. An impressive amount of studies have revealed that a causative role of Txnip in apoptotic cell death of pancreatic β-cell provides a mechanistic link between glucotoxicity and pancreatic apoptosis [7, 29, 30]. Furthermore, Txnip also plays a role in glucose and lipid metabolism in insulin sensitive tissue [26, 28]. These, along with the role of Txnip on insulin secretion and peripheral insulin sensitivity, make it an important pharmaceutical target to delay the progression of T2DM.
As discussed above, Txnip protein played an essential role on glucose uptake, metabolism, and GSIS, and geniposide could accelerate the degradation via proteasome pathway in high glucose-treated pancreatic INS-1 cells. Although the mechanisms and importance about those need to be further clarified, for the potential role of Txnip in redox-related cellular functions and pathophysiological processes, geniposide might be a promising compound to halt pancreatic β-cell apoptosis induced by hyperglycemia and the development of T2DM and its complications.
References
Stumvoll M, Goldstein BJ, van Haeften TW (2005) Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365:1333–1346
Yang H, Jin X, Kei Lam CW et al (2011) Oxidative stress and diabetes mellitus. Clin Chem Lab Med 49:1773–1782
Tiwari BK, Pandey KB, Abidi AB et al (2013) Markers of oxidative stress during diabetes mellitus. J Biomark 2013:378790
Leibowitz G, Kaiser N, Cerasi E (2011) beta-cell failure in type 2 diabetes. J Diabetes Investig 2:82–91
Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29:42–61
Maedler K, Donath MY (2004) Beta-cells in type 2 diabetes: a loss of function and mass. Horm Res 62(Suppl 3):67–73
Shalev A (2014) Minireview: thioredoxin-interacting protein: regulation and function in the pancreatic beta-cell. Mol Endocrinol 28:1211–1220
Yoshihara E, Masaki S, Matsuo Y et al (2014) Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol 4:514
Li W, Wu Z, Ma Q et al (2014) Hyperglycemia regulates TXNIP/TRX/ROS axis via p38 MAPK and ERK pathways in pancreatic cancer. Curr Cancer Drug Targets 14:348–356
Muoio DM (2007) TXNIP links redox circuitry to glucose control. Cell Metab 5:412–414
Rani S, Mehta JP, Barron N et al (2010) Decreasing Txnip mRNA and protein levels in pancreatic MIN6 cells reduces reactive oxygen species and restores glucose regulated insulin secretion. Cell Physiol Biochem 25:667–674
Minn AH, Hafele C, Shalev A (2005) Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology 146:2397–2405
Shao W, Yu Z, Fantus IG et al (2010) Cyclic AMP signaling stimulates proteasome degradation of thioredoxin interacting protein (TxNIP) in pancreatic beta-cells. Cell Signal 22:1240–1246
Guo LX, Xia ZN, Gao X et al (2012) Glucagon-like peptide 1 receptor plays a critical role in geniposide-regulated insulin secretion in INS-1 cells. Acta Pharmacol Sin 33:237–241
Liu J, Guo L, Yin F et al (2013) Geniposide regulates glucose-stimulated insulin secretion possibly through controlling glucose metabolism in INS-1 cells. PLoS ONE 8:e78315
Liu J, Yin F, Zheng X et al (2007) Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem Int 51:361–369
Guo L, Zheng X, Liu J et al (2016) Geniposide suppresses hepatic glucose production via AMPK in HepG2 Cells. Biol Pharm Bull 39:484–491
Luo Y, He F, Hu L et al (2014) Transcription factor Ets1 regulates expression of thioredoxin-interacting protein and inhibits insulin secretion in pancreatic beta-cells. PLoS ONE 9:e99049
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Afanas’ev I (2010) Signaling of reactive oxygen and nitrogen species in diabetes mellitus. Oxid Med Cell Longev 3:361–373
Buetler TM, Krauskopf A, Ruegg UT (2004) Role of superoxide as a signaling molecule. News Physiol Sci 19:120–123
Saxena G, Chen J, Shalev A (2010) Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem 285:3997–4005
Junn E, Han SH, Im JY et al (2000) Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol 164:6287–6295
Wang Y, De Keulenaer GW, Lee RT (2002) Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem 277:26496–26500
Chen J, Saxena G, Mungrue IN et al (2008) Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes 57:938–944
Chutkow WA, Birkenfeld AL, Brown JD et al (2010) Deletion of the alpha-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes 59:1424–1434
Sheth SS, Castellani LW, Chari S et al (2005) Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res 46:123–134
Parikh H, Carlsson E, Chutkow WA et al (2007) TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 4:e158
Chen J, Couto FM, Minn AH et al (2006) Exenatide inhibits beta-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem Biophys Res Commun 346:1067–1074
Chen J, Hui ST, Couto FM et al (2008) Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J 22:3581–3594
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81373459), Chongqing Science and Technology Committee (CSTC, 2015jcyjbx0064), Chongqing Science Found for Distinguished Young Scholars (2014jcyjjq10003), the Innovation of Science and Technology Leading Talent in Chongqing (2014kjcxljrc0018), and the Innovative Research Team Development Program at the University of Chongqing (CXTDX201601031).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies were in accordance with the ethical standards of the institutional research committee and the Chongqing Science and Technology Committee.
Informed consent
No informed consent.
Rights and permissions
About this article
Cite this article
Liu, C.Y., Hao, Y.N., Yin, F. et al. Geniposide accelerates proteasome degradation of Txnip to inhibit insulin secretion in pancreatic β-cells. J Endocrinol Invest 40, 505–512 (2017). https://doi.org/10.1007/s40618-016-0591-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-016-0591-9