Abstract
Purpose
Glufosinate-ammonium (GLA) is commonly used in tropical agriculture. The frequency and dosage of GLA applications far exceeded the recommended amounts, causing a substantial portion to leach into the soil, becoming a pseudo-persistent pollutant. The earthworm (Eisenia fetida) is a critical bio-indicator of soil health. The effects of pesticides on earthworms are significant to analyzing pesticides’ harm to the soil. Therefore, it is urgent to study the toxicity of GLA to earthworms.
Materials and methods
The artificial soil method was adopted to test the toxicity of GLA on earthworms from the aspects of acute toxicity, growth, reproductive toxicity, the activity of antioxidant and detoxification enzymes, and DNA damage.
Results
GLA exhibited low toxicity (LC50 > 2000 mg/kg) to earthworms in 7-day and 14-day soil tests. But at the individual level, exposure to GLA could decrease the earthworms’ weight, cocoons, and larvae, even under low-concentration stress. The effect of GLA on the individual level of earthworm may be due to the impact on the microscopic level, so the enzyme activity and DNA damage were determined. At the protein level, exposure to GLA could change the activity of superoxide dismutase (SOD), catalase (CAT), glutathione s-transferase (GST), guaiacol peroxidase (POD), and acetylcholine esterase (AChE) of earthworms. Furthermore, at the molecular level, the comet assay suggests that the olive tail moment (OTM) increased dramatically under the GLA treatments.
Conclusions
The results will be helpful for further understanding the negative influence of GLA on the earthworm and provide a foundation for the rational use of GLA and eco-environmental risks of soil pesticide contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pesticides are used worldwide to control diseases, pests, and weeds. Only a fraction of pesticides work on the targets, and a large part leaches into the soil. As a result, soil contamination is once again in the spotlight, especially herbicide contamination (Jiang et al. 2016). Glufosinate-ammonium (GLA) represents an active ingredient of herbicide products and can control many weed species, including glyphosate-resistant weeds, in fruit orchards, citrus groves, vineyards, potato fields, and non-cultivated land (Masiol et al. 2018; Lajmanovich et al. 2021). GLA belongs to the organic phosphorus herbicide family, and its mechanism of action is through the inhibition of glutamine synthetase. Plants rapidly absorb GLA through leaves that inhibit the activity of glutamine synthase and lead to the accumulation of nitrogen in plant cells. Thus, the normal and orderly reaction of the optical system is inhibited. The chloroplast structure disintegrates, and eventually, the plant dies (Ferramosca et al. 2020). Additionally, GLA is particularly soluble in water and has a half-life of 3–42 days (Carpenter and Boutin 2010). Still, the frequency and dosage of GLA applications far exceeded the recommended amounts in tropical agriculture, causing a substantial portion to leach into the soil, leading to its accumulation, and becoming a pseudo-persistent pollutant in the soil. Many studies reported that GLA had adverse influences on biota and humans (Ferramosca et al. 2020; Lajmanovich et al. 2021). However, pesticide registrations are evaluated only for acute toxicity to earthworms; the chronic toxicity of GLA is not involved (Calas et al. 2008).
The earthworm (Eisenia fetida) is a vital bio-indicator of soil health (Alves et al. 2017; Shao et al. 2018). E. fetida, inhabiting the top soil (10–20 cm), makes a crucial part of soil fertility and structure and is a highly regarded tool for a second green revolution (Shi et al. 2017). Numerous studies revealed that clarifying the influence of pesticides on earthworm behavior is significant to evaluate eco-environmental risks of soil pesticides contamination (Song et al. 2009; Li et al. 2017). It is vital to research the negative influence of herbicides on earthworms (Song et al. 2009).
Some studies have shown that most toxic compounds could cause organisms to produce and accumulate reactive oxygen species (ROS) (Yang et al. 2018). Moreover, short-term exposure to high levels of ROS may cause harmful effects, such as the oxidation of protein and genotoxicity (DNA damage) (Sauer et al. 2001). Excessive ROS also can induce the organism to produce an oxidative stress response (Banerjee et al. 2001). Earthworms have oxidative defense mechanisms, for instance, antioxidases, containing SOD, POD, CAT, and GST, among which GST can also participate in detoxification metabolism. The change of acetylcholine esterase (AChE) activity of the earthworm is one of the biological responses of pesticide exposure (Zhou et al. 2020). Many experiments have used alkaline single-cell gel electrophoresis (SCGE) to study the genotoxicity of pesticides to earthworms (Song et al. 2009; Yang et al. 2018).
Therefore, in order to better understand the biochemical responses on earthworms of exposure to GLA, it is urgent to study the genotoxicity and oxidative stress of GLA on earthworms. In our current test, the artificial soil method was adopted to test the acute toxicity, growth, and reproductive toxicity of GLA to earthworms. In addition, we measured the effects of different concentrations of GLA on SOD, CAT, POD, GST, and AChE activities in earthworms at different exposure times. The comet assay tested DNA damage in earthworms. This study will contribute to further understanding the negative influence of GLA on the earthworm and provide a foundation for the rational use of GLA and eco-environmental risks of soil pesticide contamination.
2 Materials and methods
2.1 Materials preparation
GLA (97%) were purchased from Fluorochem (UK). Earthworms were taken from an earthworm breeding base in Tianjin and raised in sterilized cow manure at room temperature. Before the beginning of the tests, earthworms were acclimated in the artificial soil for 2 weeks, then on wet filter paper for 24 h in a phytotron with 14-h light:10-h dark, 20 ± 1℃ and 80 ± 2% relative humidity.
2.2 Toxicological tests
An artificial soil test measured the acute toxicity of GLA to earthworms (OECD 1984). In acute toxicity tests, six concentrations of GLA (Fluorochem Co.) were diluted by deionized water and added into artificial soil. The final concentrations in soil were 0, 62.5, 125, 250, 500, 1000, and 2000 mg/kg. Before the test, earthworms were cleaned and put on a moist filter paper overnight to clear the intestine (300–500 mg, with obvious reproductive ring). For each treatment, the moisture content maintained approximately 60% of deionized water in artificial soil during the experiment, repeated 3 times, and 30 earthworms were detected. Finally, these treatments were maintained in a phytotron with 14-h light:10-h dark, 20 ± 1℃, 80 ± 2% relative humidity. Seven and 14 days later, the death rates of earthworms were counted.
2.3 Growth and reproduction toxicity test
E. fetida was exposed to the 0, 0.4, 2, 10, 50, and 250 mg/kg GLA (according to the acute toxicity and field survey results) for 56 days. Each treatment was repeated 3 times, and 30 earthworms were detected. After 14, 21, 28, 42, and 56 days of incubation, the weight of earthworms and the number of cocoons and larvae were counted.
2.4 Enzyme assays
The effects of GLA on SOD, CAT, POD, GST, and AChE were studied by the OECD artificial soil method (OECD 2004). Based on the field survey result and the result of the acute toxicity test, the GLA doses were 0, 0.4, 2, 10, 50, and 250 mg/kg of artificial soil. Two earthworms were gathered from each repetition for enzymatic activity and comet assays after exposure to GLA for 3, 7, 14, and 21 days. Each concentration had three repetitions and 10 earthworms in each repetition. There were no earthworms that died during tests.
The extraction of enzyme solution was as follows: earthworms were washed with deionized water, put into a pre-cooled mortar, and pestled under liquid nitrogen, then dissolved in 0.9% NaCl (pH 7.0 1:9, w/v). Following centrifugal separation at 3500 g for 10 min under 4℃, the collected supernatants were for protein determination and the enzymatic activity assay. SOD, CAT, POD, GST, and AChE activities were respectively tested by Superoxide Dismutase assay kit (Jiancheng, Nanjing, China, A001-3–2), Catalase assay kit (Jiancheng, Nanjing, China, A007-1–1), Peroxidase assay kit (Jiancheng, Nanjing, China, A084-1–1), Glutathione S-transferase assay kit (Jiancheng, Nanjing, China, A004-1–1), and Acetylcholinesterase assay kit (Jiancheng, Nanjing, China, A024-1–1), following the product specifications.
2.5 DNA damage of earthworm coelomocytes
DNA damage was measured by SCGE (Singh et al. 1998). After exposing the earthworms to GLA for 3 days, 7 days, 14 days, and 21 days, the coelomocytes were extracted from earthworms using non-invasive extraction (Eyambe et al. 1991). In general, the earthworms were washed by 0.9% NaCl (pH 7.0, 1:9, w/v), put into the extraction solution. The coelomocytes were naturally secreted in the solution, followed by centrifugal separation at 10,000 g at 4℃ for 10 min. Then, the sediment dissolved with 1 ml PBS (pH 7.4), and centrifuged at 10,000 g for 30 min under 4℃. The final cells were re-dissolved in PBS, after, and then, the SCGE was conducted.
The SCGE was carried out based on Singh et al. (1998) method, with a few minor changes. Briefly, with the compound of cell suspensions and 1.0% low melting agarose at 37℃ (1:3, v/v), the slides, which were pre-coated with 50 μl 0.8% normal melting agarose, were utterly coated slides flat. The agarose was solidified at 4℃ away from light. Then, the slides were dipped entirely in the lysis solution for 30–60 min, and cleaned them 3 times with deionized water. Later, the slides were dipped in the newly alkaline solution (pH > 13.0) for 20 min at 25℃ under dark conditions. Separated by electrophoresis and the volt was set to 25 V for 30 min. After finished, the slides were cleaned 3 times with deionized water and then wholly dipped in 0.4 M Tris–HCl (pH 7.5) for 15 min. The fluid was blotted up with filter paper; absolute ethanol was added for 60 min at 25℃. After the agarose was air-dried, slides were stained with 200 μl GelStain (1:10,000, 30 min) (Transgen, Beijing, China) in the dark. Finally, one hundred random non-overlapping cells on each slide were imaged with a fluorescence microscope (Nikon Ni/U fluorescence microscope) and used Comet Assay Software Project to analyze the pictures of cells (Końca et al. 2003). Then, the degree of DNA damage was characterized by the OTM (Dhawan et al. 2009).
2.6 Statistical analysis
All data are shown as the means of 3 replicated treatments, and a standard deviation (SD) was evaluated. The significance of the differences between the control groups and the treatment groups was calculated by one-way ANOVA (LSD-test) using SPSS v.20.0 (SPSS Inc., Chicago, IL, USA). The GraphPad Prism 7.0 was applied to determine the linear correlation between DNA damage and GLA concentration, DNA damage, and the exposure time.
3 Results
3.1 The acute toxicity of GLA to the earthworm
After exposing the earthworms to GLA for 7 days and 14 days, no earthworms died in the control or the treatment groups during tests. That is, the LC50 of GLA to the earthworm was greater than 2000 mg/kg (7 days, 14 days), indicating that the acute toxicity of GLA was low to earthworm.
3.2 Effects on earthworm growth and reproduction
The earthworms in all treatments survived after 56 days of exposure to GLA. Figure 1 shows the weight of earthworms after exposure to GLA of 14, 28, 42, and 56 days. The average weight of earthworms treated by GLA decreased gradually and prolonged exposure time. The effect was observed at all test concentrations when compared to untreated soil. Exposed to GLA for 28 days, the earthworms’ weight began to decrease significantly. After 56 days of exposure to GLA, the weight loss percentage was about 28.39%, 28.59%, 32.49%, 35.91%, and 38.04%, respectively (p < 0.001). In the control group, an insignificant effect on growth was determined (p = 0.964). The decrease in earthworms’ weight showed that GLA restrained earthworm growth.
The weight of earthworms as exposed to different concentrations and exposure time of GLA. Each data is the average of 3 replications. Error bars stand for standard deviation (SD). Different capital letters are significantly different among different exposure times under the same concentration of GLA at p < 0.05 level by LSD test. Different small letters are significantly different among different concentrations of GLA at the same exposure time at p < 0.05 level by LSD test
Table 1 shows the number of cocoons and larvae produced by earthworms exposed to different doses of GLA after 56 days. Compared to the controls, in all the GLA treatment groups, the number of cocoons and larvae had dropped significantly after 56 days (p < 0.001). The reduction in earthworms’ cocoons and larvae may be indicated that exposure to GLA at sublethal concentrations has an unhealthy effect on the reproductive system of earthworms.
3.3 Effects of GLA on antioxidant and detoxifying enzymes activities
The impact of GLA on SOD activity in earthworms could be seen in Fig. 2A. In comparison with the contrast group, the SOD activity remarkably declined by 25.83% (p = 0.026) when earthworms were exposed to 2 mg/kg GLA for 7 days. After exposure to 0.4 and 10 mg/kg GLA for 14 days, the SOD activity increased by 46.66% and 55.61% (p < 0.001), respectively. The SOD activity of the GLA treatment groups did not differ from the control on the 3rd (p = 0.782) and 21st days (p = 0.079).
Effects of GLA on the activities of SOD (A), CAT (B), POD (C), GST (D), and AChE (E) in earthworms. Data are the average of 3 replications. Error bars stand for min to max. Colors (red, orange, black, green, purple, and blue) illustrate the concentration of GLA (0, 0.4, 2, 10, 50, and 250 mg/kg), and * and ** represent significant differences at P < 0.05 or P < 0.01 level, respectively, compared to the blank
It could be seen from Fig. 2B that the CAT activities at 10 and 250 mg/kg GLA were stimulated early but were inhibited after 21 days. In comparison with the contrast, the CAT activity at 10 mg/kg was remarkably increased after 3 days (p = 0.009) and remarkably lower after 21 days (p = 0.004). On the 3rd and 7th days, at 250 mg/kg, the CAT activity remarkably increased by 59.14% and 38.35% (p < 0.001), respectively, and was considerably lower after 21 days. For exposure at 14 days, the CAT activity at 0.4 mg/kg GLA remarkably increased by 51.40% (p < 0.001). Additionally, it was shown on the 21st day, at 2, 10, and 250 mg/kg; GLA remarkably dropped compared with the contrasts (p = 0.004).
It could be seen from Fig. 2C that POD activity significantly decreased on the 7th day after exposure to GLA at 2 mg/kg (p < 0.001). In contrast to the contrast, the POD activity at 250 mg/kg was remarkably higher after 7 days (p = 0.040) and particularly lower after 14 days (p = 0.010). After exposure to 0.4, 10, and 50 mg/kg GLA for 14 days, POD activity remarkably increased by 53.55% (p < 0.001), 67.63% (p < 0.001), and 36.61% (p = 0.003), severally. It was also shown that on the 3rd (p = 0.059) and 21st (p = 0.051) days, an insignificant difference in POD activity between the GLA treatment groups and the contrast.
In Fig. 2D, in contrast with the controls, the GST activity at 2, 10, 50, and 250 mg/kg was remarkably higher after 3 days and remarkably lower after 7 days (p < 0.001). In contrast to the contrast, the GST activity at 2, 10, 50, and 250 mg/kg was noticeably higher after 3 days with increases of 32.22%, 26.07%, 31.51%, and 47.56% (p < 0.001), respectively. On the 7th day, at 2, 10, 50, and 250 mg/kg, the GST activity significantly decreased by 34.12% (p < 0.001), 29.82% (p < 0.001), 15.21% (p = 0.001), and 12.89% (p = 0.014), severally. After exposure for 14 days, in all the GLA treatment groups, there was a remarkable drop of GST activity of 14.63%, 44.31%, 15.58%, 41.85%, and 17.49%, respectively (p < 0.001). In addition, on the 21st day, compared to the controls, all the treated groups had a significant decrease of 29.09% (p < 0.001), 21.03% (p < 0.001), 38.09% (p < 0.001), 32.72% (p < 0.001), and 14.84% (p = 0.002), severally.
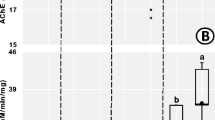
It could be seen from Fig. 2E that the AChE activity at 250 mg/kg had remarkably increased by 23.46% after 3 days (p = 0.006). After exposure to 2, 10, and 50 mg/kg GLA for 7 days, there was a remarkable drop of 43.53% (p < 0.001), 20.44% (p = 0.009), and 32.23% (p < 0.001), severally. After exposure to 0.4, 2, 50, and 250 mg/kg GLA for 14 days, there was a remarkable decrease of 26.18% (p = 0.003), 40.61% (p < 0.001), 46.67% (p < 0.001), and 42.95% (p < 0.001), severally. In addition, after exposure to 0.4 and 50 mg/kg GLA for 21 days, there was a remarkable decrease of 22.44% (p = 0.010) and 32.47% (p = 0.001), respectively.
3.4 DNA damage to earthworm coelomocytes induced by GLA
SCGE was used to test the effect of GLA on DNA damage under alkaline conditions. As shown in Figs. 3 and 4, there was no apparent DNA damage in earthworms cultured in the blank soil, whereas GLA treatments triggered DNA fragments to leave their original position and migrate to the anode to form tails under the impact of an electric field, indicating the damaging effects of GLA on the nuclei of the earthworm. The OTM values at GLA dose ranging from 0.4 to 250 mg/kg outperformed the control groups (P < 0.001). Moreover, we found that the DNA damage depended on GLA concentration. The level of DNA damage was a positive correlation with concentrations of GLA range 0 to 10 mg/kg. However, the OTM values gradually plateau when the concentration of GLA is greater than 10 mg/kg (Fig. 5). The OTM values had remarkably increased at 21 days (Fig. 4, P < 0.05). And there is a clear linear positive correlation between the OTM values with the exposure times of GLA (Fig. 6).
Effect of GLA on earthworm coelomocytes by comet assay olive tails moment. Each data is the average of 3 replications. Error bars stand for standard deviation (SD). Different capital letters are significantly different among different exposure times under the same concentration of GLA at p < 0.05 and level by LSD test. Different small letters are significantly different among different concentrations of GLA at the same exposure time at p < 0.05 level by LSD test
4 Discussion
This paper assessed the toxicity of GLA in the soil on earthworms focusing on the acute toxicity, growth, reproductive toxicity, the activity of antioxidative and detoxification enzymes, and DNA damage. GLA can competitively inhibit glutamine synthase activity and block glutamine synthesis from glutamate and ammonia, causing ammonia accumulation (Cha et al. 2017). Traditionally, acute toxicity tests have been adopted to test the harm of soil contamination to earthworms (Bandeira et al. 2019; Ma et al. 2021). The acute toxicity of earthworms is of great significance to the study of earthworm toxicology. Artificial soil with known concentrations of pesticide is normally used in earthworm eco-toxicological tests; the artificial soil could simulate the living environment of earthworms and provide relatively accurate results for the toxicity evaluation of earthworms (OECD 1984). Our results showed a possible, small influence of GLA on earthworms treated by direct contact, where the LC50 of GLA to the earthworm was greater than 2000 mg/kg (7 days, 14 days), indicating that the acute toxicity of GLA used was low to earthworms. However, Wang et al. (2012) showed that the LC50-7 days and LC50-14 days of GLA to earthworm were respectively 167.2 and 162.2 mg/kg, which were drastically different from our current study. This phenomenon might mainly be due to the different solvents used during the acute toxicity test; Wang et al. (2012) adopted acetone as a solvent, while, in this study, to be more realistic, the GLA was dissolved in deionized water. However, acute toxicity alone cannot fully reflect the toxicity of GLA to earthworms.
The growth and reproduction of earthworms are indicators of the toxicity level of pesticides in soil (Li et al. 2017). In this study, there was a remarkable decrease in weight, the number of cocoons, and larvae in all the GLA treatment groups, even under low-concentration stress. The loss of weight may be related to survival strategy (such as cutting food intake to avoid pesticides) and could seriously impact the population structure by reducing reproductive capacity (Zhu et al. 2019). And our results are similar to other reports; the loss of weight and reproductive capacity of earthworms were influenced by glyphosate, bromadiolone, and bifenthrin (Liu et al. 2015; Li et al. 2017). The influence of GLA on the individual level of earthworms may be due to the impact on the microscopic level, so the enzyme activity and DNA damage of GLA on earthworms were determined.
Herbicide accumulation can induce the organism to produce excessive-free radicals, for instance, ROS, and excessive ROS causes oxidative stress (Banerjee et al. 2001; Liu et al. 2015; Chen et al. 2017). CAT, POD, SOD, and GST activities are biomarkers to identify oxidative stress (Song et al. 2009; Jiang et al. 2021). This paper determined the antioxidant activities of CAT, POD, SOD, and GST, and the enzymatic activities were time- and concentration-dependent. After exposure to 0.4 and 10 mg/kg GLA for 14 days, SOD enzymatic activities significantly increased. However, the SOD activity of the GLA treatment groups did not differ from the blank after 21 days (Fig. 2A). In general, the production and diminution of reactive oxygen species are in kinetic equilibrium. The reason for the increase of SOD activity in the early stage may be to maintain the balance (Cheng et al. 2015), which stimulates the synthesis of SOD to remove excessive ROS. The increase of SOD activity indicates the increased production of O2− and an establishment of a protective mechanism (Ning et al. 2020). With the rise of treating time, probably due to the degradation of GLA in soil, the bioavailability of GLA was reduced, the GLA-induced oxidative stress on earthworms was gradually reduced, and the SOD activity gradually recovered to the control level. These results were inconsistent with Zhang et al. (2019) findings, and an increase in SOD activity has also been discovered in male lizards exposed to GLA-contaminated soil.
CAT plays a crucial part in the decomposition of H2O2 (Zhang et al. 2019). In the current test, CAT activities increased firstly and then decreased with increasing exposure time (Fig. 2B). The synthesis of H2O2 was increased, thus inducing the enhancement of CAT activity. However, the CAT activity was remarkably reduced with the longer exposure time. This result might be due to SOD activity that gradually recovered to the control level; the synthesis rate of H2O2 decreased. CAT and POD can effectively cooperate in decomposing H2O2 produced by SOD-disproportionated-free radicals to eliminate its oxidative toxicity to organisms. In this test, the POD activity was markedly elevated at 14 days (Fig. 2C). This could result from the POD and CAT cooperating to decompose the excessive H2O2.
GST is involved in the invertebrate haem transport and oxidative stress response (Perally et al. 2008; Bamidele et al. 2015). GST protects organisms against oxidative damage by catalyzing the binding reaction of nucleophilic glutathione with electrophilic exogenous chemicals (Lacourse et al. 2009). In the current test, GLA significantly promoted GST activity first, but with increasing exposure time, GST activities were restrained (Fig. 2D). The reduction of GST activity was likely due to consumption of GSH (glutathione) levels (Lacourse et al. 2009) or that the prolonged retention of GLA leads to increased toxicity and fulsome cumulation of ROS to harm some tissues (Xu et al. 2015). Analogous results have been observed in E. fetida exposed to deltamethrin, pirimiphos-methyl, and bifenthrin (Li et al. 2017).
AChE mainly participates in neuronal signal transmission. It can degrade acetylcholine and terminate neurotransmitter stimulation on the postsynaptic membrane to guarantee the normal transmission of nerve signals; it can also participate in cell development and maturation, facilitating neuronal development and nerve regeneration (Tang et al. 2017). In the current test, the AChE activity was remarkably restrained in GLA treatment, even in a short time and under low-concentration stress (Fig. 2E). GLA was neurovirulent and could reduce AChE activity (Tang et al. 2017). Several studies have shown that the changes in earthworm feeding and locomotor behaviors are related to the decrease of AChE activity (Tang et al. 2017; Yang et al. 2018). This result might be due to excessive stimulation of cholinergic receptors, which eventually leads to neuromuscular twitches. Analogous results have been observed in earthworms exposed to decane, undecane, and 2,2,4,6,6-pentamethyl heptane (Tang et al. 2017), although the AChE activity remarkably increased in earthworms exposed to tris (2-chloroethyl) phosphate (Yang et al. 2018).
DNA damage index was used to characterize the genetic toxicity of GLA in earthworms. The OTM is judged the most sensitive factor indicating the severity of DNA damage (Dhawan et al. 2009). In the current test, the OTM was higher significantly under the GLA treatments, and the OTM value increased as GLA concentration increased (Figs. 3 and 4). Soils contaminated by GLA cause severe DNA damage in earthworm coelomocytes, resulting in compromised immunity and increased susceptibility to pathogens.
A number of studies have shown that an increase in ROS can lead to more severe DNA damage in earthworms, ultimately leading to the break of nucleic acid chain, removal of nucleotide, and modification of nucleotide bases (Song et al. 2009; Tang et al. 2017). GLA stimulation in earthworms resulted in ROS production; firstly, excessive ROS stimulates the SOD and GST and production of O2−. The CAT and POD synergistically decompose the excessive O2− (CAT activity first increased and then decreased, when CAT activity went down, POD activity went up). With the rise of treating time, the oxidative stress on earthworms was gradually reduced, GST activity decreased due to consumption of GSH, and the SOD activity gradually recovered to the control level. GLA was neurovirulent and could reduce AChE activity. DNA damage in earthworms increased along with the exposure time of GLA.
5 Conclusion
GLA exhibited low acute toxicity (LC50 > 2000 mg/kg) to earthworms. But GLA had a significant toxic influence on earthworms’ growth and reproduction. Exposure to GLA could change the activity of SOD, CAT, GST, POD, and AChE of earthworms, and DNA damage in earthworms increased along with the exposure time of GLA. The results will be helpful for further understanding the negative influence of GLA on the earthworm and provide a foundation for the rational use of GLA and eco-environmental risks of soil pesticide contamination.
References
Alves PRL, Niemeyer JC, Cardoso EJBN (2017) Ecotoxicology and genotoxicology: non-traditional terrestrial models. In: Larramendy ML (ed) Section I: terrestrial invertebrates as experimental models chapter 1. Royal Society of Chemistry, Cambridge. The use of nonstandardized invertebrates in soil ecotoxicology pp 1–30
Bamidele JA, Idowu AB, Ademolu KO, Akinloye OA, Bamgbola AA (2015) Heavy metal accumulation and biochemical evaluation of earthworms from sawmills in Abeokuta. South-Western Nigeria Rev Biol Trop 63:1213–1221
Bandeira FO, Alves P, Hennig TB, Schiehl A, Cardoso E, Baretta D (2019) Toxicity of imidacloprid to the earthworm Eisenia andrei and collembolan Folsomia candida in three contrasting tropical soils. J Soils Sediments 20:1997–2007
Banerjee BD, Seth V, Ahmed RS (2001) Pesticide-induced oxidative stress: perspectives and trends. Rev Environ Health 16:1–40
Calas A, Richard O, Même S, Beloeil JC, Doan BT, Gefflaut T, Même W, Crusio WE, Pichon J, Montécot C (2008) Chronic exposure to glufosinate-ammonium induces spatial memory impairments, hippocampal MRI modifications and glutamine synthetase activation in mice. Neurotoxicology 29:740-747
Carpenter D, Boutin C (2010) Sublethal effects of the herbicide glufosinate ammonium on crops and wild plants: short-term effects compared to vegetative recovery and plant reproduction. Ecotoxicology 19:1322–1336
Cha YS, Kim H, Lee Y, Choi EH, Kim HI, Kim OH, Cha KC, Lee KH, Hwang SO (2017) The relationship between serum ammonia level and neurologic complications in patients with acute glufosinate ammonium poisoning: a prospective observational study. Hum Exp Toxicol 37:571-579
Chen X, Wang X, Gu X, Jiang Y, Ji R (2017) Oxidative stress responses and insights into the sensitivity of the earthworms Metaphire guillelmi and Eisenia fetida to soil cadmium. Sci Total Environ 574:300–306
Cheng C, Huang L, Ma R, Zhou Z, Diao J (2015) Enantioselective toxicity of lactofen and its metabolites in Scenedesmus obliquus. Algal Res 10:72–79
Dhawan A, Bajpayee M, Parmar D (2009) Chapter 7: detection of DNA damage in Drosophila and Mouse. Issue Toxicol 5:157–170
Eyambe GS, Goven AJ, Fitzpatrick LC, Venables BJ, Cooper EL (1991) A non-invasive technique for sequential collection of earthworm (Lumbricus terrestris) leukocytes during subchronic immunotoxicity studies. Lab Anim 25:61–67
Ferramosca A, Lorenzetti S, Giacomo MD, Murrieri F, Coppola L, Zara V (2020) Herbicides glyphosate and glufosinate ammonium negatively affect human sperm mitochondria respiration efficiency. Reprod Toxicol 99:48–55
Jiang L, Lin JL, Jia LX, Liu Y, Pan B, Yang Y, Lin Y (2016) Effects of two different organic amendments addition to soil on sorption-desorption, leaching, bioavailability of penconazole and the growth of wheat (Triticum aestivum L.). J Environ Manage 167:130–138
Jiang L, Pan B, Liang J, Wang B, Yang Y, Lin Y (2021) Earthworm casts restrained the accumulation and phytotoxicity of soil glyphosate to cowpea (Vigna unguiculata (L.) Walp.) plants. Chemosphere 279:130571
Końca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Góźdź S, Koza Z, Wojcik A (2003) A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res 534:15–20
Lacourse EJ, Hernandez-Viadel M, Jefferies JR, Svendsen C, Spurgeon DJ, Barrett J, Morgan AJ, Kille P, Brophy PM (2009) Glutathione transferase (GST) as a candidate molecular-based biomarker for soil toxin exposure in the earthworm Lumbricus rubellus. Environ Pollut 157:2459–2469
Lajmanovich RC, Attademo AM, Lener G, Cuzziol Boccioni AP, Peltzer PM, Martinuzzi CS, Demonte LD, Repetti MR (2021) Glyphosate and glufosinate ammonium, herbicides commonly used on genetically modified crops, and their interaction with microplastics: ecotoxicity in anuran tadpoles. Sci Total Environ 804:150177
Li L, Yang D, Song Y, Shi Y, Huang B, Yan J, Dong XJ (2017) Effects of bifenthrin exposure in soil on whole-organism endpoints and biomarkers of earthworm Eisenia fetida. Chemosphere 168:41–48
Liu J, Xiong K, Ye X, Zhang J, Yang Y, Ji L (2015) Toxicity and bioaccumulation of bromadiolone to earthworm Eisenia fetida. Chemosphere 135:250-256
Ma D, Yang S, Jiang J, Zhu J, Li B, Mu W, Dou D, Liu, F (2021) Toxicity, residue and risk assessment of tetraniliprole in soil-earthworm microcosms. Ecotoxic Environ Safety 213:112061
Masiol M, Giannì B, Prete M (2018) Herbicides in river water across the northeastern Italy: occurrence and spatial patterns of glyphosate, aminomethylphosphonic acid, and glufosinate ammonium. Environ Pollut Res 25:1–11
Ning YC, Zhou HR, Wang SB, Li X, Liu D, Xu WY, Zhou DX (2020) Temporal dynamics of earthworm (Eisenia fetida) microbial communities after cadmium stress based on a compound mathematical model. Environ Sci Pollut Res 27:16326–16338
OECD (1984) Test No. 207: earthworm acute toxicity tests. Organisation for Economic Co-operation and Development, Paris
OECD (2004) Test No. 222: earthworm reproduction tests (Eisenia foetida/Eisenia andrei). Organisation for Economic Co-operation and Development, Paris
Perally SR, Lacourse EJ, Campbell AM, Brophy PM (2008) Heme transport and detoxification in nematodes: subproteomics evidence of differential role of glutathione transferases. J Proteome Res 7:4557–4565
Sauer H, Wartenberg M, Hescheler J (2001) Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem 11:173–186
Shao YT, Wang J, Du ZK, Li B, Zhu LS, Wang JH, Zhang SM (2018) Toxiceffect of [Omim]BF4 and [Omim]Br on antioxidant stress and oxidative damage in earthworms (Eisenia fetida). Environ Toxicol Pharmacol 60:37–44
Shi Z, Tang Z, Wang C (2017) A brief review and evaluation of earthworm biomarkers in soil pollution assessment. Environ Pollut Res 24:13284-13294
Singh NP, McCoy MT, Tice RR, Schneider EL (1998) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Song Y, Zhu LS, Wang J, Wang JH, Liu W, Xie H (2009) DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biol Biochem 41:905–909
Tang Z, Zhang J, Yu J, Wang C, Zhang D (2017) Allelopathic effects of volatile organic compounds from Eucalyptus grandis rhizosphere soil on Eisenia fetida assessed using avoidance bioassays, enzyme activity, and comet assays. Chemosphere 173:307–317
Wang Y, Wu S, Chen L, Wu C, Yu R, Wang Q, Zhao X (2012) Toxicity assessment of 45 pesticides to the epigeic earthworm Eisenia fetida. Chemosphere 88:484–491
Xu XB, Shi YJ, Lu YL, Zheng XQ, Ritchie RJ (2015) Growth inhibition and altered gene transcript levels in earthworms (Eisenia fetida) exposed to 2,2′,4,4′-tetrabromodiphenyl ether. Arch Environ Contam Toxicol 69:1–7
Yang Y, Xiao Y, Chang Y, Cui Y, Klobučar GIV, Li M (2018) Intestinal damage, neurotoxicity and biochemical responses caused by tris (2-chloroethyl) phosphate and tricresyl phosphate on earthworm. Ecotox Environ Safe 158:78-86
Zhang L, Diao J, Chen L, Wang Z, Zhang W, Li Y, Tian Z, Zhou Z (2019) Hepatotoxicity and reproductive disruption in male lizards (Eremias argus) exposed to glufosinate-ammonium contaminated soil. Environ Pollut 246:190–197
Zhou H, Zhang T, Zhuang J, Xu M, Liu X, Shi Q, Zhou D (2020) Study on the regulation of earthworm physiological function under cadmium stress based on a compound mathematical model. Environ Toxic Pharmac 80:103499
Zhu Y, Zhang J, Liu Y, Su G, Zhu L, Lin D (2019) Environmentally relevant concentrations of the flame retardant tris(1,3-dichloro-2-propyl) phosphate inhibit the growth and reproduction of earthworms in soil. Environ Sci Technol Lett 6:277–282
Funding
This study was funded by Natural Science Foundation of Hainan Province (No. 320QN308) and Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No. 1630042017018).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Meththika Vithanage
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, B., Jiang, L., Pan, B. et al. Toxicity of glufosinate-ammonium in soil to earthworm (Eisenia fetida). J Soils Sediments 22, 1469–1478 (2022). https://doi.org/10.1007/s11368-022-03146-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03146-7