Abstract
Acetamiprid is a broad-spectrum insecticide, belonging to the neonicotinoid compounds group, which has been extensively applied throughout the globe. Recently, indiscriminate use of these compounds was reported to cause fatal impacts on non-targeted soil organisms. Hence, the present study aimed to examine the impact of acetamiprid on Indian indigenous earthworm, Perionyx excavatus. Acute toxicity revealed an LC50 concentration of 0.25 µg/cm2 for filter paper test/72 h and 400 µg/kg for artificial soil test/14 days. Oxidative stress (ROS) and various biomarkers including superoxide dismutase, catalase, glutathione S-transferase, malondialdehyde content and DNA damage were measured. The results of the biomarker responses confirmed the acetamiprid exposure can cause toxicity to P. excavatus. In addition, cell density (20 × 102 cell mL/mg) and cell viability (40%) were significantly (p < 0.05) reduced. Further, the ecotoxicological assessment made through this study can be utilized as good evidence to toxicity of neonicotinoids to non-targeted indigenous organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Neonicotinoids are systemic broad-spectrum insecticides, representing 25% of the total insecticide market in the world (Simon-Delso et al. 2015; Cossi et al. 2020). It has been stated that, around 80 to 90% of applied neonicotinoid compounds were not taken up by the crops and tends to remain in the soil. Numerous studies have determined that neonicotinoids exist as persistent contaminants in soil and remain stable in soil with a half-life of 50–1500 days (Simon-Delso et al. 2015; Bonmatin et al. 2015; Wood and Goulson 2017; Pietrzak et al. 2020). The indiscriminate use of neonicotinoids had resulted in the accumulation of its residues in soil and it affects both aquatic and terrestrial organisms (Li et al. 2018; Renaud et al. 2018). However, a considerable number of studies had documented its adverse effects over the physiological functions of the non-targeted organisms (Ma et al. 2019; Wood and Goulson 2017; Hladik et al. 2018; Yan et al. 2021). Therefore, it is important to study the deleterious impacts of neonicotinoids on non-targeted terrestrial organisms, especially in earthworms.
Acetamiprid (N-[(6-cholro-3-pyridyl) methyl]-N’ – cyano-N-methyl acetamide) is the novel insecticide belonging to chloronicotinyls subclass of neonicotinoids (Saha et al. 2017; Li et al. 2018). In recent days, acetamiprid (ACE) has been extensively used to control the lepidopteran and hemipteran pests in various crops (Murano et al. 2018). Due to its wide range of application, ACE has been extremely detected in various environmental compartments, such as soil (11–99 µg/kg) and water (0.2 to 7 µg/L) (Saha et al. 2017; Zoumenou et al. 2019). The occurrence of ACE in the environment can pose potential risks to terrestrial organisms. It has also been stated that an increase in the concentration of ACE exposure can cause alterations in physiological functions of soil living organisms especially in earthworm physiology, reproduction, and functional gene expression (Yan et al. 2021). Earthworms are exposed through direct contact of neonicotinoids with the applied granules or seeds, or with contaminated soil. Hence, the risk assessment of this neonicotinoids on nontargeted organisms, particularly earthworms, is the need of the hour.
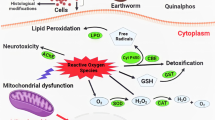
Earthworms serve as a good bioindicator and a prominent test organism for testing and assessing the toxicity of many soil pollutants (Roubalova et al. 2015; Ma et al. 2016; Yan et al. 2021). Particularly, at the macro level, behavior, growth rate, mortality, and reproduction were considered, and at the micro-level, cytotoxicity, alterations in antioxidant enzyme, and DNA damage have been considered as common indicators (Zhu et al. 2020; Saggioro et al. 2019; Lackmann et al. 2021; Yan et al. 2021). In general, Eisenia fetida earthworm model is still being used and recommended by both national and international regulatory agencies (ISD and OECD) world-wide for toxicity evaluation of soil contaminants. However, the indigenous earthworms are more sensitive than this model test species (Singh et al. 2020). Nevertheless, information on pesticide toxicity to indigenous earthworm species is not well addressed. Being unattended at the regional level, the hypothesis of testing the toxicity of ACE to indigenous earthworms through various experimental and field studies becomes imperative. Hence, the present study focussed on the assessment of toxic effects of ACE to P. excavatus, an indigenous epigeic earthworm species. We have analyzed reactive oxygen species (ROS), superoxide dismutase (SOD), glutathione S-transferase (GST), catalase (CAT), and malondialdehyde (MDA) content. In addition, immune cell toxicity, tissue damage, and DNA damage were also determined.
Materials and methods
Earthworms (P. excavatus) were obtained from local organic farm (Salem, Tamilnadu, India) and acclimatized at optimal temperature (20 ± 2℃) and photoperiod (12:12 light: dark cycle). The cocoons from the acclimatized earthworms were collected and cultured. The healthy adult earthworms with a visible clitellum and individual weight ranging from 600 to 800 mg were selected for the experiment.
Artificial soil was prepared as per OECD guideline number 207 (OECD 1984). Briefly, it was prepared by mixing 10% sphagnum peat (finely ground powder), 20% kaolin clay and 70% sand (grade 70, particle size 0.1–0.3 mm). Further its pH was adjusted to 6.0 ± 0.5 using calcium carbonate to resembling the natural soil.
Acetamiprid PESTANAL (CAS No. 160430-64-8 & lot No. BCBT9185) (99% purity) was obtained from Sigma- Aldrich.
The contact filter paper toxicity test was performed according to the OECD guideline 207 (OECD 1984). Similarly, the acute toxicity tests were also conducted with artificial soil spiked with different ACE concentrations ranging from 100, 200, 400, 800 and 1000 µg/Kg for a period of 14 days to determine LC50.
In order to assess the concentration of acetamiprid residues, the test soil was analysed using high performance liquid chromatograph (HPLC) (Jasco). HPLC separation was performed on C18 column with a flow rate of 1 ml/min. Methanol and water (55:45 v/v) was used as the mobile phase. The injection volume was 20 µl and detection was done at 255 nm. The limit of (LOD) detection for acetamiprid was 0.1 ng/L.
Subchronic toxicity tests were carried out in crystallizing dishes (1 L capacity), each containing 500 g ACE spiked soil at the 1/2,1/4,1/8 and 1/16 of LC50 concentrations (0, 25, 50, 100, and 200 µg/Kg) and moisture content were adjusted to 35% (w/w) respectively as per the OECD guidelines 207 (OECD 1984; USEPA, 2012). Earthworms (acclimatized to artificial soil overnight) were placed on the surface soil. Ten animals per replicate and three replicates, totally 30 animals were used per group. To reduce the water loss and to prevent earthworms from escaping, containers were covered with a muslin cloth. The experiments were conducted in triplicate at optimal conditions (20 ± 2 ℃) and photoperiod (12:12 light: dark cycle), for 28 days. The earthworms were sampled at 7,14, 21, and 28-days interval precisely to assess, growth inhibition rate, immune cell toxicity, histopathological alterations, and biomarker response.
The standard determination of coelomocytes viability and density were adapted from Parente et al. (2021). About 30 µL of coelomic fluid and 30 µL of trypan blue were mixed and 20 µL of this mixture were transferred into mirrored Neubauer chamber. The total number of stained and unstained cells was counted to determine the cell density and cell viability of coelomocytes.
The histopathological alterations were evaluated in earthworm midgut tissues. Samples were fixed in 10% formaldehyde for 24 h. The midgut part of the earthworms was embedded in paraffin wax and vertically dissected into sections of 6–7 mm thickness. Further, the paraffin sections were mounted on microscopic slides and subjected to Haematoxylin and Eosin (H&E) staining method (Wang et al. 2020).
ROS (reactive oxygen species) content was determined based on the 2’, 7’ – dichlorodihydrofluorescein – diacetate (DCFH-DA) method with slight modification (Han et al. 2014). The ROS content was measured using a fluorescence spectrophotometer (Jasco FP-8250, Japan) with an excitation at 488 and emission at 522 nm. ROS content was expressed as fluorescence intensity per mg of protein.
SOD (Superoxide dismutase) activity was measured based on nitro blue tetrazolium (NBT) reduction method, as described by Song et al. (2009). The activity was expressed as U/protein. CAT (Catalase) activity was assessed based on the degradation kinetics of H2O2 according to Li et al. (2018). The enzyme activity was expressed as U/mg.
The Glutathione S-Transferase (GST) activity was determined using the 1-choloro-2, 4-dinitrobenzene (CDNB) method, as described by Li et al. (2018). The GST activity was then expressed as mmol/min/mg protein. The Lipid peroxidation (MDA) content was determined according to the thiobarbituric acid (TBA) colorimetric method described by Yan et al. (2021). Level of MDA content was represented as nmol/min/mg of protein.
Genotoxicity comet assays in earthworm coelomocytes were assessed according to Yan et al. (2021). DNA damage was observed under the fluorescence microscope (Optika B 380 series). Comet images of earthworm coelomocytes were analyzed using Comet Assay Project Software (CASP).
All the experiments were performed in triplicate (n = 3). The results were shown as mean ± standard deviation (SD) and significant differences between the experimental groups were analysed by computing one-way analysis of variance (one-way ANOVA) using SPSS (Version 21.0). Tukey’s and HSD multiple comparison Post hoc tests were performed at 95% confidence interval (p < 0.05).
Results
The lethal concentration (LC50) of earthworms exposed to ACE is shown in Table 1. The LC50 values of ACE to P. excavatus earthworm, after 72 h of exposure through filter paper toxicity test was about 0.25 µg/cm2. It was statistically evaluated through probit analysis with 95% confidential limit interval 0.17–0.29. The LC50 value of ACE for P. excavatus on 14 days exposure was about 400 µg/kg in artificial soil toxicity test with 95% confidential limit interval 368–533. The acute toxicity results clearly indicates that the toxic effects were directly proportional to the ACE concentration, which finally ended up with clitellar swelling, mucus release, abridged physical movement, coiling and body fragmentation.
The nominal concentrations of ACE in test soil were analysed using HPLC. The standard curve of ACE had a good linear relationship and R2 = 0.991 within the range of studied concentration (Fig. S1). Initial concentration of ACE was shown in the Fig. S2(a). The exposure concentration of ACE was found to be moderately reduced after 28 days as shown in Fig. S2(b). It can also be found that ACE concentration got significantly reduced towards the end of the test period.
Earthworms exposed to ACE cause significant (p < 0.05) growth inhibition leading to biomass reduction and loss. The biomass of P. excavatus gradually reduced with increase in ACE exposure time. The lower ACE concentration level also affects the biomass, indicating that lesser exposure causes severe impact on P. excavatus. On the 28th day, the average biomass of earthworms in most treatment groups, exhibited a decreasing trend from its initial weight. The biomass restraint rate of earthworm growth ranged from – 8.23–5.81%.
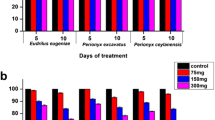
Immune cell toxicity (reduction in coelomocyte density and viability) was clearly observed after exposure to ACE at higher levels (100, and 200 µg/Kg). The total number of coelomic cells got reduced throughout the exposure period, and a decreasing level was initiated on the 14th day (Fig. 1a and b). A significant difference (p < 0.05) was noticed with reduced cells in all ACE exposed earthworm groups. The initial reduction followed by the alterations of these values indicates that the ACE exposure resulted in cytostatic or cytotoxic effects in earthworms. These results indicate that, earthworms exposed to ACE showed a continuous depletion of cell viability.
The H&E-stained sections of midgut of exposed and control earthworms were evaluated to assess the toxicity of ACE (Fig. 2). Epithelial cells of the P. excavatus in control groups were circular, smooth, and having a regular shape. However, in the case of exposed worms, the midgut epithelial cells were damaged and disorderly arranged. The highest abnormalities were recorded in highest ACE exposed groups. The epidermis was not smooth and the basophilic mucous cells were reduced. This qualitatively depicts the toxic effects of pesticides at lower concentrations.
Changes in P. excavatus ROS content when exposed to ACE in different exposure time and dose is shown in Fig. 3. ROS levels got significantly increased with prolonged exposure time and this level was discretely higher than the control (p < 0.05). These results clearly indicate that ACE can induce a greater level of ROS production, which induce oxidative stress in earthworms.
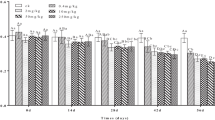
SOD activity varied randomly depending on the increase in ACE concentration and exposure time (Fig. 4a). The results showed accumulation of ROS and increased SOD activity with respect to the magnitude of ACE exposure to earthworms. A significant activation in SOD was observed during 28 days exposure. The impact of ACE exposure was most apparent at the early stage and a robust effect was exhibited at higher ACE concentrations. CAT is another key enzyme that catalyse the decomposition of H2O2 into H2O and O2, which is then further detoxified by CAT. Alterations in CAT levels in P. excavatus on ACE exposure was presented in Fig. 4b. CAT activity got significantly inhibited due to inactivation or assembly of its subunits, caused by ROS.
On the other hand, detoxification enzyme activity also altered in ACE exposed earthworms. GST is an important detoxification enzyme; it can combine electrophilic substances and glutathione to inactivate and remove contamination in second phase of metabolism in organisms. GST levels in P. excavatus were altered after exposure to ACE as shown in Fig. 4c. The MDA levels in ACE exposed groups of earthworms were significantly greater than the MDA levels of control (Fig. 4d). An increase in MDA level can reproduce a huge accretion of body’s free radicals which leads to biomass reduction and DNA damage in earthworms.
DNA damage of earthworm coelomocytes (Fig. 5a and b) was assessed through olive tail movement (OTM). The (OTM) values of the coelomocytes DNA in earthworms were illustrated in Fig. 5c. After exposure to different concentrations of ACE, the structure of the coelomocytes nucleus was damaged and DNA fragments gradually moved away from the nucleus. Furthermore, with the increase in ACE concentration, the manifestation of comet tail became more discrete, indicating that exposure to the ACE even at a low concentration causes DNA damage to earthworm coelomic cells in a dose-dependent manner. Despite this, the degree of ROS production and lipid peroxidation was significantly increased, which resulted in an increased level of DNA damage in earthworms.
Discussion
In the present study, the toxic effects of neonicotinoid on indigenous earthworm P. excavatus has been explored through enzyme biomarkers and histopathological analysis. The acute toxicity results clearly indicate the magnitude of sensitiveness of P. excavatus earthworms to insecticides when compared to the exotic species (Narayanan et al. 2022). Similar to this, an earlier study conducted by Saggioro et al. (2019), reported the LC50 value of acetamiprid to be 1.86 µg/cm2 for Eisenia andrei after 24 h of exposure. Meanwhile, we investigated the artificial soil toxicity to evaluate the lethality of earthworms in soil environment. Comparable results have also been obtained with other toxicity studies on neonicotinoid compounds, such as imidacloprid (2.26 mg/kg), dinotefuran (8.24 mg/kg) in earthworms (Liu et al. 2018; Wang et al. 2019).
ACE exposed earthworms reduces its food intake and energy stored in their body could be used for metabolic process for detoxification purpose. Hence, continuous reduction of earthworm biomass was noticed throughout the entire exposure period. Similar to this observations, Li et al. (2019) reported biomass loss and growth inhibition as the common toxicity responses in the earthworms due to pollutants.
The observed imbalance of earthworm coelomocytes may affect the immune function and earthworm survival. Similarly, Pereira et al. (2020) also reported the immune cell toxicity of Eisenia andrei when exposed to imazalil pesticide. The exposure to imazalil for a long period caused irreparable damage like cell membrane rupture, leading to performing their normal function. The same kind of immune toxicity responses were observed in our present study.
The histopathological studies are used to assess the cellular morphological alterations in earthworms with toxic pollutants (Sujeeth et al. 2023). In the present findings, the structural disintegrations were observed with ACE exposed earthworms. In line with these observations, Yan et al. (2021) reported histopathological changes in the earthworm’s intestine and midgut due to neonicotinoids stress conditions. Despite this, the alterations in the histology of the body usually depend on the body’s antioxidant system, capability to repair the damage and level of contamination (Adeel et al. 2019; Yan et al. 2021).
The exposure of pesticides to earthworms causes over production of ROS including free radicals, O2 and H2O2. In our present findings, ROS level was drastically raised after ACE exposure to earthworms. In support of this, several studies have revealed that when an organism got exposed to pesticide, its systemic function could be destroyed by an increased level of ROS, therefore it extends to increased oxidative stress in organisms (Yatoo et al. 2022; Qadri et al. 2023; Lackmann et al. 2023).
The antioxidant defence enzymes SOD and CAT are altered to protect ROS formation from pesticide exposure (Solé 2021; Sujeeth et al. 2023). In our present findings, the level of SOD and CAT activity was found to be altered in the ACE exposed earthworms. Similar to these results, the study by Li et al. (2018) found that ACE can significantly influence SOD activity in E. fetida earthworms in 28 days of exposure period. Liu et al. (2017), also reported the alteration in SOD activity and abnormal expression of SOD gene when exposed to low level of clothianidin. In line with this, Li et al. (2018) reported that earthworms exposed to ACE, showed CAT activity stimulation as a result of the increase in H2O2 content. Similarly, Chowdhary et al. (2022) also reported the alterations in CAT enzymes activity in pesticide exposed E. fetida earthworms.
GST plays a vital role in the detoxification of pesticides and xenobiotics (Fang et al. 2021). Earthworms exposed to ACE were found to have altered GST levels, indicating biotransformation of enzyme assisted GSH conjugation (Li et al. 2018). In line with this, Wang et al. (2019) reported the fluctuations of GST when exposed to neonicotinoids. Similar to these observations, several studies had reported that the neonicotinoid compounds can potentially inhibit the GST enzyme of earthworms (Liu et al. 2017; Saggioro et al. 2019).
MDA is the most important biomarker for determining the impacts of contaminants on lipid peroxidation in living organisms (Liu et al. 2018). It is the end product of lipid peroxidation (LPO) and is the most important indicator to assess cellular damage and oxidative stress of living organisms (Yan et al. 2021; Gao et al. 2022). The present findings indicate that, ACE induced the lipid peroxidation in P. excavatus earthworms. Similar to this, Yan et al. (2021) found that oxidative impairment resulting from neonicotinoid exposure in E. fetida primarily occurred in the initial stage of exposure and the MDA level got significantly increased at the end of the exposure period. However, the results of the present study demonstrate that the oxidative impairment occurred even at lower exposure bounds.
The results of present study showed that, the DNA damage (OTM) of earthworm coelomocytes was due to ACE exposure. The increase in OTM value is directly proportional to the increase in ACE concentrations. Li et al. (2018), also reported similarly that OTM values increased the increase of ACE level in E. fetida. Kaur et al. (2022) reported that low level of imidacloprid caused genotoxity in Eudrilus eugeniae and Metaphire posthuma earthworms. Hence these overall changes in biomarkers of earthworms were considered as the most important endpoint for assessing the potential adverse effects of neonicotinoid insecticides on soil organisms and the soil ecosystem.
In conclusion, the indiscriminate use of ACE in recent decades has raised concern over its toxic effects on the nontargeted organism, particularly on the earthworm. The present study explored the toxicity of ACE on P. excavatus an indigenous earthworm species. ACE triggers oxidative stress, lipid peroxidation (MDA), and genotoxicity (OTM) in earthworms. Besides, cell density (20 × 102 cell mL/mg) and cell viability (40%) were significantly (p < 0.05) reduced. ACE insecticide had caused irreversible morphological and behavioral changes and molecular damage in healthy P excavatus earthworms, making it a noteworthy indicator of pesticide pollution. Furthermore, systemic toxicity studies on other compounds of neonicotinoids on the nontargeted environmental components are also highly warranted.
References
Adeel M, Ma C, Ullah S, Rizwan M, Hao Y, Chen C, Xing B (2019) Exposure to nickel oxide nanoparticles insinuates physiological, ultrastructural, and oxidative damage: a life cycle study on Eisenia fetida. Env Poll 254: 113032.
Bonmatin JM, Giorio C, Girolami V, Goulson D, Kreutzweiser D, Krupke C, Liess M, Long E, Marzaro M, Mitchell EA (2015) Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res 22: 35–67.
Chowdhary AB, Singh J, Quadar J, Singh S, Dutta R, Angmo D, Vig AP (2022) Earthworm’s show tolerance and avoidance response to pesticide clothianidin: effect on antioxidant enzymes. Int j sci environ technol 1–10.
Cossi PF, Herbert, LT, Yusseppone MS, Pérez AF, Kristoff G (2020) Toxicity evaluation of the active ingredient acetamiprid and a commercial formulation (Assail® 70) on the non-target gastropod Biomphalaria straminea (Mollusca: Planorbidae). Ecotoxicol. Environ Saf 192: 110248.
Fang K, Han L, Liu Y, Fang J, Wang X, Liu T (2021). Enantioselective bioaccumulation and detoxification mechanisms of earthworms (Eisenia fetida) exposed to mandipropamid. Sci Total Environ 796: 149051.
Gao Y, Wang L, Zhang X, Shi C, Ma L, Zhang X, Wang G (2022). Similarities and differences among the responses to three chlorinated organophosphate esters in earthworm: evidences from biomarkers, transcriptomics, and metabolomics. Sci Total Environ 815: 152853.
Han Y, Zhu L, Wang J, Wang J, Xie H, & Zhang S (2014) Integrated assessment of oxidative stress and DNA damage in earthworms (Eisenia fetida) exposed to azoxystrobin. Ecotoxicol Environ Saf 107: 214–219.
Hladik ML, Main AR, Goulson D (2018) Environmental risks and challenges associated with neonicotinoid insecticides. Environ Sci Technol 3329–3335.
Kaur H, Hundal SS, Singh J (2022) Imidacloprid affects the reproductive performance and genotoxicity in Eudrilus eugeniae and Metaphire posthuma. Int j sci environ technol 1–10.
Lackmann C, Velki M, Bjedov D, Ečimović S, Seiler TB, Hollert H (2021) Commercial preparations of pesticides exert higher toxicity and cause changes at subcellular level in earthworm Eisenia andrei. Environ Sci Eur 33(1): 1–15.
Lackmann C, Šimić A, Ečimović S, Mikuška A, Seiler TB, Hollert H, Velki M (2023). Subcellular responses and avoidance behavior in Earthworm Eisenia andrei exposed to Pesticides in the Artificial Soil. Agriculture 13: 271.
Li B, Xia X, Wang J, Zhu L, Wang J, Wang G (2018) Evaluation of acetamiprid-induced genotoxic and oxidative responses in Eisenia fetida. Ecotoxicol Environ Saf 161: 610–615.
Li M, Yang Y, Xie J, Xu G, Yu Y (2019). In-vivo and in-vitro tests to assess toxic mechanisms of nano ZnO to earthworms. Sci Total Environ 687:71–6.
Liu T, Wang X, You X, Chen D, Li Y, Wang F (2017) Oxidative stress and gene expression of earthworm (Eisenia fetida) to clothianidin. Ecotoxicol Environ Saf 142: 489–496.
Liu T, Wang X, Chen D, Li Y, Wang F (2018) Growth, reproduction and biochemical toxicity of chlorantraniliprole in soil on earthworms (Eisenia fetida). Ecotoxicol Environ Saf 150: 18–25.
Ma T, Chen LK, Wu L, Zhang H, Luo Y (2016). Oxidative stress, cytotoxicity, and genotoxicity in earthworm Eisenia fetida at different di-n-butyl phthalate exposure levels. PLoS One11: e0151128.
Ma X, Li H, Xiong J, Mehler WT, You J (2019) Developmental toxicity of a neonicotinoid insecticide, acetamiprid to zebrafish embryos. J Agric Food Chem 67(9) 2429–2436.
Murano H, Suzuki K, Kayada S, Saito M, Yuge N, Arishiro T, Isoi T (2018) Influence of humic substances and iron and aluminum ions on the sorption of acetamiprid to an arable soil. Sci Total Environ 615: 1478–1484.
Narayanan M, Murugan JM, Kandasamy G, Kandasamy S, Nasif O, Rajendran M, Pugazhendhi A (2022). The biotransformation potential of Bacillus cereus on β-cypermethrin to protect the earthworm (Perionyx excavatus) on insecticide-contaminated soil. Arch Agron Soil Sci 68:944–55.
OECD (1984) Guideline for testing of chemicals no. 207. Earthworm, acute toxicity test. Organization for economic cooperation and development, Paris France.
Parente CE, da Silva EO, Júnior SFS, Hauser-Davis RA, Malm O, Correia FV, Saggioro EM (2021) Fluoroquinolone-contaminated poultry litter strongly affects earthworms as verified through lethal and sub-lethal evaluations. Ecotoxicol Environ Saf 207: 111305.
Pereira PC, Soares LO, Júnior SF, Saggioro EM, Correia FV (2020). Sub-lethal effects of the pesticide imazalil on the earthworm Eisenia andrei: reproduction, cytotoxicity, and oxidative stress. Environ Sci Pollut Res 27:33474–85.
Pietrzak D, Kania J, Kmiecik E, Malina G, Wątor K (2020) Fate of selected neonicotinoid insecticides in soil–water systems: current state of the art and knowledge gaps. Chemosphere 255:126981.
Qadri HA, Qamar A, Maheshwari N (2023). Oxidative stress, DNA damage, and histological alterations in Bombyx mori exposed orally to pesticide dimethoate. Physiol Entomol 48: 1–13.
Renaud M, Akeju T, Natal-da-Luz T, Leston S, Rosa J, Ramos F, Azevedo-Pereira HM (2018) Effects of the neonicotinoids acetamiprid and thiacloprid in their commercial formulations on soil fauna. Chemosphere 194: 85–93.
Roubalová R, Procházková P, Dvořák J, Škanta F, Bilej M (2015) The role of earthworm defense mechanisms in ecotoxicity studies. Invertebr Surviv J 12(1): 203–213.
Saggioro EM, do Espirito Santo DG, Júnior SFS, Hauser-Davis RA, Correia FV (2019) Lethal and sublethal effects of acetamiprid on Eisenia andrei: behavior, reproduction, cytotoxicity, and oxidative stress. Ecotoxicol Environ Saf 183: 109572.
Saha S, Mondal R, Mukherjee S, Sarkar M, Kole RK (2017) Persistence of acetamiprid in paddy and soil under West Bengal agro-climatic conditions. Environ Monit Assess 189(4): 150.
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Wiemers M (2015). Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22(1): 5–34.
Singh S, Sharma A, Khajuria K, Singh J, Vig AP (2020) Soil properties changes earthworm diversity indices in different agro-ecosystem. BMC ecology 20(1): 1–14.
Solé, M (2021). Biomarkers in earthworms. Interaction and Fate of Pharmaceuticals in Soil-Crop Systems: The Impact of Reclaimed Wastewater 311–337.
Song Y, Zhu LS, Wang J, Wang JH, Liu W, Xie H (2009) DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biol Biochem 41(5): 905–909.
Sujeeth NK, Aravinth R, Thandeeswaran M, Angayarkanni J, Rajasekar A, Mythili R, Gnanadesigan M (2023). Toxicity analysis and biomarker response of Quinalphos Organophosphate Insecticide (QOI) on eco-friendly exotic Eudrilus eugeniae earthworm. Environ Monit Assess 195: 274.
Wang X, Zhu X, Peng Q, Wang Y, Ge J, Yang G, Shen W (2019) Multi-level ecotoxicological effects of imidacloprid on earthworm (Eisenia fetida). Chemosphere 219: 923–932.
USEPA, 2012. Ecological effects test guidelines - OCSPP 850.3100: earthworm sub chronic toxicity test. EPA 712-C-016. Available at. https://nepis.epa.gov/Exe/ZyPDF.cgi/P100IREJ.PDF?Dockey=P100IREJ.PDF
Wang G, Xia X, Yang J, Tariq M, Zhao J, Zhang M, Zhang W (2020) Exploring the bioavailability of nickel in a soil system: physiological and histopathological toxicity study to the earthworms (Eisenia fetida). J Hazard Mater 383: 121169.
Wood TJ, Goulson D (2017) The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ Sci and Pollut Res 24:17285–325.
Yan X, Wang J, Zhu L, Wang J, Li S, Kim YM (2021) Oxidative stress, growth inhibition, and DNA damage in earthworms induced by the combined pollution of typical neonicotinoid insecticides and heavy metals. Sci Total Environ 754: 141873.
Yatoo AM, Ali MN, Zaheen Z, Baba ZA, Ali S, Rasool S, Sheikh TA, Sillanpää M, Gupta PK, Hamid B, Hamid B (2022). Assessment of pesticide toxicity on earthworms using multiple biomarkers: a review. Environ Chem Lett 20:2573–96.
Zhu L, Li B, Wu R, Li W, Wang J, Wang J, Zhu L (2020) Acute toxicity, oxidative stress and DNA damage of chlorpyrifos to earthworms (Eisenia fetida): the difference between artificial and natural soils. Chemosphere 255: 126982.
Zoumenou BSG, Aïna MP, Imorou Toko I, Igout A, Douny C, Brose F, Scippo, ML (2019). Occurrence of acetamiprid residues in water reservoirs in the cotton basin of Northern Benin. Bull Environ Contam Toxicol 102(1), 7–12.
Funding
This work was financially supported by Department of Biotechnology- BioCaRe Scheme (No. BT/PR18289/BIC/101/754/2016) and University Grants Commission- NFST Fellowship program (No: 201920-NFST-TAM-00427), India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elango, D., Kayalvizhi, N. & Jayanthi, P. Effects of a Neonicotinoid on Indigenous Earthworm Perionyx excavatus Biochemical and Histopathological Alterations. Bull Environ Contam Toxicol 110, 93 (2023). https://doi.org/10.1007/s00128-023-03731-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-023-03731-7