Abstract
Purpose
To evaluate atrazine behavior in an agricultural soil (adsorption–desorption, leaching) and the effects of bioaugmentation with the Arthrobacter sp. strain AAC22, as a soil remediating strategy.
Material and methods
An agricultural soil with a history of atrazine application was used. Equilibrium batch experiments allowed the investigation of the adsorption–desorption of atrazine at different soil depths, while the atrazine leaching potential was assessed using disturbed soil columns. Arthrobacter sp. strain AAC22 was selected for bioaugmentation, to remove atrazine in soil microcosms. Removal efficiency was determined by a bioassay with oat seeds.
Results and discussion
Adsorption and desorption isotherms of atrazine at different soil depths were well described by the Freundlich equation (R2 > 0.99 and R2 > 0.98, respectively). The Freundlich constant (Kf) and desorption coefficient (Kfd1–3) decreased and increased, respectively, as soil depth increased. The Kf and Kfd1–3 values were correlated positively to organic carbon (r = 0.97) and negatively to pH (r = − 0.93). In this soil, 70.2% of atrazine applied (2.5 kg ha−1) was recovered in the leachate and 7.6% remained in the soil column. The higher atrazine concentration leached can be explained by the negative hysteresis of adsorption–desorption in this soil. Bioaugmentation with AAC22 enhanced atrazine removal being nearly 70% after 2 days of treatment, and it was almost complete (> 99%) after 8 days. A bioassay demonstrated that bioaugmentation was successful and toxic by-products were not detected.
Conclusion
The adsorption–desorption and leaching experiments demonstrated the high mobility of the atrazine in the study soil. The bioaugmentation using the AAC22 strain is an effective strategy for atrazine removal in polluted soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Agricultural management practices modify the physical, chemical, and biological properties of the soil in both the short and long term, thus having a direct impact on crop development and productivity, as well as on the sustainability of agriculture (Sokolowski et al. 2020). Herbicides used in agricultural practices may directly reach the weeds but they can also be adsorbed by soil particles (Barchanska et al. 2017). Once in the soil, their distribution and retention are governed by a variety of complex and dynamic physical, chemical, and biological processes such as adsorption, desorption, transport, and degradation among others (Stipičević et al. 2015; Kanissery et al. 2019). Because adsorption–desorption and leaching processes determine the amount of herbicide that remains in soil or leaches to surface and groundwater, they must be understood and quantitatively estimated (Inoue et al. 2006).
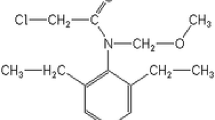
Over the past 50 years, the s-triazine herbicide atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine) has been worldwide used for agricultural purposes to control a great variety of grassy and broadleaf weeds. Atrazine is commonly found as a surface and groundwater contaminant, and residual concentrations were also detected in soil after its application (Sun et al. 2019). In fact, atrazine and its intermediate degradation product 2-hydroxyatrazine were reported to remain in soils even 22 years after application, suggesting a high persistence in soils and consequently a high risk of groundwater contamination due to gradual desorption (Jablonowski et al. 2009). Since evidence has emerged on its potentially carcinogenic and endocrine-disrupting properties, posing a risk to human health and the environment (Yang et al. 2014), the European Commission (EU) has banned its use (EC 1998) and the USA has restricted it (US EPA 2007). However, it is still extensively used in Asia, Africa, Oceania, and South America (Hansen et al. 2013). In Argentina, for instance, atrazine is the third most widely used herbicide, mainly on corn (Zea mays) and sorghum (Sorghum bicolor), with an estimated annual use of 10 − 15 million L (Alonso et al. 2018). Even though acute reports on pesticide pollution are still rather scarce, some authors have concerned to inform about the pollution levels of several pesticides (including atrazine) in soil and surface waters within Argentinian agricultural areas (Alonso et al. 2018; Bachetti et al. 2021). Hence, the intensive use and persistence of atrazine, together with the little information on its fate in the soils of the Pampean plain of the province of Córdoba (Argentina), indicates the importance to investigate the potential for environmental contamination of this herbicide.
Atrazine is moderately retained by soil colloids (clay and organic matter) and has an average organic carbon partition coefficient (KOC) of 100 mL g−1, but its persistence in soils may be long with a half-life (HL) of 28 − 115 days (Huang et al. 2013). The high persistence of s-triazines in soils suggests they have a low natural microbial degradation potential, which may be increased by the addition of microorganisms (Morgante et al. 2012). In this regard, bioaugmentation has been considered an effective and affordable option for the remediation of s-triazines from soils where the natural attenuation is slow and inefficient (Morgante et al. 2010; Zhou et al. 2013). Several bacteria are known to have the metabolic capacity necessary to degrade atrazine by a multi-step process at laboratory scale, but only few of these strains have been used for bioaugmentation strategies (Struthers et al. 1998; Rousseaux et al. 2003; Morgante et al. 2010). The usefulness of bioaugmentation in contaminated sites sometimes is constrained by the poor survival of the inoculated pollutant-degrading microbial isolates, due to factors like interaction and competition with indigenous microbial communities (Wang et al. 2013). In this regard, it is crucial to identify and test new bacterial strains with more effective atrazine removal capability on soil (Singh et al. 2018). A good candidate for bioaugmentation approach could be Arthrobacter sp. strain AAC22 (GenBank access number: KT591504). This native bacterium has been isolated from surface water in the central-southern region of Córdoba (Argentina) and encodes the well-known atz and trz catabolic genes described for s-triazine degrading pathway (Bachetti R. personal communication).
The soil microcosms are useful experimental systems to determine the potential applicability of a bioremediation strategy (Ruberto et al. 2013). In soil, the microbial biodegradation is recognized as the main mechanism for removal of atrazine (Ralebitso et al. 2002). Some atrazine-degrading microorganisms are capable of complete mineralization, while several others have a partial catabolic capability (Rousseaux et al. 2001; Singh et al. 2018). Indeed, in the environment, the deisopropylatrazine and deethylatrazine were the most frequent intermediate metabolites found as pollutants at higher concentration after 1 year of application of atrazine (Vryzas et al. 2012). In this sense, there are few toxicity reports for metabolites that are formed after bioremediation strategies in soil as well as results on atrazine residue removal (Singh et al. 2018). Some of them have determined the efficacy of a bioremediation process and the toxicity of the final degradation products on the growth parameters in atrazine-sensitive crops (Zhang et al. 2014; Silveira et al. 2017). Avena sativa L., which is a model plant recommended by international organizations to assess the standard toxicity effects of pure chemicals (OECD 2006), is sensitive to atrazine, which makes it useful to evaluate the presence of toxic by-products after treatment by bioaugmentation at microcosm scale (Chelinho et al. 2010).
Taking all of these into account, the main aims of the present work were (i) to study the adsorption–desorption and leaching capacity of the herbicide atrazine in an agricultural soil with a long-term history of atrazine application, (ii) to evaluate the degradation capacity of a native atrazine-degrading Arthrobacter sp. strain AAC22 in soil microcosms, (iii) to investigate the role of the indigenous atrazine-degrading microbial communities during the bioaugmentation assay, and (iv) to assess the effectiveness of the soil bioremediation through a bioassay with Avena sativa L. seeds.
2 Materials and methods
2.1 Chemicals
Atrazine (99% pure) was provided by Atanor S.A. (Buenos Aires, Argentina). Cyanuric acid (98% pure) was purchased from Sigma-Aldrich (Darmstadt, Germany). A stock solution of 1000 μg mL−1 of each compound was prepared in methanol. Several standard solutions, with concentrations of 0.1 − 100 µg mL−1, were injected into a capillary electrophoresis system to find out the linearity of the response by the detector and the method detection limit (MDL).
Sodium tetraborate (volumetric standard) and sodium dodecyl sulfate (SDS) (Biopack, Argentina) were used to prepare the background electrolyte. Methanol of analytical and chromatographic grade was purchased from Sintongar, Argentina.
2.2 Soil samples
The soil used in this study (typical Natracualf) is a silty clay loam (USDA NRCS 1999; USDA 2014) from an agricultural field located in the central-southern region of Córdoba (Argentina), with a 20-year history of atrazine application (S: 32°22ʹ43.11ʺ; W: 63°15ʹ45.75ʺ). Samples were collected from three layers (0 − 10, 10 − 20, and 20 − 40 cm) for the adsorption–desorption experiments, and from the top 20 cm for the leaching and bioaugmentation assays. The samples were air dried for 72 h at room temperature, homogenized, and then sieved through a 2-mm sieve. Their physicochemical properties are shown in Table 1. The pH was measured by an electrode in a soil:water ratio of 1:2.5. Total organic carbon (TOC) was analyzed by oxidation with chromic acid (Walkley and Black 1934). The sand fraction (∅ > 0.053 mm) was isolated by wet sieving to determine the proportion of total sand. The clay and silt contents were determined by the pipette method (Gee and Bauder 1979). At the time of sample collection, no residual atrazine was detected as outlined in Sect. 2.7.
2.3 Adsorption–desorption experiments
Adsorption experiments were conducted by the batch equilibration method. Duplicate samples of 5 g soil were placed in 50-mL tubes and equilibrated with 10 mL of 0.01 M CaCl2 containing atrazine at concentrations ranging from 2 to 50 μg mL−1. Since the preliminary studies showed that atrazine reached equilibrium in all the soil depths after 18 to 24 h, the tubes were shaken (120 rpm) for 24 h at 20 °C in the dark (OECD 2000). The same procedures were performed in controls without atrazine. After equilibration, the suspensions were centrifuged at 2000 g for 20 min. Three mL of supernatant was removed for analysis and filtered through a disposable 0.22 μm PVDF membrane. Atrazine concentration was determined by micellar electrokinetic chromatography (MEKC).
Desorption experiments were carried out immediately after adsorption procedures with initial atrazine concentration of 10, 30, and 50 µg mL−1. At the end of adsorption experiments, the supernatant was discarded completely and 10 mL of a 0.01 M of atrazine-free CaCl2 solution was added to the tubes. Then, the tubes were shaken for 24 h under the same condition as adsorption, and centrifuged at 2000 g for 20 min. The supernatant was collected and analyzed by MEKC. The desorption process was repeated at least three cycles, in order to desorb the majority of the adsorbed atrazine. The amount of atrazine remaining on sorbents at each desorption cycle was calculated as the difference between the initial adsorbed amount and the final desorbed amount.
The adsorption and desorption isotherms were described on the basis of the Freundlich model (Delle Site 2001), following Eq. (1):
where QS is the amount of atrazine sorbed per mass of soil (µg g−1), Ce is the equilibrium concentration of the herbicide in the liquid phase (µg mL−1), Kf is the Freundlich coefficient indicating the sorption capacity ((µg mg−1)/(µg mL−1)N), and N is a dimensionless parameter that denotes the sorption isotherm curvature. When N = 1, adsorption may be linearly proportional to the atrazine equilibrium concentration in the solution.
The normalized sorption coefficient (KOC, mL g−1) was determined regarding the organic carbon, according to the Eq. (2).
where TOC is the total organic carbon content in the soil, presented in Table 1.
The hysteresis coefficient (H) was calculated from the ratio of the desorption and adsorption isotherm parameters (Huang et al. 2013), with Eq. (3).
where Nd and Na are the empirical constants for desorption and adsorption, respectively. Theoretically, if H = 1, hysteresis is absent. On the other hand, when Nd < Na positive hysteresis occurs (H < 1) because the pesticide resists desorption from the soil. In contrast, when Nd > Na a negative hysteresis occurs (H > 1), indicating that desorption is enhanced (Yue et al. 2017).
2.4 Leaching experiment in soil columns
The downward movement of atrazine was studied in polyvinyl chloride (PVC) columns (40 cm length and 4.7 cm internal diameter), following the recommendations by the Organization for Economic Cooperation and Development (OECD) (OECD 2004). At the bottom of each column, 1 cm of glass fiber and 3 cm of quartz sand were sequentially placed to prevent soil loss during the experiment, and 400 g of soil was added at 100 g increments to reach a similar density to that of the soil under study (1.21 g cm−3). Prior to the application of atrazine, the columns were subjected to a saturation process with a 0.01 M CaCl2 solution for 24 h, followed by a 24-h drainage cycle. They were then left at room temperature, away from direct light. The pore volume (PV) of the packed columns was estimated by the weight difference between water-saturated columns and dry columns. The PV of the soil columns after saturation was 0.57 cm3 cm−3 (196.7 ± 0.7 mL). Next, 25 mL of a methanol:MilliQ water solution (10:90, V V−1) containing atrazine in an application rate of 2.5 kg ha−1 was added to the top of each column. Before leaching, the surface of the column was covered with a layer of quartz sand and glass fiber (3 cm) to minimize disturbance of soil surface. After 24 h of atrazine application, it was leached by adding 2750 mL of CaCl2 (0.01 M) with a peristaltic pump for 14 days. The leachates were collected daily from the bottom of the columns and then filtered through a nylon membrane (0.45 μm). Once leaching was completed, the columns were divided into four segments of 5 cm each.
The retardation coefficient (RC) was determined by Eq. (4) (Vega et al. 2021).
where ρ is the soil bulk density (g cm−3), η is the soil water content (cm3 cm−3), and Kd is the sorption distribution coefficient (mL g−1). The mass balance was calculated by Eq. (5).
where Ma is the mass of atrazine applied (µg), Ml is the mass of atrazine leached, Mr is the mass of atrazine retained in the column, and Md is the mass of atrazine degraded.
Finally, the constant degradation rate (χ) was calculated by Eq. (6).
where t is the duration of the experiment (days).
The half-life (HL) was calculated based on knowledge of χ by Eq. (7).
2.5 Bacterial growth
Atrazine-degrading bacterium Arthrobacter sp. strain AAC22 has been isolated from surface water (S: 32°28ʹ45.99ʺ, W: 63°23ʹ21.22ʺ) of Cabral stream, an agriculture atrazine-polluted area of the Pampean plain of Córdoba (Argentina) (Bachetti et al. 2021). The AAC22 strain contains the degradation genes trzN, atzB, and atzC, so it can degrade atrazine into non-toxic cyanuric acid, with a reported efficiency of 100%. It also has a high growth rate (µ = 0.08 h−1) in the presence of atrazine as the only nitrogen source in liquid media (Bachetti R. personal communication). AAC22 cells were grown in M9-minimal medium using 100 mg L−1 of atrazine as the sole nitrogen source. This medium contained (per liter of deionized water) 100 mL nutrient salt solution, 20 mL glucose, 1 mL MgSO4, 0.3 mL CaCl2, 1 mL thiamine, and 10 mL trace element solution. The nutrient salt solution contained 75.2 g L−1 Na2HPO4•2H2O, 30 g L−1 KH2PO4, and 5 g L−1 NaCl. The trace element solution contained 5 g L−1 EDTA, 0.8 g L−1 FeCl3•6H2O, 84 g L−1 ZnCl2, 13 g L−1 CuCl2•2H2O, 10 g L−1 CoCl2•2H2O, 10 g L−1 H3BO3, and 1.6 g L−1 MnCl2•4H2O. For solid media, 1.8% (m V−1) agar was added (Britania, Argentina). The cultures were incubated at 28 °C with agitation (150 rpm) in the M9-minimal medium. Bacterial growth was monitored by measuring the turbidity at 600 nm (turbidity600 nm). Viability was determined by counting colony-forming units (CFUs) on M9-minimal medium agar. The AAC22 cells used for bioaugmentation experiments were prepared from a late-exponential culture (1 × 107 CFU mL−1), harvested by centrifugation (11,000 rpm for 8 min) and washed twice with a phosphate saline buffer (1 × , pH 7.4).
2.6 Microcosms and bioaugmentation experiments
Microcosms were prepared in sterile plastic pots with 250 g soil (dry weight). The pots were covered with a polyethylene foil and incubated at room temperature in the dark (to prevent atrazine photodegradation). Dissipation experiments were conducted in sterile and non-sterile soil. The sterilized samples were prepared by autoclaving at 121 °C for 30 min on 3 different days. Atrazine was added to all the samples, at a final concentration of 100 mg kg−1 of dry soil. A cell suspension of Arthrobacter sp. AAC22 with a 107 CFU g−1 density was inoculated at the beginning of the experiment. Two sets of treatments were performed by triplicate as follows: (A) soil + atrazine + AAC22, and (B) sterilized soil + atrazine + AAC22. In addition, two types of controls were included: (C) soil + atrazine (natural attenuation), and (D) sterilized soil + atrazine (abiotic control). The amended soils were mixed thoroughly to achieve homogeneity. Soil moisture was maintained with sterile water at 20% of the water holding capacity, by weighing the samples every day in order to replace water lost by evaporation. Samples were periodically collected, processed immediately for microbiological analysis, and stored at − 20 °C for chemical analysis.
2.6.1 Microbiological analysis
The aerobic cultivable heterotrophic cells in the soil samples were enumerated by counting CFU on tryptic soy agar (TSA) medium (Britania, Argentina). A soil sample (1 ± 0.02 g dry soil) was placed in a conical tube (50 mL) containing 9 mL of M9-minimal medium, and vigorously shaken (150 rpm) for 2.5 h at 28 °C. Serial eight-fold dilutions were prepared, and aliquots were used to inoculate plates. The plates were incubated at 28 °C for 48 h.
The number of atrazine-degrading microorganisms (ADM) in the soil treatments (A, B, and C) was determined using the most probable number (MPN) method, with 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) as a respiration indicator (Merck, Germany) in microtiter plates (Dinamarca et al. 2007; Morgante et al. 2012). To obtain the number of MPN-TTC, 1 ± 0.02 g of soil sample (dry soil weight) was mixed with 9 mL of M9-minimal medium and shaken (150 rpm) for 2.5 h at 28 °C. The mixture was subsequently kept still for 1.5 h. Fifteen-fold serial dilutions to extinction were prepared (in triplicate) in M9-minimal medium. One hundred µL aliquots of each dilution were incubated at 28 °C for 4 days in microtiter plates. Afterwards, 20 µL of TTC solution (1% m V−1) was added to each well. The microtiter plates were incubated again at 28 °C for 4 h. The positive wells were determined after incubation by observing a change in color (red). Samples were dissolved with ethanol 96% (1 V), and the TTC formazan produced was measured with a Multiskan FC Thermo Scientific spectrophotometer (Waltham, MA, USA) at 450 nm. The MPN of ADM per gram of dry soil was determined using statistical tables designed for that purpose.
2.6.2 Bioassay
After 14 days of treatment, bioassays were performed using oat seeds (Avena sativa L.) to evaluate the success of bioremediation in soil samples from different microcosms. The seeds were surface sterilized with ethanol (70% V V−1) for 20 s and NaOCl (1% V V−1) for 90 s, and then rinsed three times in sterile distilled water. Ten of them were placed into sterile Petri dishes containing 30 g of a soil sample from each treatment. Uncontaminated soil samples were used as controls, as follows: unpolluted soil control (SC) and unpolluted sterile soil control (SSC). The Petri dishes were sealed and incubated at room temperature (25 ± 2 °C) under a 12-h light–12-h dark photoperiod for 120 h. After this time, germinated seeds were counted and early growth parameters (root and hypocotyl length) were measured. The vigor index (VI) was calculated following Aparicio et al. (2018), while the germination percentages (G %), the relative growth index (RGI), and the germination index (GI) were calculated following Zucconi et al. (1981, 1984) and Alvarenga et al. (2007), according to equations Eqs. (8) − (11):
where GSS is the number of germinated seeds in the sample, GSC is the number of germinated seeds in the control, RLS is the radicle length (mm) in the sample, and RLC is the radicle length (mm) in the control.
2.7 Analytical determinations
Atrazine was extracted from water samples by solid-phase extraction following the method described by De Gerónimo et al. (2014), with modifications. Oasis HLB cartridges (Waters, Mildford, MA, USA) were conditioned with 5 mL of methanol and 5 mL of MilliQ water. Then, the water samples were pumped through the cartridges at a flow rate of 4.0 mL min−1. Afterwards, the cartridges were rinsed with 3 mL of MilliQ water to remove matrix interferences, dried under vacuum for 20 min to remove water, and immediately eluted with 5 mL of methanol and 5 mL of ethyl acetate. The extracts were evaporated to dryness under an air flow (45 °C). The residue was reconstituted with 0.5 mL of a methanol:MilliQ water solution (40:60 V V−1), filtered through 0.22 µm and analyzed by MEKC.
Atrazine was extracted from soil samples by ultrasonic extraction (UE) (Amadori et al. 2013, with modifications). Three grams (± 0.02 g) of soil sample was weighed into conical tubes (50 mL), and 3 mL of extraction solvent (methanol, analytical grade) was added. The soil samples were homogenized in a vortex mixer and sonicated in an ultrasonic bath for 30 min (240 W and 40 kHz). Next, the suspensions were centrifuged at 5500 rpm for 15 min. The supernatant phases were collected and reserved in glass vials (20 mL). This step was performed three times and combined with the respective supernatant phases. The extracts were filtered through nylon membranes (0.45 µm). Finally, the filtrates were concentrated to dryness under an air current at 45 °C. The dry residue was dissolved with 1 mL of a methanol:MilliQ water solution (20:80 V V−1), filtered through 0.22 µm and analyzed by MEKC.
Atrazine and cyanuric acid were quantified in the water and soil extracts through MEKC, by means of Agilent Technologies 7100 (Germany), a capillary electrophoresis system equipped with a UV–Visible diode array detector. Separations were carried out with an untreated fused silica capillary (Agilent Technologies, Germany) with 75 μm I.D. and an effective length of 50 cm (total length 60 cm). The capillaries were washed with 0.1 M NaOH and the working buffer before each analysis, and with 0.1 M NaOH and MilliQ water after each analysis. The working buffer contained a 10 mM sodium tetraborate solution, 30 mM SDS, and 10% of methanol (pH 8.5) (Komarova and Kartsova 2003). The samples were injected for 30 s at 75 mbar. The stacking technique was carried out as recommended by Süsse and Müller (1996). A working voltage of 20 kV was applied for the separation and − 5 kV for the stacking. The detection wavelengths were 225 nm for atrazine and 210 nm for cyanuric acid. The concentration of each compound was quantified by integrating peak areas of different concentrations of the purified compound. The MDL for atrazine in water and soil samples was 0.1 µg L−1 and 12 µg kg−1, respectively. The recovery of the method was 95 ± 5% for water samples and 102 ± 3% for soil samples.
2.8 Statistical analyses
The data reported are mean values and standard deviations from at least duplicate determinations from two or three independent experiments carried out under similar conditions.
Quantitative parameters were interpreted by analysis of variance at significance levels of α = 0.05 and α = 0.01. The means of the parameters were compared with a Tukey test (p = 0.05 and p = 0.01), whereas their correlations were evaluated with a Pearson correlation analysis (p = 0.05 and p = 0.01). For the bioassay, three replicates were performed for each treatment and the results are an average of all three. One-way analysis of variance (ANOVA) was used to test significant differences. When such differences were found, a Tukey test was used to separate the effects. The tests were considered significantly different at p < 0.05. Statistical analyses were performed using InfoStat/P (Di Rienzo et al. 2019).
3 Results and discussion
3.1 Adsorption–desorption of atrazine in soil
The effective removal of atrazine presents constraints related to soil adsorption and the heterogeneous nature of the soil components. For this reason, those soil properties relevant to the sorption process were determined and the results are shown in Table 1. The pH values were close to neutrality. The clay content in the silt-loam soil remained relatively constant regardless of depth, but TOC was higher close to the surface (0 − 10 cm).
Freundlich parameters (Kf and N) and adsorption–desorption isotherms of atrazine in the different soil depths are presented in Table 2 and Fig. S1 (Electronic Supplementary Material). According to Fig. S1, the samples from a depth of 0 − 10 cm, with higher TOC concentration (Table 1), presented higher atrazine adsorption. Atrazine sorption is frequently correlated to TOC concentration, and hydrogen and hydrophobic bonds are the most important interactions occurring between triazines and organic matter. Hydrogen bonds occur between nitrogen atoms of the triazine ring and organic functional groups of the organic matter (Yue et al. 2017). The capacity of the soil to adsorb atrazine was well described by the Freundlich model, obtaining coefficients of determination (R2) higher than 0.99 (Table 2). Freundlich parameter Na is a measure of the nonlinearity of the adsorption isotherm. In the present study, the Na values of the Freundlich isotherm were close to one and correspond to a C-type isotherm, suggesting linear sorption as the adsorbate concentration increases (Giles et al. 1960). The C-type of isotherm has been previously reported for atrazine adsorption (Huang et al. 2015).
As described in Table 2, Kf values range from 2.01 to 1.04 (µg mg−1)/(µg mL−1)N. According to the data obtained, the Kf values followed the order 0 − 10 > 10 − 20 > 20 − 40 cm. The adsorption results are in agreement with those reported by Hang and Sereno (2002) for soils with similar physicochemical characteristics in Córdoba (Argentina). Daniel et al. (2002) reported higher Kf values to those found in this study in three representative soils of Buenos Aires province (Argentina) but with higher TOC concentration. However, after evaluating samples at different soil depths, these authors observed a behavior similar to the present study. As the sampling depth increased, the adsorption coefficient decreased. In general, the low Kf value observed for the deeper horizon (20 − 40 cm) indicates that atrazine molecules are prone to leaching into the groundwater instead of being retained or degraded close to the surface (Daniel et al. 2002). In most studies, these results were related to an increase in pH values and lower organic matter content in deeper horizons (Salazar-Ledesma et al. 2018). Moreover, microbial populations tend to decrease in these horizons, and thus, pesticide biodegradation is slower than close to the surface (Paszko and Muszyński 2017).
The KOC values were significantly different (p < 0.05) for the superficial horizons (0 to 20 cm depth) and the deeper one (20 − 40 cm) (Table 2). These values were between 64.12 and 110.00 mL g−1, which are within the range reported for atrazine in other soils from Argentina (Hang and Sereno 2002; Daniel et al. 2002).
Table 3 shows the correlation between the Kf coefficient and the soil properties. This correlation coefficient value can be used to determine the dominating factors in the adsorption process (Daniel et al. 2002). The Kf values were significantly and positively correlated with TOC concentration (r = 0.96; p < 0.01), which means that the atrazine adsorption in the soil could be attributed to organic matter (Lima et al. 2010). On the other hand, there was a high and negative correlation between pH and Kf (r = − 0.93; p < 0.01). A possible explanation is that atrazine is a weak base that becomes protonated as pH decreases, thereby increasing its adsorption in soil. Martin-Neto et al. (1994) consider that less than 1% of atrazine molecules are in a protonated form when pH values are above three, while other authors take six as the maximum value (Koskinen and Clay 1997). Therefore, the negative correlation obtained may be due to the high negative correlation between pH and TOC concentration (r = − 1.00; p < 0.01), rather than a direct effect of pH on the atrazine molecule. Given that all the horizons evaluated had pH values above seven, the impact of pH on atrazine adsorption can be discarded and atrazine may be assumed to be adsorbed as a neutral molecule (Müller et al. 2012). The clay fraction was highly and significantly correlated with Kf (r = 0.85; p < 0.05). However, the clay-silt correlation was low and not significant (r = 0.24; p > 0.05), indicating that atrazine adsorption was mainly related to TOC concentration and not to the mineral fraction in the soil.
Desorption is a key process affecting pesticide sequestration in soils and pesticide predisposition to be degraded and/or leached at different times (Lima et al. 2010; Huang et al. 2015). Herbicides with lower desorption rate may possess higher risk to the successive crops (Singh and Cameotra 2013). On the basis of the coefficient of determination values, desorption isotherms for all soil depths were adequately described by the Freundlich equation (R2 > 0.98) (Table 2; Fig. S1). The results showed that the average desorption rate of atrazine after three successive desorption cycles was in the order 0 − 10 < 10 − 20 < 20 − 40 cm. The amount of atrazine desorbed at tested soil depths increased in soils with lower Kf values. Similar results were demonstrated in soils with various physicochemical characteristics in eastern China (Huang et al. 2013).
By comparison of Kfd1–3 obtained from the different soil depths, it is possible to conclude that lower values are obtained for the horizons close to the surface with high TOC (Table 2). Larger Kfd values indicate a greater proportion of the pesticide retained by the soil (Yue et al. 2017). As presented in the adsorption study, there was a positive significant relationship between Kfd1–3 and the TOC concentration (Kfd1: r = 0.98, p < 0.05; Kfd2: r = 0.97, p < 0.05; Kfd3: r = 0.92, p < 0.05), and a negative relationship between Kfd1–3 and pH (Kfd1: r = − 0.95, p < 0.05; Kfd2: r = − 0.99, p < 0.05; Kfd3: r = − 0.96, p < 0.05) (Table 3). A higher organic matter content is usually considered not only to be responsible for a high sorption of organic compounds but also to contribute significantly to desorption hysteresis (Lima et al. 2010).
The H coefficient is a quantitative indicator of the degree of irreversible adsorption. However, there is no agreement about an absolute value of the presence or absence of hysteresis (Yue et al. 2017). Barriuso et al. (1994) defined that H values between 0.7 and 1.0 indicate the absence of hysteresis. In the current study, the H values ranging from 1.40 to 2.47 indicate negative hysteresis in all soil depths evaluated. However, the H values were significantly different (p < 0.05) for the superficial horizons (0 to 20 cm depth) and the deeper one (20 − 40 cm) (Table 2). The results of greater desorption for atrazine in the deeper horizons suggests a higher possibility of atrazine leaching into the groundwater. Several studies have reported cases of negative hysteresis in adsorption–desorption process with atrazine (Albarrán et al. 2003; Yue et al. 2017).
According to the results described so far, the high desorption capacity of atrazine in the study soil increases its probability of leaching to groundwater. Bioremediation could be an appropriate strategy to address this issue; however, adequate knowledge of atrazine mobility in this kind of soils is required as a preliminary step.
3.2 Leaching of atrazine in soil columns
The fate of atrazine in an agricultural soil was studied by means of disturbed soil columns. Figure 1 shows that atrazine breakthrough curve is skewed to the right, exhibiting an asymmetric shape and tailing. The process responsible for nonequilibrium could be chemical nonequilibrium, retarded intraparticle diffusion, or intraorganic diffusion (Montoya et al. 2006). Chemical nonequilibrium transport explains the detection of atrazine in the leaching solution after several years from the last application (Jablonowski et al. 2009).
Atrazine was detected in the first leaching event (1.11 PV) (Fig. 1). Therefore, in this study atrazine did not instantaneously reach equilibrium between the liquid and solid phase and remained in solution subject to leaching. The content of atrazine in the leachate was 70.2% after being added about 14 PV. The high percolation of atrazine is due to the low TOC concentration (1.6%), favoring leaching to the lower layers of the soil. Mendes et al. (2019) found that the highest amount of atrazine (> 73%) remained in the superficial layer (0 − 5 cm) in an Oxisol soil with a TOC concentration of 2.15%.
The interaction between the atrazine contained in the soil and the four depths evaluated (0 − 5, 5 − 10, 10 − 15, and 15 − 20 cm) revealed that leaching occurs throughout the profile (0 − 20 cm) over a 14-day leaching period. The mean atrazine concentration in the 15 − 20 cm layer was significantly higher (p < 0.05) with respect to the other depths evaluated (Fig. 2), and the high concentration detected in the deepest layer attests to the compound’s leaching power.
The mass balance calculation for the soil column indicates a 77.8% recovery of the total atrazine that was initially applied (1400 µg per column). Out of this percentage, 70.2% (982.8 µg) was recovered in the leachate, while 7.6% (106.4 µg) remained in the column (Table 4). The higher atrazine concentration leached from the silty clay loam soil can be explained by the negative hysteresis of the herbicide adsorption–desorption process. In addition, Kf and KOC were 1.64 (µg mg−1)/(µg mL−1)N and 111.6 mL g−1, respectively, in the soil tested in this study (Table 2); i.e., atrazine had moderate leaching potential through the soil column (Vryzas et al. 2007). Both the moderate leaching and the negative hysteresis suggest that the herbicide may be bioavailable to soil microorganisms, especially considering that this soil has a long-term history of application.
The HL of atrazine degradation in the typical Natracualf soil was 38.5 days based on knowledge of χ (Table 4). According to Hang and Nassetta (2003), the average degradation period of atrazine in soils of Córdoba varies between 12 and 60 days, so the results of this research are coincident. In addition, the low RC value (1.03) allows the classification of atrazine as mobile in the soil evaluated indicating leaching processes and possible groundwater contamination (Khan and Liang 1989). Similar results were reported by Vega et al. (2021) in two soils (Regosol and Calcisol) from Mexico. However, Bedmar et al. (2004) reported RC values for atrazine ranging from 7.5 to 8.8 in several soils with high TOC concentration (1.9 to 4.1%) from the province of Buenos Aires (Argentina). Several studies show that the elution capacity of atrazine in the soil profile depends on the organic matter content, agricultural management of the soil, and its chemical composition (Montoya et al. 2006; Salazar-Ledesma et al. 2018).
3.3 Biodegradation of atrazine in soil microcosms
3.3.1 Effect of the bioaugmentation of soil microcosms with native strain Arthrobacter sp. AAC22
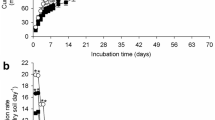
Bioaugmentation assays were carried out in soil microcosms spiked with atrazine at a final concentration of 100 mg kg−1. Figure 3 shows the results related to the changes in residual atrazine and cyanuric acid in different treatments. Atrazine was removed at a higher rate in the microcosms that were bioaugmented with Arthrobacter sp. strain AAC22 than in those that were not. On day 2 of incubation, atrazine degradation in the bioaugmented microcosms was 69.8% for treatment A and 59.9% for treatment B. Thus, the HL for atrazine in each treatment was 22 h and 33 h, respectively (Fig. 3a). When the concentration of atrazine decreased, that of cyanuric acid gradually increased (Fig. 3b). After 8 days of incubation, atrazine had been completely removed (> 99%) from the bioaugmented microcosms (Fig. 3a). The fact that the elimination percentages did not differ between these microcosms suggests that the indigenous microbial community did not affect the degradation efficiency of strain AAC22.
Residual atrazine (a) and cyanuric acid (b) in microcosms throughout the incubation period (28 days). Treatment A: soil + 1 × 107 CFU g−1 strain AAC22 + 100 mg kg−1 atrazine; treatment B: sterile soil + 1 × 107 CFU g−1 strain AAC22 + 100 mg kg−1 atrazine; control C: soil + 100 mg kg−1 atrazine; and control D: sterile soil + 100 mg kg−1 atrazine. The error bars denote standard deviation of each mean value (n = 3)
Atrazine degradation was higher in control C (natural attenuation) than in D (abiotic control) after 12 days, with a degradation rate of 34.7% by the end of the assay. However, this value was lower than those for bioaugmented treatments with AAC22 and might be an indication of the slow capability of the indigenous microbial communities to adapt to the herbicide, favoring atrazine leaching to groundwater resources. On the other hand, atrazine concentration in control D began to decrease after 8 days, although the degradation rate by the end of the assay (21.5%) was not significant (p > 0.05) with respect to treatments A and B (Fig. 3a). This might mean that atrazine was hydrolyzed in this control (Zhu et al. 2020). By contrast, total atrazine degradation occurred within 7 to 8 days in the bioaugmented microcosms, which is evidence for the high degradation efficiency of AAC22.
Figure 3b shows the variation of cyanuric acid concentration across time. Cyanuric acid is an intermediate metabolite produced during the degradation of atrazine by AAC22. After 2 days of incubation, the concentration of cyanuric acid increased in the inoculated microcosms (treatments A and B), thus confirming the capacity of the strain to transform atrazine into this metabolite (Bachetti, personal communication). Meanwhile, concentration of the same compound was very low in control C after 14 days, so the native microorganisms in the soil might be slow to degrade the herbicide after having been previously exposed and thus adapted to it. Several studies show that cyanuric acid is easily degraded in the environment and can be used as a source of nitrogen by several soil microorganisms (Fernandes et al. 2020).
3.3.2 Effect of atrazine on indigenous and degrading heterotrophic microorganisms in a soil with long-term exposure to atrazine
The effect of atrazine on indigenous microorganism in an agricultural soil with long-term exposure to the herbicide was assessed by counting cultivable heterotrophic bacteria (CFU g−1) and ADM (MPN g−1) in the microcosms corresponding to treatments A and B, and in control C (Figs. 4 and 5).
Counting of heterotrophic microorganisms (Log CFU g−1) in microcosms, on agar plates using TSA medium. Treatment A: soil + 1 × 107 CFU g−1 strain AAC22 + 100 mg kg−1 atrazine; treatment B: sterile soil + 1 × 107 CFU g−1 strain AAC22 + 100 mg kg−1 atrazine; and control C: soil + 100 mg kg−1 atrazine. The error bars denote standard deviation of each mean value (n = 3)
Estimation of catabolic activity of atrazine-degrading microorganisms (ADM count) in microcosms by the MPN method and using the TTC as indicator. Treatment A: soil + 1 × 107 CFU g−1 strain AAC22 + 100 mg kg−1 atrazine; treatment B: sterile soil + 1 × 107 CFU g−1 strain AAC22 + 100 mg kg−1 atrazine; and control C: soil + 100 mg kg−1 atrazine. The error bars denote standard deviation of each mean value (n = 3)
The dynamics of cultivable heterotrophic bacteria in the soil microcosms are shown in Fig. 4. The initial counts of these microorganisms were significantly different (p < 0.05) between the two AAC22-bioaugmented microcosms, with values of 2.2 × 107 CFU g−1 and 5.6 × 106 CFU g−1 for treatments A and B, respectively. However, these counts rose significantly (p < 0.05) to 2.2 × 108 CFU g−1 after 2 days of incubation in treatment B, and to 1.1 × 108 CFU g−1 after 7 days of incubation in treatment A. On the other hand, the initial count in control C was 1.3 × 106 CFU g−1, a number which also increased significantly after 4 days (2.2 × 107 CFU g−1), and which then remained constant until the end of the incubation period.
The ADM dynamics were determined in the different microcosms by the TTC-MPN method, developed by Dinamarca et al. (2007), for the detection and quantification of s-triazine-degrading microorganisms in the soil (Fig. 5). This method is based on the capability of microorganisms to use an s-triazine herbicide as the sole nitrogen source for growth (Dinamarca et al. 2007; Morgante et al. 2012). The initial ADM count was 5.8 × 105 and 5.6 × 105 MPN cells g−1 for treatments A and B, respectively. After 2 days of incubation, these counts increased significantly (p < 0.05), with the highest values being reached after 8 days. However, the count was higher for treatment B than for A. These results suggest that strain AAC22 has a higher growth rate and an efficient atrazine degradation in the absence of native microorganisms, probably due to lack of competition for nutrients. After 8 days, ADM began to decrease significantly (p < 0.05) in both treatments, possibly because of the poor availability of atrazine in the soil. Still, the count remained relatively high up (5.8 × 104 NMP cell g−1) to the end of the assay, which demonstrates the strong survival ability of AAC22 in the soil.
In the microcosms subjected to natural attenuation (C), the application of atrazine caused a gradual increase of the ADM, from 1.3 × 101 NMP cells g−1 (initial day) to 2.2 × 104 NMP cells g−1 on day 28. These results, which are in agreement with those previously reported, may mean that the repeated application of atrazine on crops favors the development of a microbial population with the ability to tolerate s-triazine (Bonfleur et al. 2015). Different studies have described the change that occurs in the microbial community in response to the biodegradation of s-triazines. Morgante et al. (2010) found that bioaugmentation with Pseudomonas sp. strain MHP41 promoted simazine attenuation and microbial population changes in agricultural soils from Chile. Mahía et al. (2011) reported that the dynamics of the bacterial population were significantly affected by the incubation time and the soil type after atrazine addition.
Arthrobacter sp. strain AAC22 has proved to be a suitable candidate for bioremediation of atrazine-contaminated soils in the studied area. In the present study, a single inoculation with strain AAC22 made it possible to satisfactorily remove atrazine in soils amended with 100 mg kg−1 of the herbicide after 8 days. The results obtained also offer evidence on the survival ability of AAC22 in an agricultural soil throughout the incubation period.
3.3.3 Assessment of soil bioremediation efficacy
The effectiveness of any remediation process in a contaminated environment should be verified by using a biological approach (Aparicio et al. 2018). Thus, after implementing bioaugmentation, the soil microcosms were tested through a bioassay using oat seeds (A. sativa L.). The germination of these seeds was not inhibited (100 − 93%), and non-significant differences were observed between treatments and controls after 14 days (Table 5). Fernandez et al. (2019) reported that germination is often little affected by chemical pollution, and that germination studies sometimes might not be able to predict the survival of the tested species. For this reason, we also evaluated root and hypocotyl length in A. sativa L. Although there was no inhibition in the root and shoot growth of the seedlings in controls C and D, root length showed significant differences with respect to the bioremediated treatments (Table 5). In contrast, the values for hypocotyl length were similar in treatments and controls. These results indicate that atrazine is more toxic in roots than in hypocotyls, likely because the roots are the primary organ in contact with the pollutant.
The VI facilitates the comparison between treatments because it combines data regarding germination and seedling length into a single value. This index was significantly lower for the oat seedlings from atrazine controls than for those from bioaugmented treatments and controls (SC and SSD), thus confirming that A. sativa L. serves as an indicator of the efficiency of bioremediation under the conditions studied. On the other hand, VI was significantly higher in AAC22 treatments than in control soils (Table 5). This could indicate a synergistic effect between the plant and the microorganism. In addition, the cyanuric acid produced during the biodegradation of atrazine can be converted to urea by other microorganisms present in the soil and therefore promote plant growth (Zhang et al. 2014).
In terms of GI, the values shown in Table 5 indicate the absence of phytotoxic compounds in treatments A and B according to the categories assigned by Zucconi et al. (1981, 1984). However, a moderate presence of phytotoxic compounds was detected in controls C and D. Besides, the RGI values could indicate the absence of significant effects on seedling root or root elongation stimulation, according to Alvarenga et al. (2007)
Taken together, the results of the bioassay demonstrate that Arthrobacter sp. strain AAC22 was efficient and safe for the bioremediation of atrazine-contaminated soils.
4 Conclusions
The present study analyzes the adsorption–desorption and leaching of atrazine in an agricultural soil of the Pampean plain of Córdoba (Argentina), to understand the overall fate of the herbicide in the environment. Adsorption and desorption isotherms of atrazine are well described by the Freundlich equation. The TOC concentration and pH played an important role affecting adsorption–desorption process at different soil depths. Consequently, in the studied soil the atrazine desorption raises as increasing the soil depth mainly because the TOC concentration declines in deeper layers. Negative hysteresis was verified for all tested soil depths. The vertical transport of atrazine in disturbed soil columns showed that the maximum elution peak was reached at the first week of the experimental work. To our knowledge, this is the first report concluding that atrazine is a mobile compound in soils of the Pampean plain of Córdoba (according to the RC value) and hence a potential groundwater contaminant.
This study also demonstrates that the native Arthrobacter sp. strain AAC22 increases atrazine removal in soil microcosms, without needing previous acclimatization or biostimulation. This is a relevant finding when comparing with natural attenuation processes since microorganisms present in soils with a history of atrazine application often have to undergo acclimatization prior to the onset of degradation. Thus, the process can take from several weeks to months, especially in soils with high doses of the herbicide. In addition, and based on the data obtained, prolonged microbial acclimatization can be harmful for the environment, as dispersal of the contaminant may occur by leaching and/or runoff. Besides, strain AAC22 was able to remove atrazine without generating toxic by-products and would therefore make a suitable and highly recommendable candidate for the bioremediation of soils contaminated with s-triazines. Our results support the relevance of bioaugmentation with this strain as a bioremediation strategy, especially in soils where natural attenuation is low or undetectable.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Albarrán A, Celis R, Hermosín MC, López-Piñeiro A, Ortega-Calvo JJ, Cornejo J (2003) Effects of solid olive-mill waste addition to soil on sorption, degradation and leaching of the herbicide simazine. Soil Use Manag 19:150–156. https://doi.org/10.1079/sum2002185
Alonso LL, Demetrio PM, Etchegoyen AM, Marino DJ (2018) Glyphosate and atrazine in rainfall and soils in agroproductive areas of the pampas region in Argentina. Sci Total Environ 645:89–96. https://doi.org/10.1016/j.scitotenv.2018.07.134
Alvarenga P, Palma P, Gonçalves AP, Fernandes RM, Cunha-Queda AC, Duarte E, Vallini G (2007) Evaluation of chemical and ecotoxicological characteristics of biodegradable organic residues for application to agricultural land. Environ Int 33(4):505–513. https://doi.org/10.1016/j.envint.2006.11.006
Amadori MF, Cordeiro GA, Rebouças CC, Peralta-Zamora PG, Grassi MT, Abate G (2013) Extraction method for the determination of atrazine, deethylatrazine, and deisopropylatrazine in agricultural soil using factorial design. J Braz Chem Soc 24(3):483–491. https://doi.org/10.5935/0103-5053.20130058
Aparicio JD, Saez JM, Raimondo EE, Benimeli CS, Polti MA (2018) Comparative study of single and mixed cultures of actinobacteria for the bioremediation of co-contaminated matrices. J Environ Chem Eng 6(2):2310–2318. https://doi.org/10.1016/j.jece.2018.03.030
Bachetti RA, Urseler N, Morgante V, Damilano G, Porporatto C, Agostini E, Morgante C (2021) Monitoring of atrazine pollution and its spatial-seasonal variation on surface water sources of an agricultural river basin. Bull Environ Contam Toxicol 106:929–935. https://doi.org/10.1007/s00128-021-03264-x
Barchanska H, Sajdak M, Szczypka K, Swientek A, Tworek M, Kurek M (2017) Atrazine, triketone herbicides, and their degradation products in sediment, soil and surface water samples in Poland. Environ Sci Pollut Res 24(1):644–658. https://doi.org/10.1007/s11356-016-7798-3
Barriuso E, Laird DA, Koskinen WC, Dowdy RH (1994) Atrazine desorption from smectites. Soil Sci Soc Am J 58:1632. https://doi.org/10.2136/sssaj1994.03615995005800060008x
Bedmar F, Costa JL, Suero E, Gimenez D (2004) Transport of atrazine and metribuzin in three soils of the humid pampas of Argentina. Weed Technol 18(1):1–8. https://doi.org/10.1614/WT-02-056
Bonfleur EJ, Tornisielo VL, Regitano JB, Lavorenti A (2015) The effects of glyphosate and atrazine mixture on soil microbial population and subsequent impacts on their fate in a tropical soil. Water Air Soil Pollut 226(2):1–10. https://doi.org/10.1007/s11270-014-2190-8
Chelinho S, Moreira-Santos M, Lima D, Silva C, Viana P, André S, Lopes I, Ribeiro R, Fialho AM, Viegas CA, Sousa JP (2010) Cleanup of atrazine-contaminated soils: ecotoxicological study on the efficacy of a bioremediation tool with Pseudomonas sp. ADP J Soils Sediments 10:568–578. https://doi.org/10.1007/s11368-009-0145-2
Daniel PE, Bedmar F, Costa JL, Aparicio VC (2002) Atrazine and metribuzin sorption in soils of the Argentinean humid pampas. Environ Toxicol Chem 21:2567–2572. https://doi.org/10.1002/etc.5620211207
De Gerónimo E, Aparicio VC, Bárbaro S, Portocarrero R, Jaime S, Costa JL (2014) Presence of pesticides in surface water from four sub-basins in Argentina. Chemosphere 107:423–431. https://doi.org/10.1016/j.chemosphere.2014.01.039
Delle SA (2001) Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants. A review. J Phys Chem Ref Data 30(1):187–439. https://doi.org/10.1063/1.1347984
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez LA, Tablada ME, Robledo CW (2019) InfoStat v. 2019. Cent. Transf. InfoStat, FCA, Univ. Nac. Córdoba, Argentina
Dinamarca MA, Cereceda-Balic F, Fadic X, Seeger M (2007) Analysis of s-triazine-degrading microbial communities in soils using most-probable-number enumeration and tetrazolium-salt detection. Int Microbiol 10:209–215. https://doi.org/10.2436/20.1501.01.29
EC (1998) European Commission Council Directive 98/83/EC. Off J Eur Comm 330:32–54
Epa US (2007) Atrazine - Chemical Summary. Publicacions Matem. https://doi.org/10.5565/PUBLMAT_Introduction
Fernandes AFT, Wang P, Staley C, Moretto JAS, Altarugio LM, Campanharo SC, Stehling EG, Sadowsky MJ (2020) Impact of atrazine exposure on the microbial community structure in a Brazilian tropical Latosol soil. Microbes Environ 35:1–7. https://doi.org/10.1264/jsme2.ME19143
Fernandez M, Paisio CE, Perotti R, Pereira PP, Agostini E, González PS (2019) Laboratory and field microcosms as useful experimental systems to study the bioaugmentation treatment of tannery effluents. J Environ Manage 234:503–511. https://doi.org/10.1016/j.jenvman.2019.01.019
Gee GW, Bauder JW (1979) Particle size analysis by hydrometer: a simplified method for routine textural analysis and a sensitivity test of measurement parameters. Soil Sci Soc Am J 43(5):1004–1007. https://doi.org/10.2136/sssaj1979.03615995004300050038x
Giles CH, MacEwan TH, Nakhwa SN, Smith D (1960) A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc 111:3973–3993. https://doi.org/10.1039/jr9600003973
Hang S, Nassetta M (2003) Evolución de la degradación de atrazina en dos perfiles de suelo de la provincia de Córdoba. Rev Investig Agropecu 32:57–69
Hang S, Sereno R (2002) Adsorción de atrazina y su relación con las caraterísticas sedimentológicas y el desarrollo del perfil de dos suelos de la provincia de Córdoba. Rev Investig Agropecu 31:73–87
Hansen AM, Treviño-Quintanilla LG, Márquez-Pacheco H, Villada-Canela M, Gonzalez-Marquez CL, Guillen-Garces AR, Hernandez-Antonio A (2013) Atrazine: a controversial herbicide. Rev Int Contam Ambient 29:65–84
Huang Y, Liu Z, He Y, Zeng F, Wang R (2013) Quantifying effects of primary parameters on adsorption-desorption of atrazine in soils. J Soils Sediments 13:82–93. https://doi.org/10.1007/s11368-012-0572-3
Huang Y, Liu Z, He Y, Li Y (2015) Impact of soil primary size fractions on sorption and desorption of atrazine on organo-mineral fractions. Environ Sci Pollut Res 22:4396–4405. https://doi.org/10.1007/s11356-014-3684-z
Inoue M, Oliveira R, Regitano J, Tormena CA, Constantin J, Tornisielo VL (2006) Sorption-desorption of atrazine and diuron in soils from southern Brazil. J Environ Sci Heal - Part B Pestic Food Contam Agric Wastes 41:605–621. https://doi.org/10.1080/03601230600701767
Jablonowski ND, Köppchen S, Hofmann D, Schäffer A, Burauel P (2009) Persistence of 14C-labeled atrazine and its residues in a field lysimeter soil after 22 years. Environ Pollut 157:2126–2131. https://doi.org/10.1016/j.envpol.2009.02.004
Kanissery R, Gairhe B, McAvoy C, Sims G (2019) Herbicide bioavailability determinant processes in the soil. J Bioremediation Biodegrad 10(458):2. https://doi.org/10.4172/2155-6199.1000458
Khan MA, Liang T (1989) Mapping pesticide contamination potential. Environ Manage 13:233–242. https://doi.org/10.1007/BF01868370
Komarova NV, Kartsova LA (2003) Determination of s-triazine herbicides by micellar electrokinetic chromatography using sodium dodecyl sulfate. J Anal Chem 58:785–789. https://doi.org/10.1023/A:1025099930530
Koskinen WC, Clay SA (1997) Factors affecting atrazine fate in north central U.S. soils. Rev Environ Contam Toxicol 117–165. https://doi.org/10.1007/978-1-4612-1958-3_4
Lima DLD, Schneider RJ, Scherer HW, Duarte AC, Santos EB, Esteves VI (2010) Sorption desorption behavior of atrazine on soils subjected to different organic long-term amendments. J Agric Food Chem 58:3101–3106. https://doi.org/10.1021/jf903937d
Mahía J, González-Prieto SJ, Martín A, Bååth E, Díaz-Raviña M (2011) Biochemical properties and microbial community structure of five different soils after atrazine addition. Biol Fertil Soils 47:577–589. https://doi.org/10.1007/s00374-011-0569-x
Martin-Neto L, Vieira EM, Sposito G (1994) Mechanism of atrazine sorption by humic acid: a spectroscopic study. Environ Sci Technol 28(11):1867–1873. https://doi.org/10.1021/es00060a017
Mendes KF, Shiroma AT, Pimpinato RF, Reis MR, Tornisielo VL (2019) Transport of atrazine via leaching in agricultural soil with mineral oil addition. Planta Daninha 37:1–7. https://doi.org/10.1590/S0100-83582019370100
Montoya JC, Costa JL, Liedl R, Bedmar F, Daniel P (2006) Effects of soil type and tillage practice on atrazine transport through intact soil cores. Geoderma 137:161–173. https://doi.org/10.1016/j.geoderma.2006.08.007
Morgante V, Flores C, Fadic X, González M, Hernández M, Cereceda-Balic F, Seeger M (2012) Influence of microorganisms and leaching on simazine attenuation in an agricultural soil. J Environ Manage 95:S300–S305. https://doi.org/10.1016/j.jenvman.2011.06.045
Morgante V, López-López A, Flores C, González M, González B, Vásquez M, Rosselló-Mora R, Seeger M (2010) Bioaugmentation with Pseudomonas sp. strain MHP41 promotes simazine attenuation and bacterial community changes in agricultural soils. FEMS Microbiol Ecol 71:114–126. https://doi.org/10.1111/j.1574-6941.2009.00790.x
Müller K, Duwig C, Prado B, Siebe C, Hidalgo C, Etchevers J (2012) Impact of long-term wastewater irrigation on sorption and transport of atrazine in Mexican agricultural soils. J Environ Sci Heal - Part B Pestic Food Contam Agric Wastes 47:30–41. https://doi.org/10.1080/03601234.2012.606416
OECD (2000) OECD Test Guideline 106: adsorption - desorption using a batch equilibrium method OECD Guidel Test Chem 1–44. https://doi.org/10.1787/9789264069602-en
OECD (2004) OECD Test Guideline 312: leaching in soil columns, OECD Guidelines for the Testing of Chemicals, Section 3. OECD Publishing, Paris. https://doi.org/10.1787/9789264070561-en
OECD (2006) OECD Test Guideline 208: terrestrial plant test - seedling emergence and seedling growth test. Guidel Test Chem Terr Plant Test Seedl. Emerg Seedl Growth Test. https://doi.org/10.1787/9789264070066-en
Paszko T, Muszyński P (2017) Degradation rates of alachlor, atrazine and bentazone in the profiles of Polish Luvisols. Int Agrophysics 31(3):401. https://doi.org/10.1515/intag-2016-0053
Ralebitso T, Senior E, Van Verseveld HW (2002) Microbial aspects of atrazine degradation in natural environments. Biodegradation V13:11–19. https://doi.org/10.1023/A:1016329628618
Rousseaux S, Hartmann A, Lagacherie B, Piutti S, Andreux F, Soulas G (2003) Inoculation of an atrazine-degrading strain, Chelatobacter heintzii Cit1, in four different soils: effects of different inoculum densities. Chemosphere 51(7):569–576. https://doi.org/10.1016/S0045-6535(02)00810-X
Rousseaux S, Hartmann A, Soulas G (2001) Isolation and characterisation of new Gram-negative and Gram-positive atrazine degrading bacteria from different French soils. FEMS Microbiol Ecol 36:211–222. https://doi.org/10.1016/S0168-6496(01)00135-0
Ruberto L, Mac Cormack W, Giulietti A, Merini L (2013) Microcosms: a key tool for the scaling up of soil bio/phytoremediation processes. In: Daniels J (ed) Advances in environmental research. Nova Publishers, New York, pp 201–228
Salazar-Ledesma M, Prado B, Zamora O, Siebe C (2018) Mobility of atrazine in soils of a wastewater irrigated maize field. Agric Ecosyst Environ 255:73–83. https://doi.org/10.1016/j.agee.2017.12.018
Silveira GL, Lima MGF, dos Reis GB, Palmieri MJ, Andrade-Vieria LF (2017) Toxic effects of environmental pollutants: comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere 178:359–367. https://doi.org/10.1016/j.chemosphere.2017.03.048
Singh AK, Cameotra SS (2013) Adsorption and desorption behavior of chlorotriazine herbicides in the agricultural soils. J Pet Environ Biotechnol 4:5. https://doi.org/10.4172/2157-7463.1000154
Singh S, Kumar V, Chauhan A, Datta S, Wani AB, Singh N, Singh J (2018) Toxicity, degradation and analysis of the herbicide atrazine. Environ Chem Lett 16:211–237. https://doi.org/10.1007/s10311-017-0665-8
Sokolowski AC, Prack McCormick B, De Grazia J, Wolski JE, Rodríguez HA, Rodríguez-Frers EP, Gagey MC, Debelis SP, Paladino IR, Barrios MB (2020) Tillage and no-tillage effects on physical and chemical properties of an Argiaquoll soil under long-term crop rotation in Buenos Aires, Argentina. Int Soil Water Conserv Res 8:185–194. https://doi.org/10.1016/j.iswcr.2020.02.002
Stipičević S, Galzina N, Udiković-Kolić N, Jurina T, Mendaš G, Dvoršćak M, Petrić I, Barić K, Drevenkar V (2015) Distribution of terbuthylazine and atrazine residues in crop-cultivated soil: the effect of herbicide application rate on herbicide persistence. Geoderma 259:300–309. https://doi.org/10.1016/j.geoderma.2015.06.018
Struthers JK, Jayachandran K, Moorman TB (1998) Biodegradation of atrazine by Agrobacterium radiobacter J14a and use of this strain in bioremediation of contaminated soil. Appl Environ Microbiol 64:3368–3375. https://doi.org/10.1128/aem.64.9.3368-3375.1998
Sun J, Ma X, Wang W, Zhang J, Zhang H, Wang YJ, Feng J (2019) The adsorption behavior of atrazine in common soils in Northeast China. Bull Environ Contam Toxicol 103:316–322. https://doi.org/10.1007/s00128-019-02671-5
Süsse H, Müller H (1996) Pesticide analysis by micellar electrokinetic capillary chromatography. J Chromatogr A 730(1–2):337–343. https://doi.org/10.1016/0021-9673(95)01038-6
USDA (2014) Keys to soil taxonomy. Soil Conserv Serv. https://doi.org/10.1109/TIP.2005.854494
USDA NRCS (1999) United States Department of Agriculture Natural Resources Conservation Service. Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. USDA Nat Resour Conserv Serv
Vega RC, Camacho AGR, Sampieri A, Flores VT, Moreno RDP, Gómez SES (2021) Movilidad de atrazina en dos tipos de suelo en el estado de Puebla. Rev Mex Ciencias Agrícolas 12:291–304. https://doi.org/10.29312/remexca.v12i2.2425
Vryzas Z, Papadakis EN, Vassiliou G, Papadopoulou-Mourkidou E (2012) Occurrence of pesticides in transboundary aquifers of North-eastern Greece. Sci Total Environ 441:41–48. https://doi.org/10.1016/j.scitotenv.2012.09.074
Vryzas Z, Papadopoulou-Mourkidou E, Soulios G, Prodromou K (2007) Kinetics and adsorption of metolachlor and atrazine and the conversion products (deethylatrazine, deisopropylatrazine, hydroxyatrazine) in the soil profile of a river basin. Eur J Soil Sci 58:1186–1199. https://doi.org/10.1111/j.1365-2389.2007.00913.x
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38. https://doi.org/10.1097/00010694-193401000-00003
Wang Q, Xie S, Hu R (2013) Bioaugmentation with Arthrobacter sp. strain DAT1 for remediation of heavily atrazine-contaminated soil. Int Biodeterior Biodegrad 77:63–67. https://doi.org/10.1016/j.ibiod.2012.11.003
Yang Y, Cao H, Peng P, Bo H (2014) Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J Hazard Mater 279:444–451. https://doi.org/10.1016/j.jhazmat.2014.07.035
Yue L, Ge CJ, Feng D, Yu H, Deng H, Fu B (2017) Adsorption–desorption behavior of atrazine on agricultural soils in China. J Environ Sci (china) 57:180–189. https://doi.org/10.1016/j.jes.2016.11.002
Zhang Y, Ge S, Jiang M, Jiang Z, Wang Z, Ma B (2014) Combined bioremediation of atrazine-contaminated soil by Pennisetum and Arthrobacter sp. strain DNS10. Environ Sci Pollut Res 21:6234–6238. https://doi.org/10.1007/s11356-013-2410-6
Zhou X, Wang Q, Wang Z, Xie S (2013) Nitrogen impacts on atrazine-degrading Arthrobacter strain and bacterial community structure in soil microcosms. Environ Sci Pollut Res 20:2484–2491. https://doi.org/10.1007/s11356-012-1168-6
Zhu J, Zhao Y, Fu L, Liu Z, Li X, Meng Z (2020) Application of a simazine degrading bacterium, Arthrobacter ureafaciens XMJZ01 for bioremediation of simazine pollution. Water Environ J 0:1-12. https://doi.org/10.1111/wej.12560
Zucconi F, Monaco A, Forte M (1984) Phytotoxins during the stabilization of organic matter. Composting of agricultural and other wastes. Elsevier, London, pp 73–86
Zucconi F, Pera A, Forte M, De Bertoldi M (1981) Evaluating toxicity of immature compost. Biocycle 22:54–57
Acknowledgements
NU holds a doctoral grant from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). This work was carried out with financial support from FONCyT (grant PICT Start up 0018/2016) and Instituto de Investigación, Universidad Nacional de Villa María. EA is a member of CONICET.
Funding
This research was financed by FONCyT (grant PICT Start up 0018/2016) and by the Instituto de Investigación, Universidad Nacional de Villa María (grant UNVM resolution N° 614/2018).
Author information
Authors and Affiliations
Contributions
NU, RB, VM, EA, and CM conceived and designed the research. NU, RB, and VM contributed with material preparation, data collection, and analysis. NU and EA wrote the draft manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Maria Manuela Abreu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Urseler, N., Bachetti, R., Morgante, V. et al. Atrazine behavior in an agricultural soil: adsorption–desorption, leaching, and bioaugmentation with Arthrobacter sp. strain AAC22. J Soils Sediments 22, 93–108 (2022). https://doi.org/10.1007/s11368-021-03045-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-021-03045-3