Abstract
Purpose
Adsorption and desorption are important processes that influence the transport, transformation, and bioavailability of atrazine in soils. Equilibrium batch experiments were carried out to investigate the adsorption–desorption characteristics of atrazine. The objectives of this study were to (1) determine and quantify the main soil parameters governing atrazine adsorption and desorption phenomena; (2) find the correlativity between the identified soil parameters; and (3) investigate the universal desorption hysteresis traits.
Materials and methods
Fifteen soils with contrasting physico-chemical characteristics were collected from 11 provinces in eastern China. The equilibrium time was 24 h both for adsorption and desorption experiments. Atrazine was detected by Waters 2695/UV HPLC.
Results and discussion
Adsorption isotherms of atrazine could be well described by the Freundlich equation (r ≥ 0.994, p < 0.01). The total organic carbon (TOC) was the first independent variable that described 53.0 % of the total variability of K f, followed by the pH (9.9 %), and the clay (4.0 %) and silt (1.2 %) contents, separately; while the primary soil properties that affect desorption parameters included the TOC, pH, free Fe2O3 (Fed) and the sand content, with the biggest contribution achieved by the TOC (ranged from 48.5–78.1 %). The results showed that when the content ratio of clay to TOC (RCO) was less than 40, the atrazine adsorption was largely influenced by the organic matrix, while when the RCO was greater than 40, they were vital affected by the clay content.
Conclusions
Adsorption–desorption isotherms of atrazine in soils were nonlinear. The content of TOC, clay, and iron oxides, as well as the pH value were the key soil parameters affecting the adsorption–desorption of atrazine in soil, among which the RCO especially exhibited relevance. Additionally, the desorption hysteresis existed for atrazine retention in all 15 tested soils, and the hysteretic effect enhanced with the increasing time for desorption. This would be ascribed to the heterogeneity physical–chemical properties of these soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine) is one of the most widely used herbicides for broadleaf weed and certain annual grasses controls and is popular because of its effectiveness and low cost (Huang et al. 2003; Gao and Jiang 2010). Since the 1980s, atrazine has been intensively applied in China and the application amount has increased rapidly (about 20 % per year); now, it is one of the 52 kinds of environmental priority control pesticides in China. Due to its relatively high mobility and long half-life (28–440 days) in soil, atrazine is often detected in surface and ground waters worldwide (Sherchan and Bachoon 2011), which has toxicity to aquatic organisms (Hincapie et al. 2005; Botelho et al. 2011), frogs (Hayes et al. 2002), and leads to human cancer, it could even interfere with the production of normal human hormones, which is just inferior to DDT (Rayner and Fenton 2011).
Adsorption–desorption behavior is one of the key processes affecting the fate of agrochemicals in sediment–water environment (Clausen et al. 2001; Mudhoo and Garg 2011). The role of key soil properties, such as organic carbon content, clay content, pH, and cation exchange capacity, as a determinant of pesticide adsorption–desorption behaviors has attracted much interest over the past two decades (Jenks et al. 1998; Spark and Swift 2002; Deng et al. 2007; Flores et al. 2009; Sun et al. 2010). For example, Jenks et al. (1998) constructed the adsorption and desorption isotherms for atrazine retention in a Hastings silty clay loam with six soil depths, and found that atrazine adsorption was highly correlated with organic matter content and soil pH, and desorption hysteresis was also a reflection of the relative organic matter content and pH level in each soil depth. Flores et al. (2009) researched simazine adsorption behavior by using two representative soils (a loam soil with 8.5 % organic matter content and a clay loam soil with 3.5 % organic matter content) from agricultural sites of central Chile. The results and the potentiometric profiles of both soils, suggested that simazine adsorption in loam soil was mainly governed by sinazine–organic matter interactions and in clay loam soil by sinazine–clay interactions. While the work cited above for the investigation of the correlativity among divers soil properties for controlling adsorption–desorption behaviors of pesticides were collected mainly from relatively small geographical scale regions. The information directly available for various types of natural soils from a large geographical scale region is still limited to date. Furthermore, several studies have pointed out the occurrence of hysteresis in the adsorption–desorption reactions in soils (Lesan and Bhandari 2003; He et al. 2006a, b; Anagu et al. 2011); but whether it was universal for diverse soils is not completely understood, and accurate quantification of the effects of soil properties on adsorption–desorption of atrazine in soils still seems to be lacking.
In this study, 15 representative soils, with contrasting physico-chemical properties that belong to different types of soil classification, were collected from 11 provinces across about 85 % of the total area of entire eastern China, ranging from southern latitude of 19°N to northern latitude of 47°N. Batch adsorption and desorption experiments were carried out using the standard batch equilibration method. The goal of this work was to (1) determine and quantify the main soil parameters governing atrazine adsorption and desorption phenomena; (2) find the correlativity between the identified soil parameters; and (3) investigate the universal desorption hysteresis traits.
2 Materials and methods
2.1 Chemicals and soils
Acetonitrile (HPLC grade) was purchased from Merck Co., Germany. Atrazine (with purity greater than 99.5 %) was obtained from National Standard Material Standard Sample Information Center (China). Stock solution of atrazine was prepared in acetonitrile at 1000 mg L−1, and stored in the dark at 4 °C. Working standard solutions were prepared by appropriate dilutions in 0.02 mol L−1 KCl solution (0.02 % NaN3). A Waters 2695 series HPLC with UV/Vis spectrophotometry detector was used for the quantification of atrazine.
Fifteen uncultivated soils were sampled in the surface layers (0–20 cm) in 11 provinces from south (19°N) to north (47°N) of eastern China. They were selected on the basis of contrasting taxonomy, sampling sites, and different basic properties (Table 1). Soil types were classified as Mollisols, Alfisols, Inceptisols, Ultisols, and Oxisols from north to south of China according to the USA classification. Soil samples were air-dried, sieved to 2 and 0.25 mm, stored in the dark at room temperature (20 ± 2 °C) and sheltered from humidity. No residual atrazine was detected in these soil samples.
2.2 Adsorption–desorption experiments
Adsorption experiments were carried out using the standard batch equilibration method. A mass of 2.5 g of air-dried (0.25 mm sieved) soils were mixed with 5 mL varied concentrations (C 0) of atrazine (0.1, 0.2, 0.4, 0.8, 1.0, 2.0, 4.0, 8.0, 16.0, 20.0, and 30.0 mg L−1, respectively) in 50-mL glass centrifugal tubes with a background electrolytic solution containing 0.02 mol L−1 KCl (0.02 % NaN3 was added to minimize bacterial growth and biodegradation of atrazine during batch experiments). One series of vials without soil as control was also monitored. Since the apparent equilibrium could be reached by shaking within 24 h as testified in our preliminary kinetic experiment, the tubes (with two replicates and a blank for each concentration) were shaken for 24 h with a rotary agitator (180 rpm) at 28 ± 2 °C in dark. The soil slurry was thereafter centrifuged at 3,500 rpm for 20 min using a centrifuge (Legend Plus, Thermo). Supernatant solution was sampled from each tube for atrazine analysis by Waters 2695/UV HPLC, under the optimized conditions as follows: acetonitrile/water = 60:40 as mobile phase, 1 ml/min of flow rate, 30 °C column temperature, and 221 nm detected wavelength.
Desorption experiments were conducted in sequential decant–refill steps immediately after adsorption procedures. At the end of adsorption experiments, solids were separated from the aqueous solution by centrifugation at 3,500 rpm for 20 min and liquid of supernatant was withdrawn from each tube. The tube was weighed before and after withdrawal to calculate the amount of residue. Then, about 5 mL of background solution was added into each tube to maintain the same ionic strength as that of the background solution. The new atrazine mixtures were then desorbed for a new round shaking for 24 h under the same condition as adsorption, and subsequently determined by HPLC. In total, three rounds of successive desorption procedures were carried out. Desorbed atrazine was calculated at each desorption stage. The amount of atrazine remaining on sorbents at each desorption stage was calculated as the difference between the initial adsorbed amount and the final desorbed amount. Blank samples were also processed at the same time. Atrazine degradation and adsorption loss on the centrifuge tubes was found to be negligible (<1 %).
2.3 Statistical analyses
The mass of atrazine adsorbed onto the soils was detected by measuring the mass of atrazine that disappeared from the solution phase at the end of adsorption experiment. The fraction of atrazine adsorbed on the soils was calculated by the equation:
where Q e is the quantity of atrazine adsorbed per unit weight of soil (in milligrams per kilograms); C 0 is the initial concentration of atrazine (in milligrams per liter); C e is the equilibrium concentration of atrazine at the end of the adsorption experiment (in milligrams per liter); V is the volume of solution (in liters); and M is the mass of the soil (in kilograms).
The fraction of atrazine desorbed from soils every cycle was calculated by the equation:
where Q ej is the j time’s quantity of atrazine desorbed from soils (in milligrams per kilogram); C j is the j time’s solution phase atrazine concentration of the previous equilibrium experiment (in milligrams per liter); V j is the j time’s volume of solution; C j−1 is the j−1 time’s solution phase atrazine concentration of the previous equilibrium experiment (in milligrams per liter); V j−1 and V j−2 are the j−1 and j−2 times’ volume of the soil slurry with tube.
The adsorption and desorption data are analyzed using the Freundlich equation:
where Q e is the solid-phase equilibrium concentration of atrazine (in milligrams per kilogram); C e is the liquid-phase equilibrium concentration of atrazine (in milligrams per liter); K f is the Freundlich unit capacity factor; N is the Freundlich exponent.
The content ratio of clay to total organic carbon (TOC; RCO) was calculated by the equation:
Cox et al. (1997) proposed desorption hysteresis coefficients (H) based on the ratio of desorption and adsorption isotherm parameters:
where N a and N d are the Freundlich parameter N for adsorption and desorption, respectively.
Ma et al. (1993) reported hysteresis as the maximum difference between adsorption and desorption isotherms:
where w is the hysteresis coefficient.
Although H and w are easy to use, there is great difference between practical adsorption and desorption isotherms. Zhu and Selim (2000) defined a new hysteresis parameter λ, based on the difference in the area between the adsorption and desorption isotherms:
3 Results
3.1 Adsorption and desorption isotherms
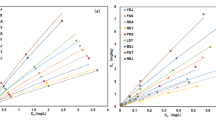
Adsorption isotherms and Freundlich parameters of atrazine in the 15 soils were presented in Fig. 1 and Table 2. The results showed that adsorption amount of atrazine in the 15 soils increased with increasing equilibrium concentrations (Fig. 1). In addition, Freundlich parameters (K f and N a) were significant, and the correlation coefficients were highly significant at 0.01 probability level (Table 2). The K f values ranged from 0.20 to 5.74 (29-fold to 0.2), and a grading in soil K f values has been observed in the following order: soil 1 > soil 10 > soil 13 > soil 9 > soil 8 ≈ soil 6 > soil 11 ≈ soil 14 ≈ soil 12 ≈ soil 7 ≈ soil 2 > soil 3 > soil 5 ≈ soil 4 > soil 15.
Since all desorption isotherms were similar in shape, only the isotherm for soil 1 was shown graphically as example (Fig. 2). Apparently, there are significant differences among adsorption and desorption isotherms, and the desorption amounts of atrazine from the tested soils increased with increasing equilibrium concentrations. Desorption isotherms also conformed well to the Freundlich equation for all the tested soils (r > 0.945, p < 0.05) (Table 2). The results showed that the average desorption rate of atrazine after three successive desorption were in the order: soil 5 > soil 15 > soil 4 > soil 2 ≈ soil 3 ≈ soil 13 > soil 14 > soil 6 ≈ soil 1 > soil 7 ≈ soil 12 > soil 11 ≈ soil 10 ≈ soil 8 > soil 9. It obviously revealed that the soils with high K f values (e.g., soils 1, 10, 9, and 8) had lower desorption rate and adsorbed more atrazine than those with low K f values (e.g., soils 15, 4, and 5).
3.2 Relation between atrazine adsorption–desorption and soil properties
Bivariate correlation analysis between soil properties and atrazine adsorption–desorption parameters (K f, K fd1−K fd3) are showed in Table 3. There was a positive significant relationship between the K f and the TOC content (r = 0.728, p < 0.01), and a negative relationship between the K f and pH (r = −0.577, p < 0.05). In addition, there were also significant relationship between desorption parameters and the TOC content (K fd1: r = 0.355, p < 0.05; K fd2: r = 0.522, p < 0.05; K fd3: r = 0.781, p < 0.01). A simple correlation analysis was also analyzed among soil properties (Table 4), which showed that the TOC content was positively correlated with the amorphous Fe2O3 (Fe0) content, and the pH was negative correlated with the Fed content, while the Fed content was positively correlated with the clay content and negative correlated with the sand content. Therefore, it is difficult to assess the exact relationship among TOC, pH, and K f if only by bivariate correlation analysis.
According to the principle of statistics, a dependent variable which is influenced by many factors cannot be explained by simple correlation analysis. Stepwise regression analysis were thus derived in order to further correlate the various soil properties with the adsorption–desorption parameters for atrazine, and leads to a linear predictive model for K f and K fd1 -K fd3, respectively (Table 5). According to Table 5, model (D) is the best regression way to describing the relationship between K f and soil properties, which had significant coefficient (p < 0.05) and described nearly 68.1 % of total variability of the K f. The TOC was the first independent variable that describe 53.0 % of total variability of the K f (model A), and followed by the pH (9.9 %), and the content of clay (4.0 %) and silt (1.2 %), respectively. Table 5 also showed that the tendency of relationship between atrazine desorption parameters and soil properties was similar in the three sequential desorption processes. The primary soil properties that affect desorption parameters were the content of TOC, Fed, and sand, as well as the pH value, with the biggest contribution achieved by the TOC (ranging from 48.5–78.1 %). The best regression model in the three desorption were model (G), model (K) and model (N), and the contribute rates to capacity of desorption were 75.1, 75.2, and 86.4 %, respectively.
3.3 Correlation of adsorption with the content ratio of clay to TOC (RCO)
Scatter diagram was used to show simple regression between the RCO values and Freundlich constants (K f) for 15 soil samples used in the present study and 21 soil samples reported by others (Spongberg and Lou 2000; Huang et al. 2003; Amaud et al. 2005; Huo 2006; Gao and Jiang 2010) (Table 6), as shown in Fig. 3. According to Fig. 3, the 15 soils used in the present study had the same trend as the 21 soil samples reported by others. Note that the K f value was RCO dependent. In most cases, the K f value sharply decreased with the increase of the RCO values when RCO < 40, and then approached relative unity in the upper RCO values range until further decrease of the K f value was not observed when RCO > 40. Therefore, the adsorption characteristics of atrazine by the soils could be separated into two groups according to the values of RCO (Fig. 4). The first group of soils with the RCO < 40 had the higher K f values, and their adsorption capability had closer positive correlation with the TOC content (r = 0.315, p < 0.05), but no relationship with the clay content (r = 0.014, p = 0.942). The second group of the soils with the RCO > 40, nearly 17 % of total tested samples, had lower K f values, and their adsorption capability had closer positive correlation with the clay content (r = 0.359, p = 0.058) than the TOC content (r = 0.009, p = 0.987).
3.4 Hysteretic effect of atrazine desorption from soils
From the current results in Table 2, adsorption parameter (N a) is bigger than desorption parameters (N d1, N d2, and N d3), and discrepancy between adsorption and desorption isotherms had been well documented in Fig. 2. According to Eqs. (5), (6), (7), if N d/N a = 1, then H = 100, ω = 0, and λ = 0. The desorption rate and the adsorption rate would be similar. There was thus no hysteresis; if N d/N a < 1, then H < 100, ω > 0, and λ > 0. The desorption rate would be less than the adsorption rate. The positive hysteresis would thus be characterized; if N d/N a > 1, then H > 100, ω < 0, and λ < 0. It would be in the case of negative hysteresis (O’Connor et al. 1980; Barriuso et al. 1994). The results from Table 7 showed that the hysteretic phenomena existed during all desorption procedures, and all of them were indicative of positive hysteresis (N d/N a < 1, H < 100, ω > 0, and λ > 0). In addition, Table 7 also revealed that desorption parameters (N d) and hysteresis index (H) increased with the increasing times of desorption circles, indicating that desorption hysteresis effect and desorption activation energy enhanced with the increasing times of sequential desorption procedures, and desorption of atrazine became increasingly difficult.
4 Discussion
4.1 Adsorption
The Freundlich equation was developed mainly to allow for an empirical account of the variation in adsorption energy with concentration of an adsorbate on an energetically heterogeneous surface. The adsorption capacity of atrazine on different soils has been estimated by comparing Freundlich parameters K f in previous studies (Hamaker and Thompson 1972; Si et al. 2006). The results showed that the higher the K f value, the stronger adsorption of atrazine in soil. According to Table 1, adsorption isotherms of atrazine could also be well described by Freundlich model, and the largest K f value was observed in the Argiustoll (the silty clay loam with high organic matter content), while the minimal K f value was found in Haplustalf (the silt loam with low organic matter content). The 15 soils could be divided into three groups: the first group of soil 1 and soil 10 with the strongest adsorption capacity, the second group of soils 13, 9, 8, 6, 11, 14, 12, 7 and 2 with a moderate adsorption capacity, and the third group of soils 3, 5, 4 and 15 with the weakest adsorption capacity (Fig. 1) The values of parameter N a, which can be used as an index of nonlinearity, were not equal to 1 (Table 2), suggesting a significant nonlinearity for atrazine adsorption isotherm in the 15 soils. Clay and Koskinen (1990a) also got similar results on atrazine adsorption.
4.2 Desorption
Desorption is an inverse process of adsorption and a key process affecting pesticide sequestration behaviors in soils. Desorption controls the predisposition of a pesticide to be degraded and/or leached at different times (Dur et al. 1998). It is important to assess desorption since it may reflect some of the interactions involved between the chemical and the various soils (Amaud et al. 2005). The current results showed that desorption isotherms could also be well described by Freundlich model for the 15 soils (r ≥ 0.945, p < 0.05) (Table 2). The order of the average desorption rate of atrazine is obviously different from that of adsorption. For example, soils 1, 10, 9, and 8 had higher K f values but lower desorption rate, while soils 15, 4, and 5 had lower K f values but higher desorption rate. These results indicate that in soils with low K f values, less atrazine will be adsorbed, and the adsorbed atrazine would also be less strongly held, resulting in a greater tendency to leach.
Hysteresis is one of several manifestations of non-ideal behavior of hydrophobic organic chemicals in soils (Huang et al. 1998; Deng et al. 2005). The adsorption of atrazine in soil is irreversible when the N a is greater than N d (Pusino et al. 2004), and the ratio of Freundlich exponents (N d/N a) gives an indication of adsorption–desorption hysteresis (Palvostathis and Mathavan 1992; Carrizosa et al. 2001). The major finding of the current result suggested that some fraction of adsorbed atrazine may be irreversibly bound to all of the 15 soils, and indicated clearly the considerable positive hysteresis (the N d /N a values ranged from 0.13–0.88; Table 2, Fig. 2 and Table 7). Sorption–desorption hysteresis has been explained variously as the result of nonattainment of equilibrium (Huang and Weber 1997; Barriuso et al. 2004), degradation (Armstrong and Chesters 1968; Schiavon 1988), irreversible binding to soil constituents such as organic matrices or inorganic structures (Bhandari et al., 1996; Altfelder et al. 2000; Oren and Chefetz 2005; Liao and Xie 2007), or any combination of these. In the present study, degradation was not measured but nonattainment of equilibrium (a relative equilibration time of 24 h was considered the most convenient) may result in hysteresis. Moreover, we observed that the soils with contrasting properties resulted in varying degrees of hysteresis effect (Tables 1 and 7). This may also suggest that the heterogeneity of soil properties have contributed to atrazine hysteresis phenomenon. In a similar study, Weber et al. (1998) found that particle-scale soil organic matter heterogeneity significantly affected the extent of hysteresis of hydrophobic organic contaminants in soils and sediments. Clay and Koskinen (1990b) observed more atrazine desorption from a Plano soil at pH 6.1 than at pH 4.5. Less desorption at the lower soil pH was attributed to greater protonation and ionic bonding of atrazine.
4.3 Primary parameters affecting adsorption–desorption behavior
Several studies have suggested that various soil parameters, including the TOC content, pH, clay silicates, and aluminum and iron oxides, should affect the adsorption and desorption behavior of atrazine and other s-triazine herbicides by soil samples (Stevenson 1972; Huang et al. 1984). It is reported that soil organic matter contains a variety of functional groups such as hydrophobic, hydrophilic, and free radicals that can strongly entrap atrazine (Hamaker and Thompson 1972). Borggaard and Streibig (1988) also showed that atrazine adsorption is highly correlated with total organic matter, especially with pyrophosphate-extractable organic matter. He et al. (2006b) founded that the pH was an important factor affecting adsorption behavior of pentachlorophenol in soils. When the pH increases, the soil surface tends to be negatively charged, and the organic molecules also tend to be ionized and negatively charged. This is due to the repulsion of same-sex charge, which will be detrimental to the adsorption of organic molecules on the soil surface (Wang et al. 1999). Characteristics of organic matter in different soil particle size composition are very different and may indirectly affect the adsorption–desorption behavior of organic molecules (Chen and Chiu 2003; Chefetz et al. 2004). However, accurate information about the relative importance of the various soil parameters to these behaviors still seems to be lacking. In our study, we found that the TOC was the most important factor for affecting atrazine adsorption behavior (accounted for 53.0 % of the variation in K f value), then the pH (9.9 %), and the contents of clay (4.0 %), and silt (1.2 %), separately. The TOC was also the first parameters affecting atrazine desorption behavior as it could describe the variation of K fd value ranging from 48.5–78.1 %, and followed by the pH (ranged from 3.8–22.4 %), the Fed content (ranged from 1.1–12.0 %), and the sand content (ranged from 4.2–7.2 %).
As indicated by the determination coefficient (R 2), about 31.9 % of the total variability in the K f and about 13.6–24.9 % of the total variability in the K fd, could not be described by the best stepwise regression models. This indicated that even with the combination of soil properties of the contents of TOC, clay, silt, Fed, and sand, as well as the pH value, adsorption and desorption processes of atrazine in soils could not be assessed exactly. It was reported that the amorphous hydroxyl-Fe and -Al components had high capacity for the retention of the phenolic acids, and provided adsorption sites for atrazine (Huang et al. 1977, 1984). The non-crystalline to poorly crystalline Fe and Al components are often qualified by the term of “active”, because they have high specific surface and proton donor functional groups (Kwong and Huang 1979; Huang and Wang 1997; Huang et al. 2002). In this study, a positive relationship of the content between Feo and TOC was observed in the tested soils (r = 0.627, N = 15, p < 0.05) (Table 4). Therefore, the amorphous minerals were likely interacted with the organic matter in soils, and thereby could affect the interfacial behavior of atrazine in soils such as adsorption and desorption.
Our investigation indicated that 53.0 % of the total relevance for K f values was correlated with the TOC content, and only very small contribution was from the clay content (Table 5). However, the previous study of Wang et al. (1999) verified that K f had better correlation with the RCO than with the TOC only for the adsorption of acetanilide herbicides. More recently, our previous studies have also testified that the RCO could act as a good parameter to predict the adsorption of butachlor in soils (Liu et al. 2008; He et al. 2011). In this study, by investigation on natural different type soils that showed contrasting characteristics and were collected from large geographical scale region that make up about 85 % of the total area of entire eastern China, the results also indicated that the RCO value would be helpful to understand the atrazine adsorption mechanism in soils. Moreover, Mersie and Seybold (1996) suggested that desorption capability of atrazine in soil could reflect the stability of atrazine; the higher the stability, the better practice restoration effect. Therefore, the RCO may be used to direct the application process of atrazine to achieve adequate weed control and the environmental protection and remediation. That is, in the soils with the RCO < 40, atrazine might have a higher adsorption capability. The application rates should thus be adjusted upward, and the bioavailability and mobility of atrazine in these soils should be considered; while in the soils with the RCO > 40, atrazine might have a lower adsorption and desorption capacity and apparently higher bioactivity, leachability, and volatility. The application rates of atrazine should thus be adjusted downward for preventing the environmental risks.
5 Conclusions
Based on investigation of contrasting natural soils from large geographical scale region that make up about 85 % of the total area of entire eastern China, this study indicated that the adsorption and desorption data were of major importance for understanding the overall fate of atrazine in soils. Adsorption and desorption isotherms of atrazine in soils were nonlinear, and could be well described by the Freundlich equation. The TOC content, pH, and inorganic fraction (such as clay and amorphous sesquioxides) played an important role affecting both adsorption and desorption processes of atrazine in soils, among which the TOC content did a vital contribution. The content RCO was observed as a useful parameter for predicting the atrazine behavior in soils. For soils with high organic matter content (RCO < 40), the strong binding between atrazine molecules and soil organic compounds can prevent the atrazine from release, thus reducing its bioavailability; for soils with low organic matter content (RCO > 40), high bioactivity and leachability of atrazine deserved to be taken into account for potential environmental risk control. Positive hysteresis was verified for all of the tested soils. The hysteretic effects enhanced with the increasing times of sequential desorption and atrazine desorption became increasingly difficult.
References
Altfelder S, Streck T, Richter J (2000) Nonsingular sorption of organic compounds in soil: the role of slow kinetics. J Environ Qual 29:917–925
Amaud B, Richard C, Michel S (2005) A comparison of five pesticides adsorption and desorption processes in thirteen contrasting field soils. Chemosphere 61:668–676
Anagu I, Ingwersen J, Drori Y, Chefetz B, Streck T (2011) Modeling concentration-dependent sorption–desorption hysteresis of atrazine in a sandy loam soil. J Environ Qual 40:538–547
Armstrong DE, Chesters G (1968) Adsorption catalyzed chemical hydrolysis of atrazine. Environ Sci Technol 2:683–689
Barriuso E, Laird DA, Koskinen WC, Dowdy RH (1994) Atrazine desorption from smectites. Soil Sci Soc Am 58:1632–1638
Barriuso E, Koskinen WC, Sadowsky MJ (2004) Solvent extraction characterization of bioavailability of atrazine residues in soils. J Agric Food Chem 52:6552–6556
Bhandari A, Novak J, Berry D (1996) Binding of 4-monochlorophenol to soil. Environ Sci Technol 30(7):2305–2311
Borggaard OK, Streibig JC (1988) Atrazine adsorption by some soil samples in relation to their constituents. Acta Agric Scandinavica 38:293–301
Botelho RG, Santos JBD, Fernandes KM, Neves CA (2011) Effects of atrazine and picloram on grass carp: acute toxicity and histological assessment. Toxicol Environ Chem 94:121–127
Carrizosa MJ, Koskinen WC, Hermosin MC, Cornejo J (2001) Dicamba adsorption–desorption on organoclays. Appl Clay Sci 18:223–231
Chefetz B, Bilkis YI, Polubesove T (2004) Sorption–desorption behavior of triazine and phenylurea herbicides in Kishon river sediments. Water Research 38:4383–4394
Chen JS, Chiu CY (2003) Characterization of soil organic matter in different particle-size fractions in humid subalpine soils by CP/MAS 13C-NMR. Geoderma 117:129–141
Clausen L, Fabricius I, Madsen L (2001) Adsorption of pesticides onto quartz, calcite, kaolinite, and α-Alumina. J Environ Qual 30:846–857
Clay SA, Koskinen WC (1990a) Characterization of alachlor and atrazine desorption from soils. Weed Science 38:74–80
Clay SA, Koskinen WC (1990b) Adsorption and desorption of atrazine, hydroxyatrazine, and S-glutathione atrazine on two soils. Weed Science 38:262–266
Cox L, Koskinen WC, Yen PY (1997) Sorption–desorption of imidacloprid and its metabolites in soil. J Agric Food Chem 45:1468–1472
Deng JC, Jiang X, Wang F, Lu X, Yu GF, Yan DY, Bian YR (2005) Hysteretic characteristics of atrazine desorption from fluvo-aquic soil. Environ Sci 26(6):137–142
Deng JC, Jiang X, Lu X, Yu GF, Wang F, Zhang B (2007) Atrazine adsorption behavior on a fluvo-aquic soil as influenced by contact periods. Pedosphere 17(6):786–791
Dur JC, Gouy V, Calvet R, Belamie R, Chaplain V (1998) Influence of adsorption–desorption phenomena on pesticide runoff measured under controlled conditions. Comptes Rendus de I’Academie des Sciences-Series IIA-Earth Planetary Science 327(6):405–411
Flores C, Morgante V, Gonzalez M et al (2009) Adsorption studies of the herbicide simazine in agricultural soils of the Aconcagua Valley, central Chile. Chemosphere 74:1544–1549
Gao HJ, Jiang X (2010) Effect of inintial concentration on adsorption–desorption characteristics and desorption hysteresis of hexachlorobenzene in soils. Pedosphere 20(1):104–110
Hamaker JW, Thompson JM (1972) Adsorption of organic chemicals in the soil environment. Dedder, New York. pp 49–143
Hayes BH, Collins A, Lee M et al (2002) Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. PNAS 99(8):5476–5480
He Y, Xu JM, Wang HZ, Ma ZH, Chen JQ (2006a) Detailed sorption isotherms of pentachlorophenol on soils and its correlation with soil properties. Environ Res 101(3):362–372
He Y, Xu JM, Wang HZ, Zhang QC, Muhammad M (2006b) Potential contributions of soil minerals and organic matter to pentachlorophenol retention in soils. Chemosphere 65(3):497–505
He Y, Liu ZZ, Zhang J, Wang HZ, Shi JC, Xu JM (2011) Can assessing for potential contribution of soil organic and inorganic components for butachlor sorption be improved? J Environ Qual 40:1705–1713
Hincapie M, Maldonado MI, Oller I et al (2005) Solar photocatalytic degradation and detoxification of EU priority substances. Catal Today 101:203–210
Huang PM, Wang MK (1997) Formation chemistry and selected surface properties of iron oxides. Adv Geoecol 30:241–270
Huang WL, Weber WJ (1997) A distributed reactivity model for sorption by soils and sediments. 10. Relationships between desorption, hysteresis, and the chemical characteristics of organic domains. Environ Sci Technol 31(9):2562–2569
Huang PM, Wang TSC, Wang MK, Wu MH, Hsu NW (1977) Retention of phenolic acids by noncrystalline hydroxyl-aluminum and -iron compounds and clay minerals of soils. Soil Sci 123:213–219
Huang PM, Grover R, Mckercher RB (1984) Components and particle size fractions involved in atrazine adsorption by soils. Soil Sci 138:20–24
Huang W, Hong Y, Weber WJ (1998) Hysteresis in the sorption and desorption of hydrophobic organic contaminants by soils and sediments. 1. A comparative analysis of experimental protocols. J Contam Hydrol 31:129–148
Huang PM, Wang MK, Kampf N, Schulze DG (2002) Aluminum hydroxides. In: Dixon JB, Schulze DG (eds) Soil mineralogy with environmental applications. Soil Science Society of America, Madison, WI, pp 261–289
Huang G, Li Q, Zhang X (2003) Adsorption and desorption of atrazine by three soils. Environ Contam Toxicol 71:655–661
Huo LJ (2006) Experimental study on adsorption, degradation and leaching of atrazine and its metabolites in sandy loamy soil. Dissertation, China, pp 9–16
Jenks BM, Roeth FW, Martin AR (1998) Influence of surface and subsurface soil properties on atrazine sorption and degradation. Weed Science 46:132–138
Kwong NKKF, Huang PM (1979) Surface reactivity of aluminum hydroxides precipitated in the presence of low molecular weight organic acids. Soil Sci Soc Am J 43:1107–1113
Lesan HM, Bhandari A (2003) Atrazine sorption on surface soils: time-dependent phase distribution and apparent desorption hysteresis. Water Res 37(7):1644–1654
Liao M, Xie XM (2007) Adsorption of metsulfuron and bensulfuron on a cationic surfactant-modified paddy soil. Pedosphere 17(1):101–108
Liu ZZ, He Y, Xu JM, Huang P, Jilani G (2008) The ratio of clay content to total organic carbon content is a useful parameter to predict adsorption of the herbicide butachlor in soils. Environ Pollut 152:163–171
Ma LW, Southwick LM, Willis GH, Selim HM (1993) Hysteresis characteristic of atrazine adsorption–desorption by a Sharkey soil. Weed Science 41:627–633
Mersie W, Seybold C (1996) Adsorption and desorption of atrazine, deethylatrazine, deisopropylatrazine, and hydroxyatrazine on levy wetland soil. J Agr Food Chem 44:1925–1929
Mudhoo A, Garg VK (2011) Sorption, transport and transformation of atrazine in soils, minerals and composts: a review. Pedosphere 21(1):11–25
O’Connor GA, Wierenga PJ, Cheng HH, Doxtader KG (1980) Movement of 2,4,5-T through large soil columns. Soil Sci 130:157–162
Oren A, Chefetz B (2005) Sorption–desorption behavior of polycyclic aromatic hydrocarbons in upstream and downstream river sediments. Chemosphere 61:19–29
Palvostathis SG, Mathavan GN (1992) Desorption kinetic of selected volatile organic compounds from field contaminated soils. Environ Sci Technol 26:532–538
Pusino A, Pinna MV, Gessa C (2004) Azimsulfuron sorption–desorption on soil [J]. J Agr Food Chem 52:3462–3466
Rayner JL, Fenton SE (2011) Atrazine: an environmental endocrine disruptor that alters mammary gland development and tumor susceptibility. Environ Breast Cancer 167–183
Schiavon M (1988) Studies of the movement and the formation of bound residues of atrazine, of its chlorinated derivatives, and of hydroxyatrazine in soil using 14C ring-labeled compounds under outdoor conditions. Ecotox Environ Safe 15:55–61
Sherchan SP, Bachoon DS (2011) The presence of atrazine and atrazine-degrading bacteria in the residential, cattle farming, forested and golf course regions of Lake Oconee. J Appl Microbiol 111(2):293–299
Si YB, Zhang J, Wang SQ, Zhang LG, Zhou DM (2006) Influence of organic amendment on the adsorption and leaching of ethametsulfuron-methyl in acidic soils in China. Geoderma 130:66–76
Spark KM, Swift RS (2002) Effect of soil composition and dissolved organic matter on pesticide sorption. Sci Total Environ 298:147–161
Spongberg AL, Lou G (2000) Adsorption of atrazine and metolachlor in three soils from Blue Creek wetlands, Waterville. Ohio Sciences of Soils 5:1
Stevenson FJ (1972) Organic matter reactions involving herbicides in soil. J Environ Qual 1:333–343
Sun K, Gao B, Zhang ZY, Zhang GX, Zhao Y, Xing BS (2010) Sorption of atrazine and phenanthrene by organic matter fractions in soil and sediment. Environ Pollut 158:3520–3526
Wang QQ, Yang WC, Liu WP (1999) Adsorption of acetanilide herbicides on soils and its correlation with soil properties. Pestic Sci 55:1103–1108
Weber WJ, Huang WL, Hong Y (1998) Hysteresis in the sorption and desorption of hydrophobic organic contaminants by soils and sediments: 2. Effects of soil organic matter heterogeneity. J Contam Hydrol 31:149–165
Zhu HX, Selim HM (2000) Hysteresis behavior of metolachlor adsorption–desorption in soils. Soil Sci 165(8):632–645
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (40901113), the Natural Science Foundation of Guangdong Province (8451064001001121), the Project Supported by Zhejiang Provincial Natural Science Foundation of China (R5110079) and the Fundamental Research Funds for the Central Universities (2011FZA6020).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Bernd Markert
Rights and permissions
About this article
Cite this article
Huang, Y., Liu, Z., He, Y. et al. Quantifying effects of primary parameters on adsorption–desorption of atrazine in soils. J Soils Sediments 13, 82–93 (2013). https://doi.org/10.1007/s11368-012-0572-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-012-0572-3