Abstract
The objective of this study was to evaluate the adsorption capacity of atrazine and the effects of different environmental conditions such as temperature, pH, Ca2+ and biochar on the adsorption characteristics of atrazine in different types of soil using the intermittent adsorption method. The kinetic experiment showed that the adsorption of atrazine in albic, black and saline–alkaline soils reached equilibrium within 24 h. In the thermodynamics experiment, the Freundlich model effectively described the adsorption characteristics of atrazine in all three types of soil, indicating that the adsorption process forms multi-molecular layers. Lower soil pH conditions were more favorable for the absorption of atrazine. The addition of appropriate concentrations of Ca2+ or biochar could promote the adsorption of atrazine by the soil. Biochar could promote the fixation of atrazine in soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Atrazine is a persistent and widely used triazine-based organochlorine pesticide. It was usually applied to corn pre- and post-emergence most falling on the soil surface during the application process, causing soil pollution (Salazar-Ledesma et al. 2018; Brondi et al. 2011; Lizotte et al. 2017). Under high rates of atrazine applications, the soils adsorption capacity become saturated with atrazine adsorption, while the remaining un-adsorbed atrazine pollutes surface water and groundwater through surface runoff and leaching (Alsharekh et al. 2018). Adsorption behavior of atrazine in soils is not only the key factor to control the translocation, transformation and bioavailability of atrazine in soil, but also directly affects the pollution effect of atrazine in soils (Yang et al. 2018a, b; Kaur et al. 2017). Albic, black and saline–alkaline soils are common farmland soil types in northeast China. Albisol is a kind of clay soil with obvious acidic (pH value is 4–6) and lower organic matter content (Arias et al. 2005). Black soil is mostly neutral pH, characterized by an fertile humus layer from the accumulation of organic matter due to low decomposition rates (Liu et al. 2015). Saline–alkali soil is a strongly alkaline soil with low content of organic matter and their key properties are high sodium levels and alkaline conditions (Wu et al. 2013).
Studies on northeast Chineses farmlands soils indicate atrazine adsorption and distribution was influenced by differences in mineral types, pH and organic content (Jiang et al. 2016). The adsorption characteristics of atrazine in soils containing different mineral types were analyzed by Huang who found that clay minerals strongly influenced adsorption capacity of atrazine (Huang et al. 2014). However, comparative studies on the effects of pH and different soil types on the absorption of atrazine were rarely reported (Paszko 2012). Calcium ions have higher exchangeable alkali saturation in soil, and have stronger exchange desorption and effectiveness. The addition of calcium ions can reduce the solubility of atrazine and improve the adsorption capacity of atrazine in soils. Chemical modifiers such as calcium chloride and calcium humate were commonly used to alter excessive acidity or alkalinity of soil (Gao et al. 2015). Biochar usually used as soil improvement could reduce the bioavailability and leachability of organic pollutants in soils (Lu et al. 2015). We identified few literature exists on the influence of pH, Ca2+ and biochar on atrazine adsorption in common Chinese soils. Therefore, the main purpose of our study is to explore the adsorption behavior of atrazine in white mud, black soil and saline–alkali soil, and to analyze the influence of external factors such as pH, Ca2+ and biochar on atrazine adsorption.
Materials and Methods
Analytical-grade atrazine with a purity of 99.9% was purchased from Dr. Ehenstofer, GmbH (Germany). Calcium chloride, hydrogen chloride and sodium hydroxide were all of analytical grade. Biochar was pyrolyzed from corn straw using a muffle furnace (Type sx2-15-10 Jiangsu Zhengfei electric furnace factory). The soil was collected from corn fields in Yongji county, Gongzhuling city and Daan county of Jilin province. Top-soils were sampled from a depth of 0–20 cm and the basic soil properties are summarized in Table 1.
The actual concentration of atrazine was measured using a UV detector and a C18 column liquid chromatograph (ZORBAX Eclipse xdb-c18150 mm × 4.6 mm). The mobile phase was composed of methanol and deionized water (60:40, v/v) at a flow rate of 1.0 mL min−1. The column temperature was set at 30°C, with a UV detection wavelength of 222 nm and specific retention time of 5.6 min (Deng et al. 2014; Qin et al. 2019). The minimum detection limit for atrazine was 0.3 ng.
The time required to balance liquid and solid matrices was measured by intermittent experiments. Three soil samples 2 g were placed in a 50 mL polypropylene centrifuge tube, with atrazine (5 mg L−1) added at volume ratio of 1:5. Adsorption experiments were performed at room temperature (25°C) (Deng et al. 2014). To ensure the stability of ionic strength, the background solution was 0.01 mol L−1 calcium chloride, at pH 6.0. The contact times assessed between soil and solution were 10, 20, 30, 60, 120, 240, 360, 480, 720 and 1440 min, respectively. The mixtures were centrifuged for 10 min at 10,000 r min−1. The supernatant passes through a 0.45 µm filter. Then the concentration of atrazine in the supernatant was determined. In the isothermal adsorption test, atrazine solution at concentrations of 0, 1, 2, 5, 10, 15, 20, 25 and 30 mg L−1, was added to the centrifuge tube. Tubes were shaken at 25°C for 24 h, pH 6.0 and then centrifuged for 10 min at 10,000 r min−1. The supernatant was filtered through a 0.45 µm membrane filter, prior to the determination of atrazine concentrations. Repeat isothermal adsorption experiments were performed at temperatures of 15°C, 25°C and 35°C (Xu et al. 2016). The pH values of atrazine solutions were adjusted to 3.0, 5.0, 7.0, 9.0 and 11.0 with 1 mol L−1 HCl and NaOH. The atrazine concentration was set at 5 mg L−1. Atrazine solutions with different concentrations of Ca2+ background solution were prepared with CaCl2, at concentrations of 0.1, 0.2, 0.4, 0.5 and 0.8 mol L−1, respectively. Test the concentration of 10 mL of atrazine solution in centrifuge tub, which were centrifuged at 25°C for 10 min 10,000 r/min. The effects of different biochar concentrations were assessed on the adsorption of atrazine in the soil, with the addition of biochar to soils at levels of 0.05%, 0.25%, 0.5%, 0.75% and 1%. Mixtures were placed in centrifuge tube and mixed thoroughly by glass rod. The atrazine concentration was set at 5 mg L−1, temperature was 25°C. All tests were performed in triplicate.
In order to describe the adsorption characteristics, the quasi-second-order kinetic model (1) and Elovich model (2) were used to fit the dynamic data for atrazine in the three soil types:
where qt (mg/g) and qe (mg/g) are the amount of atrazine adsorbed at a specific time t (min) and equilibrium conditions, respectively; k is the adsorption rate constant of the quasi-second-order kinetic equation; a is the constant related to the initial velocity of the adsorption reaction; b is the constant associated with the adsorption activation energy.
Langmuir (3) and Freundlich (4) models were used to fit the adsorption isotherm data:
where, qe (mg kg−1) is the amount of atrazine adsorbed by a mass of soil, Ce (mg L−1) is the equilibrium concentration of atrazine; Qmax (mg kg−1) is the maximum adsorption amount of atrazine; KL (L mg−1) represents the adsorption affinity constant; KF represents the adsorption coefficient, where larger values represent a stronger binding ability between the adsorbent and substance; n is the adsorption strength constant.
Thermodynamic parameters of the adsorption process were obtained using the following equations:
where, KF is the Freundlich constant; T (K) as the absolute temperature; R is the gas constant (8.314 J mol−1 K−1); Δ S (kJ mol−1 K−1) is the standard entropy change; Δ H (kJ mol−1) is the standard enthalpy change; Δ G (kJ mol−1) is the Gibbs free energy; △H and △S were obtained from the slopes and intercepts of a linear regression of lnK, 1/T. All the data were fitted and analyzed using origin 8.5.
Results and Discussion
Figure 1 showed the adsorption kinetics of atrazine on the three types of soil. The adsorption rate was relatively fast for all three soils, from 0 to 60 min. At this time, the adsorption amount of albic, black and saline–alkaline soils were 9.45 mg kg−1, 7.05 and 3.37 mg kg−1 accounting for 95.9%, 93.7% and 94.8% of the total adsorption amount respectively. The increasing rate of adsorption gradually slowed between 60 and 480 min for all test soils. When the adsorption time reached 1440 min, the amount of atrazine adsorbed in the soil stabilized. Therefore, the equilibrium adsorption time was set as 24 h in this experiment. At this time, the capacity of atrazine adsorbed in albic, black and saline–alkaline soils were 9.77 mg kg−1, 7.52 and 3.45 mg kg−1, respectively. Adsorption kinetics can be used to characterize the rate and efficiency of solute adsorption under controlled conditions (Tang et al. 2012). The rapid adsorption process can be attributed to the adsorption of atrazine on the surface of minerals (Lladó et al. 2015). With increased contact time, the blank adsorption sites gradually decrease and the rate of adsorption reduces. Slow adsorption may be related to the gradual diffusion of organic compounds to soil micropores or highly cross-linked areas of soil organic matter covering the adsorption sites on internal mineral surfaces (Lupul et al. 2015). The adsorption process for atrazine in all three different soils could be well simulated by the quasi-second-order kinetic equation. It could be seen from Table 2 that the quasi-second-order kinetic equation coefficient r values ranged from 0.957 to 0.976, showing a better fit than the coefficient r values established using the Elovich model, which ranged from 0.885 to 0.931.
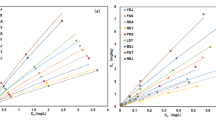
The adsorption isotherms of atrazine in three types of soil were shown in Fig. 2. The pattern of adsorption followed a similar trend in all three soil types. As the concentration of atrazine increased, the absorption of atrazine in the three soils gradually increased. When atrazine concentrations were 30 mg L−1 and the adsorption equilibrium was reached, the adsorption capacities of albic, black and saline–alkaline soils were 36.18 mg kg−1, 30.08 mg kg−1, 13.34 mg kg−1 respectively. The Freundlich model was often used to describe the adsorption of organic matter by soil and was mainly used to describe adsorption surfaces with non-uniform energy distribution, including the non-uniformity of adsorbent surface, adsorption energy and exponential distribution of adsorption point, among other factors. The Langmuir model was used to describe single-layer adsorption on a completely smooth and uniform matrix surface (Sahu et al. 2016). The correlation coefficients of adsorption isotherm fitted by the Freundlich and Langmuir models were shown in Table 3. The atrazine adsorption isotherm for all three soil types can be well fitted by both the Freundlich and the Langmuir model, with correlation coefficients all above 0.98, indicating an extremely significant degree of correlation (p < 0.01). The Freundlich model had a better fitting effect overall, which is consistent with the findings reported by Lin et al. (2017). The results showed that adsorption may occur on heterogeneous surfaces, in the form of multi-layer adsorption. According to the Freundlich model adsorption constant (Kf), the adsorption capacity of the three soil types to atrazine could be ranked in the order: albic soil > black soil > saline–alkali soil. All Kf values were less than 5. It indicated that atrazine is highly mobile in soils and could easily pollute groundwater. According to the Langmuir model adsorption constant (Qm), the maximum amount of atrazine adsorption by albic soil was 82.21 mg kg−1, black soil was 82.13 mg kg−1 and saline–alkaline soil was 26.43 mg kg−1, with this trend being consistent with the change in Kf.
Albic and black soils had a high organic matter contentand high clay mineral content as well as large surface area, which could provide more adsorption sites for atrazine (Huang et al. 2014). In the process of saline–alkali soil adsorption, due to its low organic matter content and strong alkalinity, atrazine interacts with inorganic minerals rather than organic matter. Therefore, the adsorbed amount of atrazine in saline–alkali soil is far less than that in albic and black soils, further proving that the adsorption mechanism of atrazine in soil may be related to factors such as soil pH and organic matter (Huang et al. 2018). The fitting of atrazine adsorption thermodynamics for all three types of soil are shown in Table 4. The Freundlich model exhibited better fitting, with decreased Qm values observed with rising temperatures from 15 to 35°C by 44.09%, 50.49% and 53.66% in albic soil, black soil and saline–alkaline soil, respectively. At low temperature, the adsorption coefficient was low (Kf = 0.657–2.970), indicating that a weak atrazine adsorption capacity in all three kinds of soil. To clarify the adsorption mechanisms, the previously discussed thermodynamic parameters mentioned were calculated, as shown in Table 5. In the process of adsorptionΔG was < 0, proving that the chemical reaction was spontaneous, with ΔG increasing with rising temperature. The value of ΔH being less than 0 showed that atrazine adsorption from water into the solid phase of all three kinds of soil was an exothermic process releasing a large amount of heat (Wei et al. 2018). In addition, the water solubility of atrazine in soil gradually increased with the increase in temperature (Jia et al. 2013). The higher the solubility of atrazine was, the stronger the interaction between atrazine and aqueous solutions there was, and the more difficult it was to separate the adsorbed substance from aqueous solution. With increased temperatures, the reaction is carried out in the direction of heat absorption and the adsorption capacity is weakened. The hydrogen bonding lifetimes of atrazine-water show decrease (Yang et al. 2018a, b). These results further proved that lower temperatures support a greater degree of atrazine adsorption on the soil surface.
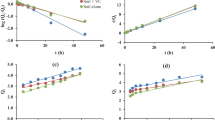
The effect of pH on atrazine adsorption by all three types of soil was presented in Fig. 3, showed that the amount of atrazine adsorbed gradually decreased with increasing pH values in the range of 3.0 to 11.0. The reason may be that atrazine is a weakly alkaline pesticide with a pKa of 1.68. When the pH is close to the pKa, atrazine exists partly in the form of a cation and partly in the form of non-ion. As the pH value of the solution decreases, proton exchange and ion exchange gradually increased, and at this time, the adsorption of atrazine by soil was dominated by ion adsorption (Sherif et al. 2014). When the pH value increased, the hydrogen bond breaks, the organic polymer is anionic, the soil cationic state decreases, and the protonation gradually weakens, and atrazine adsorption mechanism occurs at the molecular level (Lin et al. 2017). However, cationic adsorption is one of the main mechanisms of atrazine adsorption in soil (Wang et al. 2018). Therefore, atrazine has a lower adsorption capacity at a higher pH. Huang (2013) studied the adsorption behavior of atrazine in a large number of soils in eastern China, and found that the adsorption coefficient Kf of soil and atrazine increased with the increase of H+.
The influence of different background solution CaCl2 concentrations on the amount of atrazine adsorbed in the three soil types is shown in Fig. 4. With increased Ca2+ concentrations, the amount of atrazine adsorbed by all the three soils gradually increased. When the concentration of Ca2+was greater than 0.4 mol L−1, the adsorption capacity of atrazine in the soil was significantly higher. The reason may be that the increase of ion strength enhances the hydrophobicity of atrazine, weakens the electrostatic interaction between atrazine and soil, and promotes the absorption of atrazine in soil. In addition, Ca2+ may compete with atrazine for solvent molecules, which reduces the solubility of atrazine and produces salting out, and also increases the adsorption capacity of atrazine in soil. However, the increase effect was limited. When the concentration of Ca2+was greater than 0.5 mol L−1, possibly because Ca2+ and atrazine exhibited competitive adsorption, clay mineral oxides competed with soil organic matter for cation exchange adsorption, and the adsorption point was gradually saturated (Zhang et al. 2017).
The effects of addition of different amounts biochar to the three types of soil on atrazine adsorption are shown in Fig. 5. With the increasd of biochar, atrazine adsorption in all three types of soil gradually increased. Organic matter is one of the important adsorption domains of organic pollutants in soils. The adsorption of atrazine by biochar is a result of hydrophobic interactions and pore filling (Zhang et al. 2013a, b). With the increase of organic matter content, the hydrophobicity was enhanced, the adsorption sites in soil increase correspondingly, and the adsorption amount of atrazine in soil gradually increases (Deng et al. 2017). The study by Deng et al. (2017) found that the high affinity for atrazine on cassava waste biochar governed by hydrophobic nature of biochars, which was characterized by lower (N + O)/C ratios. The content of organic matter in saline–alkaline soil was low and therefore, the adsorption increased most after the addition of organic matter. The content of organic matter in saline–alkaline soil was low and therefore, the adsorption increased most after the addition of organic matter.
The adsorption of atrazine effect was best when the pH value was 3.0. At this time, the adsorption capacity of white soil is 11.16 mg kg−1, and that of black soil is 7.45 mg kg−1. The adsorption capacity of saline–alkali soil is 4.16 mg kg−1. Compared with the isothermal adsorption test, the adsorption capacity of atrazine in soil was improved by 27.25%, 11.69% and 13.04%, respectively. When Ca2+ concentrations were 0.5 mol L−1, the adsorption capacity reached the maximum level. The adsorbed amounts were 12.95 mg kg−1 for albic soil, 11.10 mg kg−1 for black soil and 5.05 mg kg−1 for saline–alkaline soil, which were 32.58%, 47.55% and 53.00% higher than isothermal adsorption, respectively. When the Ca2+ concentration was greater than 0.5 mol l−1, the adsorption amount of atrazine in soils showed a downward trend. Atrazine has the best adsorption effect when biochar content was 0.05%. The adsorption amounts in albic soil, black soil and saline–alkaline soil were 13.47, 8.87, 4.33 mg kg−1 respectively. Compared with the treatments without the addition of biochar, the growth rates increased by 26.03%, 19.89% and 27.86% respectively.
In summary, the results obtained in this study showed that the adsorption of atrazine in the three different agricultural soils was generally following the order albic soil > black soil > saline soil. Atrazine concentration in soil, ambient temperature, addition of calcium ions and biochar were significantly correlated with the adsorption capacity (p < 0.01). The pH value of background solution was significantly negatively correlated with the adsorption capacity of atrazine on soil. Therefore, appropriate soil acidification, as well as the addition of calcium ions and biochar to the soil can reduce the mobility of atrazine in the soil. The results of the experiment provided a basis for the residual dynamics of atrazine in the soil, and provided a scientific basis for the treatment of atrazine pollution in the soil of northeast China.
References
Alsharekh A, Swatzell LJ, Moore MT (2018) Leaf composition of American Bur-Reed (Sparganium americanum Nutt.) to determine pesticide mitigation capability. Bull Environ Contam Toxicol 100(4):576
Arias-Estévez M, Soto-González B, López-Periago E, Cancho-Grande B Cancho-Grande, Simal-Gándara J (2005) Atrazine sorption dynamics in acid-surface soils. Bull Environ Contam Toxicol 75(2):264–271
Brondi SHG, Macedo AND, Vicente GHL, Nogueira ARA (2011) Evaluation of the QuEChERS method and gas chromatography-mass spectrometry for the analysis pesticide residues in water and sediment. Bull Environ Contam Toxicol 86(1):18–22
Deng H, Feng D, He JX, Li FZ, Yu HM, Ge CJ (2017) Influence of biochar amendments to soil on the mobility of atrazine using sorption-desorption and soil thin-layer chromatography. Ecol Eng 99:381–390
Deng H, Yu HM, Chen M, Ge CJ (2014) Sorption of atrazine by biochar prepared from manioc wastes in tropical soils. Adv Mater Res 878:433–442
Gao YJ, Wang ZH, Wang S, Jin JJ, Cao HB, Dai J, Yu R (2015) Effects of calcium chloride on winter wheat yield and uptake of Ca and Zn in calcareous soil. J Plant Nutr Fertil 97(4):15–22
Huang Y, Liu Z, He Y, Li Y (2014) Impact of soil primary size fractions on sorption and desorption of atrazine on organo-mineral fractions. Environ Sci Pollut Res 22(6):4396–4405
Huang Y, Liu Z, He Y, Zeng F, Wang R (2013) Quantifying effects of primary parameters on adsorption–desorption of atrazine in soils. J Soils Sediments 13(1):82–93
Huang H, Zhang C, Zhang P, Cao M, Xu G, Wu H et al (2018) Effects of biochar amendment on the sorption and degradation of atrazine in different soils. Soil Sediment Contam 27(8):643–657
Jang YF, Mu ZF, Yves UJ, Sun H, Hu XF, Zhang HY, Liu PY (2016) Study on the adsorption behavior of atrazine onto loess soil in northwest China. Res Environ Sci 29(4):547–552
Jia D, Wang L, Shao X, Li C (2013) Solubility of atrazine in binary mixtures of water + ethanol or 1-propanol from 283.15 to 343.15 k. J Solut Chem 42(5):1051–1062
Kaur P, Makkar A, Kaur P, Shilpa (2017) Temperature dependent adsorption–desorption behaviour of pendimethalin in punjab soils. Bull Environ Contam Toxicol 100(1):167–175
Lin Y, Ge CJ, Feng D, Yu HM, Deng H, Fu BM (2017) Adsorption-desorption behavior of atrazine on agricultural soils in China. J Environ Sci 57(7):180–189
Liu J, Sui Y, Yu Z, Shi Y, Chu HY, Jin J, Liu XB, Wang GH (2015) Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol Biochem 83:29–39
Lizotte R, Locke M, Bingner R, Steinriede RW, Smith S (2017) Effectiveness of integrated best management practices on mitigation of atrazine and metolachlor in an agricultural lake watershed. Bull Environ Contam Toxicol 98(4):447–453
Lladó Jordi, Lao-Luque C, Ruiz B, Fuente E, Solé-Sardans Montserrat Dorado, A D, (2015) Role of activated carbon properties in atrazine and paracetamol adsorption equilibrium and kinetics. Process Saf Environ Prot 95:51–59
Lu W, Kang C, Wang Y, Xie Z (2015) Influence of biochar on the moisture of dark brown soil and yield of maize in northern China. Int J Agric Biol 17(5):1007–1012
Lupul I, Yperman J, Carleer R, Gryglewicz Grażyna (2015) Adsorption of atrazine on hemp stem-based activated carbons with different surface chemistry. Adsorp J Int Adsorp Soc 21(6–7):489–498
Paszko T (2012) Effect of pH on the adsorption of carbendazim in polish mineral soils. Sci Total Environ 435–436(7):222–229
Qin X, Liu Y, Huang Q, Liu YY, Zhao LJ, Xu YM (2019) In-situ remediation of cadmium and atrazine contaminated acid red soil of South China using sepiolite and biochar. Bull Environ Contam Toxicol 102:128
Sahu MK, Mandal S, Yadav LS, Dash SS, Patel RK (2016) Equilibrium and kinetic studies of Cd(II) ion adsorption from aqueous solution by activated red mud. Desalin Water Treat 57(30):14251–14265
Salazar-Ledesma M, Prado B, Zamora O, Siebe C (2018) Mobility of atrazine in soils of a wastewater irrigated maize field. Agr Ecosyst Environ 255:73–83
Taha Sherif M, Amer Mohamed E, Elmarsafy Ashraf E, Elkady Mohamed Y (2014) Adsorption of 15 different pesticides on untreated and phosphoric acid treated biochar and charcoal from water. J Environ Chem Eng 2(4):2013–2025
Tang WW, Zeng GM, Gong JL, Liu Y, Wang XY, Liu YY (2012) Simultaneous adsorption of atrazine and Cu (II) from wastewater by magnetic multi-walled carbon nanotube. Chem Eng J 211–212(22):470–478
Wang X, Li Q, Li M, Yu L (2018) Interference adsorption mechanisms of dimethoate, metalaxyl, atrazine, malathion and prometryn in a sediment system containing coexisting pesticides/heavy metals based on fractional factor design(resolution v) assisted by 2d-qsar. Chem Res Chin Univ 34(3):397–407
Wei X, Wu Z, Wu Z, Ye BC (2018) Adsorption behaviors of atrazine and Cr (III) onto different activated carbons in single and co-solute systems. Powder Technol 329:207–216
Wu Y, Li Y, Zheng C, Zhang Y, Sun Z (2013) Organic amendment application influence soil organism abundance in saline alkali soil. Eur J Soil Biol 54:32–40
Xu C, Wen D, Zhu Q, Zhu H, Huang D (2016) Effects of peanut shell biochar on the adsorption of Cd(II) by paddy soil. Bull Environ Contam Toxicol 98(3):1–7
Yang F, Zhang SS, Sun LL, Zhang Y (2018) Facile synthesis of highly porous “carbon sponge” with adsorption and co-adsorption behavior of lead ions and atrazine. Environ Sci Pollut Res 25:1–12
Yang X, Cheng K, Jia GZ (2018) Molecular dynamics simulation of temperature-dependent atrazine aqueous solution. J Mol Liq 256:456–461
Zhang P, Sun H, Yu L, Sun T (2013a) Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: impact of structural properties of biochars. J Hazard Mater 244–245:217–224
Zhang X, Wang H, Li Z (2013b) Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res Int 20(12):8472–8483
Zhang Y, Cao B, Zhao L, Sun L, Gao Y, Li J, Yang F (2017) Biochar-supported reduced graphene oxide composite for adsorption and coadsorption of atrazine and lead ions. Appl Surf Sci 427:147–155
Acknowledgement
This research was supported by the National Natural Science Foundation (31672051), The National Key Basic Research Program (2016YFD0200203), The National Key Projects (2016YFC0501201).The authors thank anonymous reviewers for their comments on this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, J., Ma, Xl., Wang, W. et al. The Adsorption Behavior of Atrazine in Common Soils in Northeast China. Bull Environ Contam Toxicol 103, 316–322 (2019). https://doi.org/10.1007/s00128-019-02671-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02671-5