Abstract
Purpose
Much attention is directed to the accumulation of mercury and methylmercury (MeHg) in rice grown on Hg-contaminated paddy fields, since they pose a risk to the health of both people and wildlife. Ultimately, measures to control the accumulation of Hg and MeHg in rice should become a key focus of research on this topic. The objective of this study is to test whether Hg and MeHg accumulation are reduced in polished rice when iron and sulfur are added together to the paddy fields during the cultivation of rice.
Materials and methods
In this experimental study, rice plants were grown in pots amended with sulfur and iron. Rice paddy soil contaminated by mercury chloride in the sewage water used for irrigation was simulated in this experiment by Hg-contaminated soil. The total mercury (THg) content added to the soil as mercury chloride reached 120 mg kg−1 as the weight of the dry soil. Two levels of iron (0 and 200 mg kg−1 as FeCl2) as well as sodium sulfate and cysteine (Cys) were added via watering. Two 30-day rice seedlings were transplanted into a root-bag (20 cm long, 12 cm wide), filled with fine quartz sand and cultured in a plastic barrel filled with 4.5 kg of waterlogged soil. When the rice plants matured, samples were taken.
Results and discussion
The addition of iron and sulfur to the Hg-contaminated paddy soil increased the dry weight of rice grains. The THg and MeHg mainly concentrated in the upper soil layer, and the concentrations of MeHg in the treatments with iron and sulfur were especially higher than that of the control. The addition of iron, sulfate and iron, and cysteine and iron decreased THg concentrations in polished rice by 17.7, 38.3, and 21.3%, respectively. The addition of iron, sulfate and iron, or cysteine and iron decreased the MeHg concentration up to 29.9, 36.4, and 48.2% in polished rice, respectively. Thus, we infer that the coupling of sulfur and iron (II) plays an important role in decreasing the accumulation of MeHg in polished rice.
Conclusions
The addition of iron and sulfur together decreased the concentration of THg and MeHg in polished rice. These results can help control concentration levels of MeHg in rice; however, further study of the mechanism of the interaction of sulfur and iron in Hg-contaminated paddy soil should be conducted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mercury (Hg) is a global pollutant as well as a genotoxic and phytotoxic metal. MeHg is one of the most toxic species of Hg, which can be easily bioaccumulated and biomagnified in food chains (Li et al. 2014). Rice (Oryza sativa L.) is found to accumulate methylmercury (MeHg) when grown in mercury (Hg)-contaminated paddy soil (Meng et al. 2010; Meng et al. 2014; Shu et al. 2016). This is of particular concern since rice is a crucial food crop worldwide, and the quality of rice could have great impacts on the health of people as well as wildlife that also feeds on the rice (Abeysinghe et al. 2016). Therefore, the issue demands a greater focus on how to control the concentrations of mercury and MeHg in rice (Rothenberg et al. 2012; Shu et al. 2016; Szakova et al. 2016; Wang et al. 2016b). Soil application with Se could reduce inorganic mercury (IHg) accumulation in rice (Tang et al. 2017). Biochar amendment (Shu et al. 2016) and amendment of sulfate with Se into soils reduce MeHg accumulation in rice (Wang et al. 2016b). Water management (Peng et al. 2012; Rothenberg et al. 2016) and to deliberate selection of rice cultivars are effective measures to reduce total Hg and MeHg accumulation in rice grains (Peng et al. 2012).
Several studies have shown that iron is an important factor influencing Hg and MeHg migration and transformation (Slowey and Brown 2007; Han et al. 2008; Feyte et al. 2010; Ulrich and Sedlak 2010; Li et al. 2016a; Tang et al. 2017). Iron oxides in soil are found mainly in crystalline and amorphous form since the nature of surface charge adsorbs metal ions and nonmetal ions. Iron (hydr)oxides such as goethite can adsorb Hg (II) primarily as a bidentate sorption complex on the goethite surface (Bonnissel-Gissinger et al. 1999; Kim et al. 2004). Moreover, the goethite surface may be a catalyst site for mercury transformation, as it reduces Hg (II) to Hg (0) (Wiatrowski et al. 2009). At the same time, mercury adsorbed by iron oxides is released during iron oxide reduction processes (Slowey and Brown 2007) and directly affects the methylation of mercury. Mercury methylation, or the conversion of mercury into MeHg, is mainly attributed to sulfate-reducing bacteria (SRB) and iron-reducing bacteria (IRB) (Yu et al. 2012; Kaschak et al. 2014; Liu et al. 2014; Su et al. 2016) as well as abiotic processes (Weber 1993).
In general, mercury methylation depends on the biological activities of SRB and IRB as well as the bioavailability of mercury (Ullrich et al. 2001; Avramescu et al. 2011; Frohne et al. 2012; Jonsson et al. 2012), even though more about the complexity of microbial involvement in the cycling of mercury continues to emerge (Bravo, et al. 2017). Sulfate addition enhances SRB activity and promotes mercury methylation. Sulfides (S2−), a key factor in the methylation process, are also generated through sulfate (SO4 2−) reduction related to microbial activity (Frohne et al. 2012). At high sulfide concentrations, Hg forms soluble bi- and poly-sulfide complexes such as HgSH+, Hg(SH)2, Hg(SH)S−, HgS2 2−, Hg(Sx)2 2−, or Hg(Sx)OH−, depending on pH, redox potential (Eh) conditions, and S0/S2− concentrations (Jay et al. 2000; Ullrich et al. 2001). Furthermore, Hg(SH)2 (aq) and HgOHSH0 can be used in the SRB methylation of mercury (Lehnherr 2014). Sulfur indirectly influences mercury speciation, which affects mercury bioavailability and methylation levels (Corrales et al. 2011). Meanwhile, the speciation of iron and sulfur occurs by interaction under conditions of dry and wet alternation. In anoxic environments, inorganic sulfur mainly appears in the form of HS−, S2−, or S, and iron exists in the form of Fe2+, allowing FeS or FeS2 to form readily. When FeS adsorbs onto Hg, the methylation of Hg decreases (Liu et al. 2009; Jeong et al. 2010; Ulrich and Sedlak 2010). However, the extent to which the interaction of iron and sulfur influences the migration and transformation of mercury and MeHg in the soil-rice system remains largely unknown.
Earlier studies have shown that THg and MeHg accumulation in plants can be reduced by water management, deliberate selection of rice cultivars (Peng et al. 2012), biochar (Shu et al. 2016) and selenium amendment (Wang et al. 2014; Shu et al. 2016). Iron is an abundant element in soil, and sulfur could enter the field in several ways, such as through chemical fertilizers, farm manure, irrigation, and acid rain. The objective of this study is to test whether Hg and MeHg accumulation could be reduced in polished rice when iron and sulfur are added together. In the experiments, rice seedlings were watered with mercury-contaminated water (which simulated waste water) and were treated with two iron levels (no additional iron and additional iron) as well as two kinds of sulfur, sulfate in inorganic and cysteine in organic. Sulfate and cysteine were selected to study because sulfate is one of the main input forms to paddy soil and cysteine bounded to Hg (Schaefer and Morel 2009), which have influence on mercury transformation.
2 Materials and methods
2.1 Soil
The soil for this experiment was collected between depths of 0 to 30 cm in an abandoned paddy field of long-term agricultural use in the suburbs of Hengyang in Hunan Province, China. The soil was air-dried and sieved through a 2-mm mesh. The pH was 5.78 (soil/water = 1:2.5, w/w), and the organic matter content was 2.62%. The THg, total iron, and total sulfur content of the soil were 0.21, 691.25 mg kg−1, and 0.39%, respectively.
2.2 Pot experiment
Rice variety Zhongyou #838 was selected for planting, and rice seedlings were cultivated in a large plastic tray (40 cm × 60 cm) for normal seedling. Germination occurred within 1 week, and the trays were then transferred outside for about 4 weeks until the shoots were approximately 6–8 cm height. Rice plants were planted in pots and supplied with sulfur and iron. Hg-contaminated soil simulated the rice paddy soil contaminated by mercury chloride in four successive man-made sewage water irrigations. The THg content of the mercury chloride added into the soil reached 120 mg kg−1 as the weight of the dry soil. Two levels of iron (0 and 200 mg kg−1 as FeCl2) as well as sodium sulfate and cysteine were added via watering. Two 30-day rice seedlings were transplanted into a root-bag (20 cm long, 12 cm wide), filled with 1 kg fine quartz sand and cultured in a plastic barrel filled with 4.5 kg of soil. Each treatment was replicated three times. For 12 weeks, the pots were arranged randomly and were rotated regularly to ensure uniform conditions, and the plants were watered to 0.5 cm above the surface of the soil.
2.3 Samples treated
The soil samples were taken from upper layer (0–10 cm) and sublayer (10–20 cm) in each pot. The well-washed rice plants were divided into root, stalk, leaf, and rice grain. The soil and plant samples were then stored in a refrigerator at −17 °C prior to being freeze-dried. Rice grain samples were air-dried for 2–3 weeks and the hull was removed (Huller, JLGJ4.5, China). Brown rice was milled removing the bran layers and germ (Rice mill, JNMJ3, China), setting the timer at 40 s for all samples. Rice plant tissues and polished rice were ground to 100 mesh (IKA-ALL basic, IKA, Germany), and the grinder was cleaned by using anhydrous alcohol after every sample. Freeze-dried soil samples were homogenized to 150 meshes with a mortar. During the course of sample preparation, precautions were taken in order to avoid any cross-contamination, including the use of anhydrous alcohol to clean the pestle and mortar after a sample was treated.
2.4 Sample analyses
Total Hg in the soil and plant samples was determined using CV-AFS. MeHg contents in these samples were measured using gas chromatography (GC)-CVAFS detection after aqueous methylation, purging and trapping (Liang et al. 1994), as specified by Method 1630 (U.S. EPA 2001a). Quality control for THg and MeHg determination in samples was conducted using duplicates, method blanks, matrix spikes, and certified reference materials. The method detection limits were 10 ng kg−1 for THg and 2 ng kg−1 for MeHg in tissues of rice samples as well as in soil samples. The relative standard deviation for analysis of duplicate samples was less than 8.2% for THg and MeHg. Recoveries from the reference materials and matrix spikes were in the range of 90–110% in the total Hg and MeHg analyses. The certified international reference materials, including National Research Centre for Certified Reference Materials, rice (GBW08508), and International Atomic Energy Agency, sediment (IAEA-405) were used for quality control of rice plant and soil sample analysis. Total sulfur of the samples was determined by CHNS element analyzer (vario MACRO cube, Germany). Quality control for total sulfur determination in samples was conducted using duplicates, and the relative standard deviation for analysis of duplicate samples was less than 4.5%. Certified reference material was measured every ten samples, and Certified Reference Materials plant (AP2026) was bought from National Research Centre.
2.5 Calculation and statistical analysis
Statistical significance was determined by Tukey’s multiple comparison test of one-way analysis of variance (ANOVA) (p = 0.05). Correlation analysis was carried out by bivariate correlation (2-tailed) and test of significance in IBM SPSS Statistics 24. Some data processing was carried out using Micro EXCEL 2016.
3 Results
3.1 Dry weight of rice grain as affected by Fe2+ and sulfur treatments
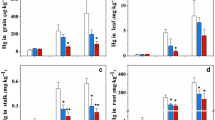
The dry weight of rice grain was increased by addition of sulfur, ferrous ion or both to the soil through watering (Fig. 1). The average dry weight of rice treated by addition of iron was 1.61 times that of the control. The dry weight of rice grain was significantly affected by the addition of ferrous chloride (p = 0.024); however, the influence of the sodium sulfate and cysteine on the dry weight was not significant (p = 0.823), and the interaction of sulfur and iron did not show a significant difference (p = 0.282).
Effects of the addition of iron (II) and sulfur on the dry weight of rice grain. Note: Control represents no addition of iron (II) and sulfur, S represents the addition of 200 mg kg−1 of sodium sulfate, Fe + S represents the addition of 200 mg kg−1 of iron (II) and sulfate, Fe + Cys represents addition ferrous iron and cysteine. The bar represents the standard error. Different letters indicate significant differences (one-way ANOVA with Tukey’s test, p < 0.05)
3.2 The distribution of THg and MeHg in soil
Mercury was mainly deposited in the upper layer soil, and THg concentrations varied from 24.6 to 57.5 mg kg−1 in these treatments (Fig. 2). The results showed that more mercury was concentrated in the upper layer soil treated with sulfur or iron as compared to that of the control. Soil amendment with Fe significantly influenced THg concentration in upper layer soil (p = 0.031), and sulfur amendments had a similar effect (p = 0.028). The interaction effect of sulfur and iron was also significant (p = 0.035). The THg concentrations in sublayer soils were far lower than those in the upper layer. The highest THg concentration in the sublayer was only 2.86 mg kg−1. The influence of sulfur on THg concentration in sublayer soil was significant (p = 0.032), but neither iron (p = 0.709) nor the interaction of sulfur and iron (p = 0.053) had a significant effect.
THg (a) and MeHg (b) concentration in different treatments in upper layer and sublayer soil. Note: Control represents no addition of iron (II) and sulfur, S represents the addition of 200 mg kg−1 of sodium sulfate, Fe + S represents the addition of 200 mg kg−1 of iron (II) and sulfate, Fe + Cys represents 200 mg kg−1 of iron (II) and cysteine, and the bar represents the standard error. Different letters indicate significant differences (one-way ANOVA with Tukey’s test, p < 0.05)
The MeHg concentrations in the upper layer soil were higher than in the sublayer, with the highest MeHg concentration in the upper layer reaching 110.7 ng g−1 in the soils amended with cysteine. The MeHg concentrations in control soils were lower than in any of the amended soils. The influences of both iron (p = 0.001) and sulfur (p = 0.001) on MeHg concentrations in the upper layer soil were significant, and the interaction of iron and sulfur was also a significant effect (p = 0.025). But the MeHg concentrations in sublayer soils did not show any significant influence from the addition of iron (p = 0.07), sulfur (p = 0.711) or their interaction (p = 0.121). Table 1 shows the ratio of MeHg to THg in upper layer and sublayer soils with different treatments. The ratios varied from 0.76 ± 0.17 to 12.54 ± 2.54, with clear indications that sulfur and iron influenced Hg transformation and translocation in the soil.
3.3 THg concentrations in different parts of rice plants
The THg concentration in different parts of the rice plant varied significantly from greatest to least in the following order: root > leaf > stalk > polished rice. The addition of Fe (II) ions increased the THg concentration in both root and leaf. By contrast, the THg concentration in polished rice decreased with addition of ferrous ion, as the concentration was only 1.16 mg kg−1. The addition of Fe (II) had significant effects on THg concentrations in the roots (p = 0.027), leaves (p = 0), and polished rice (p = 0). The two types of sulfur used, sulfate and cysteine, affected mercury accumulation in different parts of the rice plant. Cysteine had a large impact on the THg in roots, with concentrations reaching 229.2 mg kg−1, while sulfate had little impact. Sulfate increased the concentration of THg in the roots and stalks while cysteine decreased the concentration of THg in the stalks and leaves. When it comes to the THg concentrations in polished rice, however, neither sulfate nor cysteine had a significant influence, with concentrations ranging from 1.41 to 1.54 mg kg−1. Sulfur addition did not significantly influence the THg concentration in root (p = 0.091), stalk (p = 0.071), leaf (p = 0.193), and polished rice (p = 0.252).
When sulfur and iron (II) were added to the irrigation water, the interaction of sulfate with iron (II) and cysteine with iron (II) enhanced the concentration of THg in the roots and leaves. The concentration of THg in the roots treated with sulfate and iron (II) reached 174.5 mg kg−1, which was much higher than the THg value of the control (71.2 mg kg−1). Interestingly, when the soils were treated with sulfate and iron, the concentration of THg in polished rice decreased. Soil treated with iron (II) that had interacted with sulfate or cysteine resulted in THg reduction ratios of 38.3 and 21.3% in polished rice, respectively (Fig. 3). The interaction effect of iron and sulfur had a significant effect on THg in roots (p = 0) and polished rice (p = 0.018); however, their interaction was not significant on stalk (p = 0.392) and leaf (p = 0.262) THg.
Concentrations of THg in root, stalk, leaf, and polished rice in different treatments: Control represents no addition of iron (II) and sulfur, S represents the addition of 200 mg kg−1 of sodium sulfate, Fe + S represents the addition of 200 mg kg−1 of iron (II) and sulfate, Fe + Cys represents 200 mg kg−1 of iron (II) and cysteine, and the bar represents the standard error. Different letters indicate significant differences in one tissue of rice plant (one-way ANOVA with Tukey’s test, p < 0.05)
3.4 MeHg concentrations in different parts of rice plants
MeHg concentrations in different parts of the rice plant varied from 17 to 1060 ng g−1; the MeHg concentration in the leaves was lowest while that of polished rice was highest (Fig. 4). When the soils were treated with iron, the concentrations of MeHg in polished rice decreased with the addition of iron. These results showed that iron addition is a potential method for decreasing MeHg concentrations in polished rice. Statistical analysis showed that the concentration of MeHg in stalks (p = 0) and polished rice (p = 0.01) was significantly affected by the addition of Fe, while Fe had no significant influence on the MeHg concentration in root (p = 0.10) or leaf (p = 0.755). In polished rice, sulfate increased MeHg accumulation, but cysteine inhibited MeHg accumulation. However, the effect was reversed in the roots. No significant effect from sulfur additions was observed on MeHg concentrations in stalk (p = 0.074), leaf (p = 0.55), or polished rice (p = 0.092), but the effect on MeHg concentration in roots was significant (p = 0.01). The concentration of MeHg in polished rice is of considerable concern. When the soils were treated with iron (II) that had interacted with sulfate and cysteine, the reduction ratios of MeHg in polished rice were 36.4 and 48.2%, respectively (Fig. 4). Similar results were observed in the roots, stalks, and leaves. The interaction of iron and sulfur had significant effects on MeHg concentrations in root (p = 0) and stalk (p = 0.03); however, there were no significant interactive effects on MeHg concentrations in leaf (p = 0.148) or polished rice (p = 0.251). The concentration of MeHg in different parts of the rice plant went from greatest to least in the following order: polished rice > root > stalk > leaf.
Concentrations of MeHg in root, stalk, leaf, and polished rice in different treatments: Control represents no addition of iron (II) and sulfur, S represents the addition of 200 mg kg−1 of sodium sulfate, Fe + S represents the addition of 200 mg kg−1 of iron (II) and sulfate, Fe + Cys represents 200 mg kg−1 of iron (II) and cysteine, and the bar represents the standard error. Different letters indicate significant differences in one tissue of rice plant (one-way ANOVA with Tukey’s test, p < 0.05)
4 Discussion
4.1 Iron and sulfur increase the weight of rice grains
The yield of rice grain is decreasing in the mercury contaminated paddy soil (Liu et al. 2015). In this experiment, the dry weights of rice grain were increased with the addition of sulfate, cysteine, and ferrous iron (Fig. 1). More iron plaque formation on the roots with iron addition can decrease the toxicity of mercury to rice plants (Huang and Deng, 2016) because iron plaque could adsorb mercury and MeHg on the root (Liu et al. 2016). At the same time, sulfate addition may promote the formation of iron plaque on the roots of rice plants (Hu et al. 2007), as more Hg was adsorbed by iron oxides on the root. Li et al. also found that Hg exists in the form of R-S-Hg-S-R in rice roots (Li et al. 2016).
4.2 Iron and sulfur effect on translocation and transformation of mercury in soil
The concentration of THg in the upper layer soil was always higher than in the sublayer soil (Fig. 2). This phenomenon is the result of THg entering the soil surface by watering, and the slow rate of mercury migration. Ferrous ion could easily change into ferric iron at the soil surface, and form iron oxides. Mercury absorbed by these iron oxides will be kept in the upper layer soil. Sulfate can be reduced to sulfide under anoxic conditions, and the sulfide can be deposited with mercury ion (Slowey et al. 2007).
We found that MeHg concentrations in the upper layer soil were also higher than in the sublayer soil (Fig. 2). MeHg is mainly derived from net methylation of mercury. The ratio of MeHg/THg showed the effect of iron and sulfur on mercury methylation (Table 1). Iron promoted the methylation of mercury, yielding higher MeHg/THg ratios. This result is consistent to some literature (Mehrotra and Sedlak 2005; Hana et al. 2008). The effect of iron on methylation could be due to the fact that oxidation of the added ferrous iron decreased the surface water pH almost 1.2–2.5 units (data not shown). The lower pH may enhance rhizosphere Hg(II) bioavailability and bacterial uptake, increasing microbial Hg(II)-methylation (Winfrey and Rudd 1990; Miskimmin et al. 1992). Both sulfate and cysteine enhanced the concentration of MeHg in upper lay soil. Sulfate also plays an important role in mercury methylation. Sulfate could enhance activities of sulfate-reducing bacteria (SRB), the principal methylator under anoxic conditions, and consequently increase net MeHg production in soil (Gilmour et al. 1992; Wang et al. 2016a). As for cysteine addition, formation of a mercury-cysteine complex promotes both the uptake of inorganic mercury by the bacteria and the enzymatic formation of methylmercury (Schaefer and More 2009). Other authors have found that sulfur can also increase methylation (Pak et al. 2010; Orem et al. 2011). The addition of iron and sulfate together had almost no effect on methylation of mercury compared with the control. In anoxic condition, FeS is formed by sulfide as the reduction of sulfate with ferrous iron, which can greatly inhibit the formation of MeHg probably by the reduction of bioavailable neutral mercury complexes via formation of charged Hg(II)-polysulfide complexes (Liu et al. 2009).
4.3 Iron effect on THg and MeHg accumulation
The results showed that iron addition promoted THg and MeHg concentration in the roots (Fig. 3). Iron plaque formation on the roots of the rice plant was often found to have a significant correlation with the concentration of iron (II) (Xing et al. 2006). The iron plaque could adsorb Hg and MeHg, especially MeHg, and the amount of Hg adsorption positively correlated with the amount of iron plaque on the roots (Li et al. 2016). To a certain extent, the concentration of THg in polished rice decreased with the addition of iron, and iron plaque inhibited Hg migration, which is consistent with the previous study (Li et al. 2016). Furthermore, the addition of iron (II) decreased the concentration of not only THg but also of MeHg in polished rice. The adsorption and co-precipitation of Hg and iron oxides could inhibit Hg and MeHg transformation.
4.4 Effect of sulfur on mercury and MeHg accumulation
The results indicated that the concentration of THg in the roots of the rice plants after being treated with sulfur was higher than that of the control, and the effect of cysteine was even stronger than that of sulfate. Sulfate addition may promote the formation of iron plaque on the roots of rice plants (Hu et al. 2007), as more Hg was adsorbed by iron oxides on the root. Li et al. also found that Hg exists in the form of R-S-Hg-S-R in rice roots (Li et al. 2016). This study showed that the MeHg concentration of the rice plants treated with cysteine was higher than that of the plants treated with sulfate and that of the control. Cysteine has been found to increase the level of mercury in microbial cells and promote the methylation of mercury (Merritt and Amirbahma 2009). A likely reason for this is the formation of a mercury-cysteine complex that promotes both the uptake of inorganic mercury by the bacteria and the enzymatic formation of methylmercury (Schaefer and More 2009). Cysteine may have a larger effect on MeHg because sulfate can be reduced to sulfide in anoxic conditions, which would then precipitate with Fe2+ in paddy soils, which inhibits the migration of MeHg. On the other hand, the addition of sulfate allows for iron oxide growth on the roots to reduce Hg2+ to Hg0, which reduces the concentration of mercury ions and could also weaken mercury methylation.
4.5 Interaction of iron and sulfur and its effect on mercury and MeHg accumulation
The experiments showed that the addition of both iron and sulfur enhanced the concentration of THg in the roots and leaves. This result indicates that the interaction of iron and sulfur increased the accumulation of Hg in roots and leaves. Iron plaque on the roots of rice mainly consists of goethite and lepidocrocite (Chen et al. 1980). By reducing Fe (III) in the goethite of iron plaque, S (−II) can oxidize to form S (0) and polysulfides that subsequently facilitate the dissolution of HgS(S) (Slowey and Brown 2007), which enhances Hg reactivity. Figure 2b shows that the MeHg concentration was increased in soil treated with sulfate and iron, cysteine and iron. The results indicate sulfate and iron, cysteine and iron could improve Hg methylation. However, both iron with sulfate and cysteine decreased the MeHg concentration in polished rice. Because MeHg does not form a solid MeHg-sulfide phase, surface complexes with FeS(S) may be of great importance for the solubility and bioavailability of MeHg under suboxic and anoxic conditions (Skyllberg and Drott 2010). As we known, in anoxic condition, FeS is formed by sulfide as the reduction of sulfate or cysteine with ferrous iron in the soil. At the same time, the amount of iron plaque on the root can be increased by iron and sulfur amendment (Hu et al. 2007; Li et al. 2016), and the formation of iron plaque reduces MeHg uptake and significantly inhibits the MeHg translocation from the roots to the shoots in rice plants for MeHg adsorbed by iron plaque on the root surface (Li et al. 2016). To further understand the role of sulfur on translocation and accumulation of MeHg, the relationship of MeHg concentration and total sulfur in different parts of rice was analyzed (Table 2). A significant positive correlation was observed between MeHg concentrations of polished rice and stalk to the total sulfur of stalk; however, the MeHg concentrations of stalk and polished rice had a significant negative correlation with the total sulfur in the roots. The sulfur inhibited the transfer of MeHg from root to stalk. The results showed that increasing the total sulfur of roots can decrease the MeHg concentration in polished rice.
5 Conclusions
The addition of iron and sulfur greatly influenced the migration and accumulation of Hg and MeHg in the soil-rice system. The addition of iron contributed to greater amounts of Hg that accumulated in the roots, stalks, and leaves; however, the addition of iron also decreased concentrations of THg in polished rice. The interaction of sulfur and iron decreased the concentration of MeHg in polished rice, which provides a way to control the concentration of MeHg. Further studies should investigate the appropriate proportion of iron to sulfur to supply in rice plant treatments, and the mechanism for controlling the accumulation of MeHg and Hg should be researched extensively in the future.
References
Abeysinghe K, Ao M, Goodale E, Yang XD, Xu XH, Xu ZD, Qiu GL (2016) Mercury and selenium in arthropods and their bioaccumulation across food webs. Chin J Ecol 35(4):1031–1037 (in Chinese)
Avramescu M, Yumvihoze E, Hintelmann H, Ridal J, Fortin D, Lean DR (2011) Biogeochemical factors influencing net mercury methylation in contaminated freshwater sediments from the St. Lawrence River in Cornwall, Ontario. Canada Sci Total Environ 409(5):968–978
Bonnissel-Gissinger P, Alnot M, Lickes J, Ehrhardt J, Behra P (1999) Modeling the adsorption of mercury(II) on (hydr)oxides II:α-FeOOH (goethite) and amorphous silica. J Colloid Interf Sci 215(2):313–322
Bravo AG, Bouche S, Tolu J. Erik Bjorn E, Alejandro MR, Bertilsson S (2017) Molecular composition of organic matter controls methylmercury formation in boreal lakes. Nat Commun doi: 10.1038/ncomms14255
Chen CC, Dixon JB, Turner TF (1980) Iron coatings on rice roots: mineralogy and quantity influencing factors. Soil Sci Soc Am J 44:635–639
Corrales J, Naja GM, Dziuba C, Rivero RG, Orem W (2011) Sulfate threshold target to control methylmercury levels in wetland ecosystems. Sci Total Environ 409(11):2156–2162
Feyte S, Tessier A, Gobeil C, Cossa D (2010) In situ adsorption of mercury, methylmercury and other elements by iron oxyhydroxides and organic matter in lake sediments. Appl Geochem 25(7):984–995
Frohne T, Rinklebe J, Langer U, Du Laing G, Mothes S, Wennrich R (2012) Biogeochemical factors affecting mercury methylation rate in two contaminated floodplain soils. Biogeosciences 9(1):493–507
Han S, Obraztsova A, Pretto P, Deheyn DD, Gieskes J, Tebo BM (2008) Sulfide and iron control on mercury speciation in anoxic estuarine sediment slurries. Mar Chem 111(3–4):214–220
Hu ZY, Zhu YG, Li M, Zhang LG, Cao ZH, Smith FA (2007) Sulfur (S)-induced enhancement of iron plaque formation in the rhizosphere reduces arsenic accumulation in rice (Oryza sativa L.) seedlings. Environ Pollut 147(2):387–393
Huang TY, Deng H (2016) Effects of reduced nitrogen and phosphorus applications and iron plaque formation on root growth of rice seedlings under mercury stress. Chin J Ecol 9:2417–2421 (in Chinese)
Jay JA, Morel FMM, Hemond HF (2000) Mercury speciation in the presence of polysulfides. Environ Sci Technol 34(11):2196–2200
Jeong HY, Sun K, Hayes KF (2010) Microscopic and spectroscopic characterization of Hg(II) immobilization by Mackinawite (FeS). Environ Sci Technol 44(19):7476–7483
Jonsson S, Skyllberg U, Nilsson MB, Westlund P, Shchukarev A, Lundberg E, Bjoern E (2012) Mercury methylation rates for geochemically relevant Hg-II species in sediments. Environ Sci Technol 46(21):11653–11659
Kaschak E, Knopf B, Petersen JH, Bings NH, König H (2014) Biotic methylation of mercury by intestinal and sulfate-reducing bacteria and their potential role in mercury accumulation in the tissue of the soil-living Eisenia foetida. Soil Biol Biochem 69:202–211
Kim CS, Rytuba JJ, Brown GE (2004) EXAFS study of mercury(II) sorption to Fe- and Al-(hydr)oxides. J Colloid Interf Sci 271(1):1–15
Lehnherr I (2014) Methylmercury biogeochemistry: a review with special reference to Arctic aquatic ecosystems. Environ Rev 22(3):229–243
Li WC, Ouyang Y, Ye ZH (2014) Accumulation of mercury and cadmium in rice from paddy soil near a mercury mine. Environ Toxicol Chem 33(11):2438–2447
Li YY, Zhao JT, Zhang BW, Liu YJ, Xu XH, Li YF, Li B, Gao YX, Chai ZF (2016) The influence of iron plaque on the absorption, translocation and transformation of mercury in rice (Oryza sativa L.) seedlings exposed to different mercury species. Plant Soil 398(1–2):87–97
Liu H, Ma W, Dai JL (2015) Research progress of mercury pollution on rice. J Shan Dong Jian Zhu University 30:170–176 (in Chinese)
Liu JG, Valsaraj KT, Delaune RD (2009) Inhibition of mercury methylation by iron sulfides in an anoxic sediment. Environ Eng Sci 26(4):833–842
Liu YR, Zheng YM, Zhang LM, He JZ (2014) Linkage between community diversity of sulfate-reducing microorganisms and methylmercury concentration in paddy soil. Environ Sci Pollut Res 21(2):1339–1348
Meng B, Feng XB, Qiu GL, Cai Y, Wang DY, Li P, Shang LH, Sommar J (2010) Distribution patterns of inorganic mercury and methylmercury in tissues of rice (Oryza sativa L.) plants and possible bioaccumulation pathways. J Agr Food Chem 58(8):4951–4958
Meng M, Li B, Shao JJ, Wang T, He B, Shi JB, Ye ZH, Jiang GB (2014) Accumulation of total mercury and methylmercury in rice plants collected from different mining areas in China. Environ Pollut 184:179–186
Peng XY, Liu FJ, Wang WX, Ye ZH (2012) Reducing total mercury and methylmercury accumulation in rice grains through water management and deliberate selection of rice cultivars. Environ Pollut 162:202–208
Rothenberg SE, Anders M, Ajami NJ, Petrosino JF, Balogh E (2016) Water management impacts rice methylmercury and the soil microbiome. Sci Total Environ 572:608–617
Rothenberg SE, Feng X, Zhou WJ, Tu M, Jin BM, You JM (2012) Environment and genotype controls on mercury accumulation in rice (Oryza sativa L.) cultivated along a contamination gradient in Guizhou, China. Sci Total Environ 426:272–280
Schaefer JK, More FMM (2009) High methylation rates of mercury bound to cysteine by Geobacter sulfurreducens. Nat Geosci 2:123–126
Shu R, Wang YJ, Zhong H (2016) Biochar amendment reduced methylmercury accumulation in rice plants. J Hazard Mater 313:1–8
Skyllberg U, Drott A (2010) Competition between disordered iron sulfide and natural organic matter associated thiols for mercury(II)—an EXAFS study. Environ Sci Technol 44(4):1254–1259
Slowey AJ, Brown GE (2007) Transformations of mercury, iron, and sulfur during the reductive dissolution of iron oxyhydroxide by sulfide. Geochim Cosmochim Ac 71(4):877–894
Su YB, Chang WC, Hsi HC, Lin CC (2016) Investigation of biogeochemical controls on the formation, uptake and accumulation of methylmercury in rice paddies in the vicinity of a coal-fired power plant and a municipal solid waste incinerator in Taiwan. Chemosphere 154:375–384
Szakova J, Buresova A, Praus L, Garcia-Sanchez M, Holeckova Z, Gabriel J, Sysalova J, Cervenka R, Komarek J, Grohova S, Tlustos P (2016) The response of mercury (Hg) transformation in soil to sulfur compounds and sulfur-rich biowaste application. Environ Earth Sci 75(5847)
Tang WL, Dang F, Evans D, Zhong H, Xiao L (2017) Understanding reduced inorganic mercury accumulation in rice following selenium application: selenium application routes, speciation and doses. Chemosphere 169:369–376
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31(3):241–293
Ulrich PD, Sedlak DL (2010) Impact of iron amendment on net methylmercury export from tidal wetland microcosms. Environ Sci Technol 44(19):7659–7665
Wang X, Tam NFY, Fu S, Ametkhan A, Ouyang Y, Ye ZH (2014) Selenium addition alters mercury uptake, bioavailability in the rhizosphere and root anatomy of rice (Oryza sativa). Ann Bot-London 114(2):271–278
Wang YJ, Dang F, Zhao JT, Zhong H (2016a) Selenium inhibits sulfate-mediated methylmercury production in rice paddy soil. Environ Pollut 213:232–239
Wang YJ, Wei ZB, Zeng QL, Zhong H (2016b) Amendment of sulfate with Se into soils further reduces methylmercury accumulation in rice. J Soils Sediments 16(12):2720–2727
Weber JH (1993) Review of possible paths for abiotic methylation of mercury in the aquatic environment. Chemosphere 26(11):2063–2077
Wiatrowski HA, Das S, Kukkadapu R, Ilton ES, Barkay T, Yee N (2009) Reduction of Hg(II) to Hg (0) by magnetite. Environ Sci Technol 43(14):5307–5313
Xing CH, Cai MZ, Liu P, Xu GD (2006) The role of iron and manganese plaques on wetland plant roots in environment and ecology. Ecol Environ 15(6):1380–1384 (in Chinese)
Yu R, Flanders JR, Mack EE, Turner R, Mirza MB, Barkay T (2012) Contribution of coexisting sulfate and iron reducing bacteria to methylmercury production in freshwater river sediments. Environ Sci Technol 46(5):2684–2691
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 41273152), Hunan Province Natural Science Foundation (No. 14JJ2121), and Opening Fund of the State Key Laboratory of Environmental Geochemistry (grant no. SKLEG2017907, SKLEG2017912).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Dong-Mei Zhou
Rights and permissions
About this article
Cite this article
Zhong, S., Qiu, G., Feng, X. et al. Sulfur and iron influence the transformation and accumulation of mercury and methylmercury in the soil-rice system. J Soils Sediments 18, 578–585 (2018). https://doi.org/10.1007/s11368-017-1786-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1786-1