Abstract
Recent studies showed that rice is the major pathway for methylmercury (MeHg) exposure to inhabitants in mercury (Hg) mining areas in China. There is, therefore, a concern regarding accumulation of Hg in rice grown in soils with high Hg concentrations. A soil pot experimental study was conducted to investigate the effects of Hg-contaminated soil on the growth of rice and uptake and speciation of Hg in the rice. Our results imply that the growth of rice promotes residual fraction of Hg transforming to organic-bound fraction in soil and increased the potential risks of MeHg production. Bioaccumulation factors deceased for IHg but relatively stabilized for MeHg with soil total mercury (THg) increasing. IHg in soil was the major source of Hg in the root and stalk, but leaf was contributed by Hg from both atmosphere and soil. Soluble and exchangeable Hg fraction can predict the bioavailability of IHg and MeHg in soils, and that can provide quantitative description of the rate of uptake of the bioavailable Hg. Soluble and exchangeable Hg fraction in paddy soil exceeding 0.0087 mg kg−1 may cause THg concentration in rice grain above the permissible limit standard, and MeHg concentration in paddy soil more than 0.0091 mg kg−1 may have the health risks to humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a highly toxic heavy metal and is a well-known global contaminant that can affect the health of people and ecosystems. However, human activities such as mining, smelting, and coal combustion disperse Hg, mainly inorganic mercury (IHg), which can be methylated by bacteria to produce methylmercury (MeHg), a potent neurotoxicant (Ullrich et al. 2001). MeHg is known to biomagnify strongly in the food chain, with the resulting concentrations in certain fish species up to 106 times higher than ambient water Hg concentrations (Stein et al. 1996). Based on the results of research conducted in North America and Europe, the most common MeHg exposure pathway to humans is believed to be the consumption of fish, fish products, and marine mammal tissues (Clarkson 1998), and the average absorption rate of MeHg by human body is about 95 % (WHO 1990).
However, owing to the contamination from Hg mining and smelting activities, as well as the discharge of industrial effluent and wastewater irrigation in agriculture, Hg pollution of soils and crops are becoming serious problems worldwide (Dai et al. 2013; Shi et al. 2013; Kotnik et al. 2014; Zagar et al. 2014). Recent studies have shown that rice (Oryza sativa L.) grown from local areas near Hg mines or abandoned Hg mining sites could contain elevated concentrations of IHg and MeHg, which have exceeded the maximum total mercury (THg) level (0.020 mg kg−1) recommended by the Chinese National Standards Agency (CNSA) (Feng et al. 2008; Horvat et al. 2003; Qiu et al. 2006). Horvat et al. (2003) reported that THg and MeHg concentrations in rice grown at abandoned Hg mining areas in Guizhou Province, China, ranged from 0.011 to 1.1 and 0.0016 to 0.17 mg kg−1, respectively. Although rice consumption contributes to a low level of MeHg exposure in non-mining areas, southern China (Li et al. 2012), it is the main pathway for MeHg exposure to the population in Hg mining areas and in certain inland areas in China where rice consumption accounted for 94–96 % of MeHg exposure while fish contributed little (Feng et al. 2008; Zhang et al. 2010a, b; Li et al. 2011).
Many studies have conducted on the transport and transformation of Hg in the soil-rice system to predict the metal concentration in rice plant tissues growing in field conditions (e.g., Feng et al. 2008; Rothenberg et al. 2011; Rothenberg and Feng 2012; Li et al. 2013; Rocha et al. 2014). For example, physiochemical and biochemical conditions of paddy soils promote the formation of MeHg, which was enriched by roots, then translocated to the aboveground parts of the rice plant and eventually to grains during the ripening period (Meng et al. 2011). The root surface acted as a potential Hg barrier and consequently reduced the translocation of Hg ion mass through the root system to the aboveground parts (Meng et al. 2012). However, the exact mechanisms of IHg and MeHg accumulation in rice and soil Hg ecotoxicological risk are still unknown.
Previous research focused mostly on THg and MeHg in different rice tissues in Hg mining areas. The distribution and bioaccumulation pathways of IHg and MeHg in rice plants during the rice growing season have not been studied in detail under different soil concentration gradients. A large study in an Hg mining area has previously reported that the distribution pattern of MeHg and IHg in rice plant is inconsistent under different soil contamination gradients in the natural fields (Rothenberg et al. 2011; Zhang et al. 2012; Li et al. 2013), one of the potential mechanisms of which may be resulted in the bioavailable Hg fraction in soils. However, the influence of bioavailable Hg fraction to rice plants has not been studied yet. To a certain extent, soil THg concentrations help us understand the degree of Hg pollution in the paddy soils, but limited information was provided to explore the environmental risk of Hg in soil.
At present, no studies were conducted to explore the relationship between soil Hg fractions and Hg species in rice plants, so the mechanism remains unknown. In the current study, a greenhouse experiment was conducted (1) to study the mechanisms and processes of IHg and MeHg in rice plants under different soil Hg concentration gradients during the rice growing seasons, (2) to characterize the variation of soil Hg fractions and its influence on the bioaccumulation of rice plants, and (3) to estimate the risks of soil MeHg to human health.
Materials and methods
Greenhouse experiment
Pot experiments were performed in a greenhouse made of vinyl at ambient temperatures (14–33 °C) under sunlight from June to October in 2011, in Guiyang, China. The local rice cultivar seedlings were selected for the study as it is widely grown in Huaxi District of Guiyang. Based on our previous surveys, Hg-contaminated soil was collected from a rice paddy field (0–20-cm depth) close to a large Hg smeltery of Wanshan in Guizhou province. Regional background soil was collected from the experimental field of Guizhou University. The composition of the soils is presented in Table 1. Bulk soil was air-dried, sieved to <2 mm, and thoroughly homogenized and then the two different types of soils were weighted and mixed in different rations, forming four concentration treatments of 3, 6, 14, and 24 mg kg−1 and a control group (CG). Based on the results of a previous view, Hg-contaminated soil from a rice paddy field at artisanal Hg mining areas was about 22 mg kg−1 (Meng et al. 2010), which was similar with our sets. The soil in pots was submerged 3–4 cm below the water level and then irrigation was stopped 15 days before rice mature (last batch of samples) to simulate field rice growing conditions. Uniform, about 30-day old seedlings collected from paddies of the background areas (Huaxi District) were transplanted into PVC pots (25 cm in diameter and 15 cm deep) containing 5.0-kg soils pretreated with fertilizers (two plants per pot). Twelve replicates were conducted for each treatment group.
Sample collection and preparation
Three rounds of sampling were conducted during the rice growing season, at the elongating, heading, and ripening stages. For each treatment group, rice plants and the corresponding soil of four replicated experiment plots were collected during each sampling campaign. Whole rice plants were harvested and washed thoroughly with tap water and then with deionized water. The roots, stalks, leaves, and panicles (heading stage) and grains (ripening stage) were separated. The tissue samples were air-dried to constant weight, and the soil samples were stored in a refrigerator at −17 °C prior to being freeze-dried. The mass of each rice plant tissue sample was recorded in the laboratory after drying. All the samples were ground passing through 100 mesh and stored in polyethylene bags to avoid cross-contamination.
Analytical methods and quality control
THg concentrations in plant and soil samples were measured by Lumex RA-915+ multifunctional Hg analyzer (Lumex Ltd., Russia) coupled with a pyrolysis attachment (PYRO-915+). Each sample was determined twice, and the average concentration was got. In this study, the soil Certified Reference Material (IGGE IRMA China, 290 ± 40 ng g−1) and citrus leaf CRM (GBW10020, 150 ± 20 ng g−1) were used for quality control. Detection limits were 0.5 ng g−1 for both soil and plant samples (Zhou et al. 2013). The standard deviations obtained from nine replicated determinations of standards were 8.8 % for soil and 4.0 % for citrus foliage. An average THg concentration of 296 ± 18 ng g−1 was obtained from IGGE IRMA and 148 ± 7 ng g−1 citrus leaf. The recoveries ranged from 92.4 to 113.1 % for soil materials and 94.3 to 103.2 % for plants. The relative error was <5 % in duplicate samples (n = 5).
For MeHg analyses, plant samples were prepared using KOH-methanol/solvent extraction (Liang et al. 1996), whereas soil samples were prepared using CuSO4-methanol/solvent extraction (Liang et al. 2004). MeHg concentration was determined using aqueous ethylation, purge, trap, and GC-CVAFS detection following US EPA method 1630 (2001). Quality assurance and quality control were conducted using duplicates, method blanks, matrix spikes, and certified reference material (TORT-2 and IAEA-405). The analytical detection limit was 0.05 ng g−1. Recoveries on matrix spikes of MeHg in soil and plant samples were in the range of 90–107 % and 86–108 %, respectively. An average MeHg concentration of 0.152 ± 0.013 mg kg−1 (n = 10) and 0.00542 ± 0.0007 mg kg−1 (n = 6) was obtained from TORT-2 and IAEA comparable with the certified concentration of 0.149 ± 0.011 mg kg−1 and 0.00549 ± 0.00053 mg kg−1, respectively. The relative error was <10 % in duplicate samples (n = 5). Inorganic Hg (IHg) was obtained by subtracting MeHg from THg.
The sequential extraction of soil samples was based on the method of Wang et al. (2011). The steps were as follows:
-
(1.)
Soluble and exchangeable fractions—1 g of soil was weighted and added with 8 ml 1 M Mg(NO3)2 (adjusted to pH = 7 with HNO3) then shook for 1 h end over end. Centrifugation was done for 20 min and the supernatant was analyzed
-
(2.)
Specifically sorbed fraction—an 8-mL extractant of 1 M NaOAc (dissolved with HOAc to pH = 5) was added to the residual of step 1 and shook for 5 h at room temperature. Subsequently, centrifugation was done for 20 min and the supernatant was analyzed
-
(3.)
Oxide-bound fraction—20 ml of 0.4 M NH2OH · HCl (dissolved in 25 % HOAc) solution was added to the residual of step 2, heated for 6 h at 96 °C in water bath, and, subsequently, centrifuged for 20 min, and the supernatant was analyzed
-
(4.)
Organic-bound fraction—an 8-mL extractant of 30 % H2O2 (adjusted to pH 2 with HNO3) was added to the residual of step 3 and heated for 2 h at 85 °C in water bath; then, another 3-ml extractant was added and digestion continued for 3 h at 85 °C in water bath. Subsequently, centrifugation was done for 20 min and the supernatant was analyzed
-
(5.)
Residual fraction—a 10-ml aqua regia (concentrated HNO3:HCl = 1:3(v/v)) was added to the residual of step 4 and heated for 1 h at 95 °C in water bath
The correlation between the sum concentration of all fractions and soil concentration determined by Lumex is r 2 = 0.983 (p < 0.001).
Results and discussion
Hg fractions in soils
Average THg and MeHg inputs by irrigation water (the campus tap water, THg 5.8 ± 1.9 ng L−1 and MeHg 0.042 ± 0.014 ng L−1, n = 5) is negligible compared to the concentrations of THg and MeHg in the soils. In this study, the concentrations of THg in the pot soils were relatively stable, also indicating no Hg contamination from other sources in the greenhouse experiments.
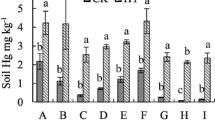
The THg treatment levels of 3, 6, 14, and 24 mg kg−1 were above the domestic environmental quality standard for agricultural soil at 1.5 mg kg−1 (CNEPA 1995). The ratios of THg concentration associated with each fraction in the pot soils at the three periods are shown in Fig. 1. Throughout the three rice growth periods, the average percentage of Hg concentration in the organic-bound fraction was the highest and up to 61.71 %, followed by the residual fraction with an average percentage of 37.42 %. The oxide-bound fraction accounted for a smaller proportion of 0.89 %, while the soluble and exchangeable and specifically absorbed fractions were significantly small, accounted for only 0.05 and 0.03 %, respectively. The relative partitioning of various Hg fractions accorded to the sequence was as follows: organic-bound fractions > residual fractions >> oxide-bound fraction > soluble and exchangeable fractions≈specifically sorbed fractions. The ratio of organic-bound fraction to THg in pot soils of this study was much higher than that of field rice paddy soil in the Hg mining areas, where the fraction of organic-bound Hg was only 1–9 % of the THg in the soil and the residual Hg was the main Hg fraction (Lin et al. 2010). This major difference could be attributed to the different sources of soil. In this study, the soil was collected from Hg smelting-impacted areas leading to high Hg deposition and Hg-polluted wastewater irrigation, which could translate to organic-bound fraction easily (Gunda and Scanlon 2013). Furthermore, higher total organic matter (TOM) concentrations in the pots (range from 9.23 to 9.68 %), compared to field rice paddy soil in the Hg mining regions (Zhang et al. 2010b), may be another reason for this difference. Changes in soil physical and chemical properties under pot culture conditions could also lead to the difference in soil Hg fractions.
There was no obvious variation of THg in the soluble and exchangeable, specifically sorbed, and oxide-bound fractions during the rice growing periods (Fig. 1). However, the statistically significant THg variations were found in the organic-bound and residual fractions during the different rice growing stages. The THg in the organic-bound fraction ranged from 50.0 to 60.1 %, averaging by 57.5 %, during the elongation stage. But it increased significantly (p < 0.05) to 60.8∼66.9 % with an average of 63.2 % in the soil sample at the ripening stage. Correspondingly, the THg in the residual fraction declined from 38.0∼54.4 % in the elongation stage to 32.1∼39.0 % in the ripening stage, resulting in an average reduction of 5.3 %. The significant increase of the organic-bound fraction indicated that the residual fraction of soil Hg was transformed to more organic-bound bioavailable forms, which could promote the transfer of Hg from soil to plants. Although the form of Hg in the strong organic-bound fraction of soil Hg differs from Hg directly absorbed on plants, possible transformation under certain conditions as well as its long-term effects should not be ignored. These were similar to previous researches which suggested that plant growth or planting patterns changed the physical, chemical, and biological properties of soil significantly, and this change could lead to transformation of heavy metal fractions during the process of crop growing (Shuman and Wang 1997; McGrath et al. 1997).
The mean concentrations of MeHg in soil collected from treatments 3, 6, 14, and 24 mg kg−1 were 4.4 ± 0.99, 5.4 ± 1.1, 6.6 ± 1.1, and 11.4 ± 2.0 ng g−1 (Fig. 2). They were significantly higher than that of the MeHg in the CG at 0.58 ± 0.24 ng g−1 (p < 0.01 for all) during the rice growing season. However, the ratio of MeHg to THg in soils of this study was much higher than that in field rice paddy soils in Hg mining areas (Feng et al. 2008; Horvat et al. 2003; Qiu et al. 2006; Meng et al. 2011, 2012; Zhang et al. 2010a, b). TOM has been shown to be an important factor for MeHg production in aquatic systems in boreal and temperate areas (Ullrich et al. 2001). In the present study, however, the higher MeHg in the pot soils may be related to the higher ratio of organic-bound Hg fraction in pot soil compared to that in field rice paddy soils. Although a previous study reported that the new added Hg is rapidly methylated in the paddy soils (Meng et al. 2012), the elevated organic-bound Hg could be another reason for higher MeHg in pot soils. Therefore, the growth or planting patterns of rice not only facilitated the translation of residual fractions to organic-bound fractions but also increased the risk of MeHg production in soils.
Figure 2 also shows that MeHg in the soil varied among the rice growing periods and reached a maximum at the heading stage. A previous study showed that rice paddies (pots) can be more conductive to MeHg production (Rothenberg and Feng 2012) and are generally considered as net sources of MeHg (Skyllberg 2008). In this anoxic environment, paddy soil, as in a typical ephemeral wetland, has a high potential of Hg methylation and results in the production of MeHg. Therefore, MeHg concentrations increased in the rice elongating periods compared to heading periods. Interestingly, MeHg in pot soil decreased in the ripening period (p < 0.05). It is necessary to note that irrigation was ceased 15 days before collecting the last batch of samples as stated above. This may change the soil redox condition to a relatively less anaerobic environment, leading to a lower Hg methylation rate and even demethylation. Alternatively, MeHg accumulation may reverse in the ripening stage, resulting in a gradual decrease.
Hg in rice plants
IHg distribution and accumulation in tissues of rice plants
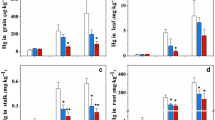
Throughout the growing season, the concentrations of IHg in rice plants varied significantly. The average concentrations of IHg in the roots of rice in treatments with 3, 6, 14, and 24 mg kg−1 of Hg were significantly higher than those of IHg in the roots of CG (Fig. 3a). A significant positive correlation between IHg in rice root and IHg in soil was observed (r 2 = 0.89, p < 0.01) (Table 2), suggesting that IHg in pot soils could serve as a major source of IHg in root. The results agreed well with previous studies in which IHg levels in roots were significantly correlated with the corresponding soil IHg concentration (Zhang et al. 2010b; Meng et al. 2010, 2012). However, the much lower IHg concentrations in root compared to those in soil suggested that rice root surface may have a similar iron plaque to block IHg absorption through the root system, as other metals (Abedin et al. 2002; Hu et al. 2014). Statistical analysis also revealed a positive correlation between IHg in the soil and stalk (Table 2) during the rice growing seasons, suggesting that IHg in pot soils could serve as a source of IHg in rice tissues. The IHg transformation from the root to shoot may be related to the absorption of Hg from soil solution through ion exchange, solute transport, root hair surface contact, etc. and should be further tested.
Previous studies demonstrated that Hg in the leaves of plants was mainly accumulated from the atmosphere rather than from the soil (Ericksen and Gustin 2004; Stamenkovic and Gustin 2009). However, Hg could also be absorbed by roots and then translocated to the aerial part of the plant (Wang et al. 2012). Statistical analyses revealed a positive correlation between the IHg in the leaf and soil (r = 0.92 and r = 0.95, p < 0.01 for the first two growing stages). However, no similar variation and no significant correlation between IHg levels in the leaves and soil was found in the rice ripening season (Fig. 3a), implying that the IHg in the leaves is controlled not only by soil Hg concentration but also by atmospheric Hg.
The average THg concentrations in the whole grains were 5.3 ± 1.5, 8.3 ± 0.96, 11 ± 1.3, and 15 ± 1.7 ng g−1, respectively, for CG and treatments with 3, 6, 14 mg kg−1 Hg. These concentrations were lower than the maximum permissible limit of 0.02 mg kg−1 issued by Chinese National Standard Agency (1994). Lower THg concentrations in grains may relate to lower bioavailable Hg in soil as presented above. However, the concentration of THg obtained from the treatments of 24 mg kg−1 Hg reached 22 ± 1.7 ng g−1, exceeding the permissible limit and posing a potential threat to the health of humans.
The masses of IHg (ng pot−1) in the tissues of rice plants collected from each plot during the rice growing season are shown in Fig. 4a. Analyses of the IHg mass in the plant tissues increased in the order grain < panicle < stem < leaf < root. The apparent accumulation of IHg through rice plants was in agreement with the findings of Meng et al. (2012). In the same growing season, but different Hg doses, the IHg mass in whole rice plants were significantly elevated with increasing Hg concentrations in soil, which was attributed to the elevated IHg mass in roots, as IHg mass in aerial tissues were relatively stable, especially in leaves. This could be probably due to the fact that the GEM concentrations at the green house were essentially the same or similar to the compensation point for the aerial rice plant.
MeHg distribution and accumulation in tissues of rice plants
Figure 3b displays the variation of MeHg concentrations in rice plant of the plot experiment. Rice seedlings contained the same level of MeHg as IHg before being transplanted. However, throughout the rice growing season, the mean concentrations of MeHg in the tissues of the Hg-added treatments were significantly higher than that of MeHg in CG. A significantly positive correlation was observed between MeHg concentrations in rice plants and the corresponding soil (P < 0.001) (Table 2) and agreed well with previously reported data (Meng et al. 2010, 2011). MeHg could be translocated more easily than IHg in other crop plants (Schwesig and Krebs 2003; Henriques et al. 2013), and MeHg in rice plants probably mainly originated from soil through root uptake. In the ripening stage, rice grains recorded the highest concentration of MeHg compared to other aerial tissues. Our analytical data showed that MeHg levels in the tissues of rice plants followed the trend: root > grain > stalk > leaf > panicle for all the rice growing seasons under the same Hg treatment.
Higher MeHg concentration in the root compared to grains in this experiment not the same as previously reported (Feng et al. 2008; Zhang et al. 2010a, b) may be related to several factors. For example, higher organic fraction Hg in the soil is the main reason of higher MeHg in roots, based on the facts that higher organic-bound fraction of Hg created higher MeHg in the pot soils and then rice roots potentially absorb MeHg from soil. Furthermore, a study by Zhang et al. (2012) in an Hg mining area has previously reported that the distribution pattern of MeHg in rice plant is inconsistent under different soil contamination gradients in the natural fields because of an extremely high binding affinity between Hg and selenium (Se) in the rhizospheres and/or roots, and one of the potential mechanisms of this inconsistence may be associated with the interactions. Moreover, rice properties may also be of importance. For instance, genotypic variation may affect rice grain accumulation of MeHg (Peng et al. 2012) and cultivar selection also changed THg and MeHg accumulation in seeds of rice grown in Hg-contaminated regions (Li et al. 2013). It should be noted that the uptake of MeHg by rice plant found in this study might be different from that in the field as exact field environment.
The masses of MeHg (ng pot−1) in the tissues of rice plants collected from each plot during the rice growing season are shown in Fig. 4b. Results from this study demonstrate that MeHg formed in soil could readily move across root barriers and bio-accumulate in the aboveground parts of rice plants as Meng et al. (2011) suggested that MeHg in soil is first absorbed by the plant roots after rice plants are transplanted then the MeHg in roots translocated to store in leaf and stalk before seeding formed, and finally, MeHg is transferred to grains from leaves and stalks during the ripening stage, which is completely consistent with our results.
Bioaccumulation factors
The bioaccumulation factors (BAFs, i.e., organism/soil concentration ratios) are a quotient concentration of Hg in rice plant tissues to Hg in corresponding soils, as shown in Fig. 5. A higher BAF indicates an accumulation of Hg concentrations of the soils by rice plants. Studies have reported BCFs of MeHg of >1 and IHg <0.5 for rice grains in Hg-contaminated areas (Zhang et al. 2010b; Meng et al. 2010, 2011; Peng et al. 2012). Mean BAFs for IHg was 0.0098 ± 0.0029 in the CG and significantly decreased from 0.0013 ± 0.00040 to 0.00052 ± 0.00024 with the soil Hg increasing. Corresponding BAF for MeHg was 12 ± 5.6 for CG and no significant differences among the added Hg soil treatments, which was from 0.82 to 1.4. Hence, the BAF of MeHg was on average more than 1195 times than those of IHg, and the maximum was 2795 times in the treatment of 14 mg kg−1 (Fig. 5).
Much lower BAFs of IHg rice grain fractions suggest that rice root has a similar barrier on the root surface and strong binding force to IHg and root cells have a lower permeability to heavy metal elements such as IHg. IHg would be blocked by endodermis casparian strips while transporting from the roots to the stele resulting in most of the stored Hg in the root bound to cell wall (80 %) in plants (Wang and Greger 2004). However, almost all rice grain samples in our study have BAFs for MeHg exceeding 1 (Fig. 5), and this is consistent with previous studies (Zhang et al. 2010b; Horvat et al. 2003). Results from this study demonstrate that MeHg formed in soil could readily move across root barriers and bio-accumulate in the aboveground parts of rice plants, implying that the mechanisms of IHg and MeHg uptakes into the tissues of rice plant are completely different. Our observations clearly implied that MeHg in the paddy soil could be more easily absorbed and transferred to rice grain compared to IHg as previous studies on other plants (Gnamus et al. 2000; Schwesig and Krebs 2003).
Soil ecotoxicological risk and food safety
This is the first study, to the best of our knowledge, to explore the relationship between soil Hg fractions and Hg species in rice plants. Hence, knowledge of the relationship between Hg fraction and speciation would help us to know the potential risk of Hg in soil to rice plants as well as human health.
Soluble and exchangeable Hg fraction is a potentially good indicator of Hg bioaccessibility in soils compared to other fractions and presents the highest environmental risk, as this fraction of soil Hg can be readily transformed into more toxic complexes such as organic Hg (Zagury et al. 2009). Rice plants may absorb considerable Hg in soluble and exchangeable fraction from pot soil with rice growing as other plants (Wang et al. 2011). However, the concentration of Hg in this fraction was not significantly changed during the process of rice growing (p > 0.05). The growth of rice took up soluble and exchangeable Hg fraction directly resulting in reducing the bioavailable Hg in soil, while other fractions of Hg were transformed to this more bioavailable forms. Furthermore, this transformation could promote the transfer of Hg from soil to plants and remaining the soil ecotoxicological risk. It is proven that sulfur-reducing bacteria (SRB) and Desulfovibrio desulfuricans ND132 and Geobacter sulfurreducens PCA with hgcA and hgcB genes were facilitated in rice paddy soil (Parks et al. 2013), which has a high potential for Hg methylation. Another risk would be that IHg (mainly in soluble and exchangeable forms) can generate MeHg in the environment by SRB and D. desulfuricans under the ensuing anoxic conditions (Ullrich et al. 2001; Rothenberg and Feng 2012). Regression analyses showed that soluble and exchangeable fraction (X) in pot soil were statistically significant with THg and MeHg accumulated in the grain (THggrain = 1.6501 X soil + 5.7088 and MeHggrain = 0.8695 X soil + 3.1386, r 2 = 0.9693 and 0.972, p < 0.001 for both, respectively) (Fig. 6a, b), which may imply that the soluble and exchangeable fraction is bioavailable to rice plants.
Further research is needed to experimentally test this relationship and to establish critical levels of THg and MeHg in rice grains based on bioavailable concentration in soil. However, the reported use of soluble and exchangeable Hg fraction concentration in soil can be implied to predict uptake fluxes throughout a rice growing season prior to rice cultivation or seed formation. Analysis of this fraction could allow for assessment of the risk of rice contamination that might become apparent if the crop is allowed to cultivate or reach maturity, as this fraction remain stable during the whole rice growth period. This would be a relatively inexpensive and easy monitoring method that could guide the action of change crop species or land use patterns before food crop is cultivated. This would effectively reduce the risk of Hg-contaminated rice entering the food chains in areas where the rice are the most important routes to human exposure.
THg concentration in most crops exists mainly in inorganic forms (WHO 1991). However, in this study, the ratios of MeHg/THg in grains, with a mean of 55 % in the Hg-added treatments, were much higher than those from the remaining tissues of rice plants. Organic forms of Hg are of greater concern, due to their higher toxicity and absorption rate (95 %) by the human body (WHO 1990). Hence, the higher ratios of MeHg/THg in grains pose a potential threat to human health.
From the perspective of food safety, the bioaccumulation process of IHg and MeHg in rice is a physiological mechanism of concern. Although a permissible limit of THg in food was prescribed by the Chinese National Standard Agency, the risk of excessive concentration in rice grains caused by Hg concentrations in soil is still unknown. Even for MeHg, there are no guidelines in rice, as the presence of any MeHg in a food crop is an undesirable situation. Generally, humans are exposed to MeHg through highly enriched fish and seafood, but recent studies confirmed the risk of MeHg from rice consumption (Zhang et al. 2010a). Therefore, the risk of MeHg contamination in rice grain assessment urgently needed to examine rice uptake of MeHg from soil, while rice is a staple food for billions in China and around the world.
Regression analyses also showed that THg in pot soil was a statistically significant factor (r 2 = 0.8191, p < 0.001) for THg accumulated in the grain (THggrain = 0.0007 THgsoil + 0.006) (Fig. 7a). When the concentration of Hg in rice grain is the national limit concentration (0.02 mg kg−1), the corresponding concentration of Hg in soil is 20 mg kg−1 calculated by linear equations. But it should be noted that to a large extent, THg in rice grains is not only determined by the THg in soil, other processes also contributed, such as bioavailable Hg fraction in the soil as stated above, which is geographically and temporally varied. If we used the relationship between soluble and exchangeable fraction and THg in grains (Fig. 6a), this Hg fraction should not exceed 0.0087 mg kg−1 according to this standard.
For MeHg, we assumed that the daily consumption of rice is 600 g by a 60-kg person in the mining areas (Li et al. 2009) and there is no MeHg lost during cooking (Horvat et al. 2003), although most, if not all, of the CH3HgCys in uncooked rice is converted to other forms of MeHg by cooking, a complex that is thought to be responsible for the transfer of MeHg across the blood-brain and placental barriers, the identity and toxicity of which remain elusive (Li et al. 2010). While MeHg concentration in rice was 0.010 mg kg−1, MeHg intakes would be getting the USEPA (1997) reference dose (RfD) of 0.10 μg kg−1 bw day−1. According to the regression equation between soluble and exchangeable fraction and MeHg in grains, we can predict that this Hg fraction is exceeding 0.0079 mg kg−1, MeHg concentration in rice would exceed 0.010 mg kg−1. However, lower MeHg exposure levels were calculated using the average rice ingestion rate (270 g day−1) in 2008 in China (Rothenberg et al. 2011), the concentration at risk of MeHg concentration in rice was 0.022 mg kg−1. The BAFs of MeHg in rice grains remained relatively stable and indicated that MeHg in soil can be a good prediction of MeHg in rice grains. Based on the regression equation (MeHggrain = 0.9323 MeHgsoil + 0.0015) (Fig. 7b), MeHg concentration in soil reaches 0.0091 mg kg−1, indicating some risks to local human health in the mining areas.
These relationships may be useful for the assessment of an ecological risk that might be apparent from Hg-contaminated soil where rice is the dominant field crops. However, it should be that this concentration was set on the basis of laboratory-controlled experiments rather than in natural fields with more complex conditions, where they vary both spatially and temporally. Thus, further research is needed on natural sources to experimentally test these relationships and to establish critical levels of IHg and MeHg in soil contributed to rice grains as rice is known to be the main pathway for human MeHg exposure in Hg-contaminated areas.
Conclusion
Results of this investigation reveal that the growth of rice promotes transformation of Hg from residual fraction to organic-bound fraction in pot soil, increasing the potential risks of rice plant uptake and MeHg production. The IHg accumulation in the rice plant follows the order of root > leaf > stalk > panicle > grain. However, highly toxic MeHg accumulation in rice is completely different from the case of IHg, following the order of root > grain > panicle > stalk > leaf. During the rice growing seasons, IHg in soil is the predominant source for the root, stalk, panicle, and seed concurrently, but IHg in the leaf was influenced by both the air and soil. IHg in aerial parts of rice plants remained stable; however, MeHg in aerial parts was transferred to the seed in the ripening stage. The highly bioavailable fraction of soluble and exchangeable Hg in soil can be implied to predict uptake flux throughout a rice growing season. The BAFs of IHg in rice grains decreased with soil Hg increasing, but MeHg stabilized with soil MeHg increasing. Regression analyses showed that soluble and exchangeable fraction and MeHg in paddy soil exceed 0.0087 mg kg−1 and 0.0091 mg kg−1, rice grain may have the risk of exceeding the permissible limit and some risks to human health.
References
Abedin MJ, Feldmann J, Mehang AA (2002) Uptake kinetics of arsenic species in rice (Oryza sativa L.) plants. Plant Physiol 128:1120–1128
Clarkson TW (1998) Human toxicology of mercury. J Trace Elem Exp Med 11:303–317
CNEPA (Chinese National Environment Protect Agency) (1995) Environmental quality standard for soils. Beijing, China: GB 15618–1995. (In Chinese)
CNSA (1994) Tolerance limit of mercury in foods. Chinese National Standard Agency. pp. 171–173. (In Chinese)
Dai ZH, Feng XB, Zhang C, Wang JF, Jiang TM, Xiao HJ, Li Y, Wang X, Qiu GL (2013) Assessing anthropogenic sources of mercury in soil in Wanshan Hg mining area, Guizhou, China. Environ Sci Pollut Res 20:7560–7569
Ericksen JA, Gustin MS (2004) Foliar exchange of mercury as a function of soil and air mercury concentrations. Sci Total Environ 324:271–279
Feng XB, Li P, Qiu GL, Wang SF et al (2008) Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou province, China. Environ Sci Technol 42:326–32
Gnamus A, Byrne AR, Horvat M (2000) Mercury in the soil–plant–deer–predator food chain of a temperate forest in Slovenia. Environ Sci Technol 34:3337–3345
Gunda T, Scanlon TM (2013) Topographical influences on the spatial distribution of soil mercury at the catchment scale. Water Air Soil Pollut 224:1511
Henriques B, Rodrigues SM, Coelho C, Cruz N, Duarte AC, Römkens PFAM, Pereira E (2013) Risks associated with the transfer of toxic organo-metallic mercury from soils into the terrestrial feed chain. Environ Int 59:408–417
Horvat M, Nolde N, Fajon V, Jereb V, Logar M, Lojen S, Jacimovic R, Falnoga I, Qu L, Faganeli J, Drobne D (2003) Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci Total Environ 304:231–256
Hu Y, Duan GL, Huang YZ, Liu YX, Sun GX (2014) Interactive effects of different inorganic As and Se species on their uptake and translocation by rice (Oryza sativa L.) seedlings. Environ Sci Pollut Res 21:3955–3962
Kotnik J, Sprovieri F, Ogrinc N, Horvat M, Pirrone N (2014) Mercury in the Mediterranean, part I: spatial and temporal trends. Environ Sci Pollut Res 21:4063–4080
Li P, Feng X, Qiu G, Shang L, Li G (2009) Human hair mercury levels in the Wanshan mercury mining area, Guizhou Province, China. Environ Geochem Health 31:683–691
Li L, Wang F, Meng B, Lemes M, Feng X, Jiang G (2010) Speciation of methylmercury in rice grown from a mercury mining area. Environ Pollut 158:3103–3107
Li P, Feng X, Shang L, Qiu G, Meng B, Zhang H, Guo Y, Liang P (2011) Human co-exposure to mercury vapor and methylmercury in artisanal mercury mining areas, Guizhou, China. Ecotoxicol Environ Saf 74:473–479
Li P, Feng X, Yuan X, Chan HM, Qiu G, Sun GX, Zhu YG (2012) Rice consumption contributes to low level methylmercury exposure in southern China. Environ Int 49:18–23
Li B, Shi JB, Wang X, Meng M, Huang L, Qi XL, He B, Ye ZH (2013) Variations and constancy of mercury and methylmercury accumulation in rice grown at contaminated paddy field sites in three provinces in China. Environ Pollut 181:91–97
Liang L, Horvat M, Cernichiari E, Gelein B, Balogh S (1996) Simple solvent extraction technique for elimination of matrix interferences in the determination of methylmercury in environmental and biological samples by ethylation gas chromatography cold vapor atomic fluorescence spectrometry. Talanta 43:1883–1888
Liang L, Horvat M, Feng X, Shang L, Li H, Pang P (2004) Re-evaluation of distillation and comparison with HNO3 leaching/solvent extraction for isolation of methylmercury compounds from sediment/soil samples. Appl Organomet Chem 18:264–270
Lin Y, Larssen T, Vogt RD, Feng X (2010) Identification of fractions of mercury in water, soil and sediment from a typical Hg mining area in Wanshan, Guizhou province, China. Appl Geochem 25:60–68
McGrath SP, Shen Z, Zhao F (1997) Heavy metal uptake and chemical changes in the rhizosphere of Thlaspi caerulescens and Thlaspi ochroleucum grown in contaminated soils. Plant Soil 188:153–159
Meng B, Feng X, Qiu G, Cai Y, Wang D, Li P, Shang LH, Sommar J (2010) Distribution patterns of inorganic mercury and methylmercury in tissues of rice (Oryza sativa L.) plants and possible bioaccumulation pathways. J Agric Food Chem 58:4951–4958
Meng B, Feng X, Qiu G, Liang P, Li P, Chen C, Shang L (2011) The process of methylmercury accumulation in rice (Oryza sativa L.). Environ Sci Technol 45:2711–2717
Meng B, Feng X, Qiu G, Wang D, Liang P, Li P, Shang L (2012) Inorganic mercury accumulation in rice (Oryza sativa L.). Environ Toxicol Chem 31:2093–2098
Parks JM, Johs A, Podar M, Bridou R, Hurt RA, Smith SD, Tomanicek SJ, Qian Y, Brown SD, Brandt CC, Palumbo AV, Smith JC, Wall JD, Elias DA, Liang LY (2013) The genetic basis for bacterial mercury methylation. Science 339:1332–1335
Peng XY, Liu FJ, Wang WX, Ye ZH (2012) Reducing total mercury and methylmercury accumulation in rice grains through water management and deliberate selection of rice cultivars. Environ Pollut 162:202–208
Qiu G, Feng X, Wang S, Shang L (2006) Environmental contamination of mercury from Hg-mining areas in Wuchuan, northeastern Guizhou, China. Environ Pollut 142:549–558
Rocha LS, Lopes I, Lopes CB, Henriques B, Soares AMVM, Duarte AC, Pereira E (2014) Efficiency of a cleanup technology to remove mercury from natural waters by means of rice husk biowaste: ecotoxicological and chemical approach. Environ Sci Pollut Res 21:8146–8156
Rothenberg SE, Feng X (2012) Mercury cycling in a flooded rice paddy. J Geophys Res 117, G03003. doi:10.1029/2011JG001800
Rothenberg SE, Feng X, Dong B, Shang L, Yin R, Yuan X (2011) Characterization of mercury species in brown and white rice (Oryza sativa L.) grown in water-saving paddies. Environ Pollut 159:1283–1289
Schwesig D, Krebs O (2003) The role of ground vegetation in the uptake of mercury and methylmercury in a forest ecosystem. Plant Soil 253:445–455
Shi JB, Meng M, Shao JJ, Zhang KG, Zhang QH, Jiang GB (2013) Spatial distribution of mercury in topsoil from five regions of China. Environ Sci Pollut Res 20:1756–1761
Shuman LM, Wang J (1997) Effect of rice variety on zinc, cadmium, iron and manganese content in rhizosphere and non-rhizosphere soil fractions. Commun Soil Sci Plan 28:23–36
Skyllberg U (2008) Competition among thiols and inorganic sulfides and polysulfides for Hg and MeHg in wetland soils and sediments under suboxic conditions, illumination of controversies and implications for MeHg net production. J Geophys Res 113:536–554
Stamenkovic J, Gustin MS (2009) Nonstomatal versus stomatal uptake of atmospheric mercury. Environ Sci Technol 43:1367–1372
Stein ED, Cohen Y, Winer AM (1996) Environmental distribution and transformation of mercury compounds. Crit Rev Environ Sci Technol 26:1–43
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293
USEPA (United States Environmental Protection Agency) (1997) Mercury study report to the congress, volume V: health effects of mercury and mercury compounds. United States Environmental Protection Agency, Washington
USEPA Method 1630 (2001) Methyl mercury in water by distillation, Aqueous ethylation, purge and trap, and CVAFS Draft January 2001. US Environmental Protection Agency, Office of Water, Office of Science and Technology Engineering and Analysis Division (4303), 1200 Pennsylvania Avenue N.W., Washington, DC 20460, pp. 1–41
Wang Y, Greger M (2004) Clonal differences in mercury tolerance, accumulation, and distribution in willow. J Environ Qual 33:1779–1785
Wang J, Feng X, Anderson CWN, Qiu G, Li P, Bao Z (2011) Ammonium thiosulphate enhanced phytoextraction from mercury contaminated soil-results from a greenhouse study. J Hazard Mater 186:119–127
Wang J, Feng X, Anderson CWN, Wang H, Zheng L, Tu T (2012) Implications of mercury speciation in thiosulfate treated plants. Environ Sci Technol 46:5361–5368
WHO (1990) Environmental health criteria 101-methylmercury. World Health Organization, Geneva
WHO (1991) Environmental health criteria118—inorganic mercury. World Health Organization, Geneva
Zagar D, Sirnik N, Cetina M, Horvat M, Kotnik J, Ogrinc N, Hedgecock IM, Cinnirella S, De Simone F, Gencarelli CN, Pirrone N (2014) Mercury in the Mediterranean. Part 2: processes and mass balance. Environ Sci Pollut Res 21:4081–4094
Zagury GJ, Bedeaux C, Welfringer B (2009) Influence of mercury speciation and fractionation on bioaccessibility in soils. Arch Environ Contam Toxicol 56:371–379. doi:10.1007/s00244-008-9205-7
Zhang H, Feng X, Larssen T, Qiu GL, Vogt RD (2010a) In inland China, rice, rather than fish is the major pathway for methylmercury exposure. Environ Health Perspect 118:1183–1188
Zhang H, Feng X, Larssen T, Shang L, Li P (2010b) Bioaccumulation of methylmercury versus inorganic mercury in rice (Oryza sativa L.) grain. Environ Sci Technol 44:4499–4504
Zhang H, Feng X, Zhu J, Sapkota A, Meng B, Yao H, Qin H, Larssen T (2012) Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativa L.). Environ Sci Technol 46:10040–10046
Zhou J, Feng X, Liu H, Zhang H, Fu X, Bao Z, Wang X, Zhang Y (2013) Examination of total mercury inputs by precipitation and litterfall in a remote upland forest of Southwestern China. Atmos Environ 81:364–372
Acknowledgments
This research was financially supported by National Key Basic Research Program of China (2013CB430002), National Natural Science Foundation of China (41461097), and talented person team program of Guizhou province (Qian ke he No. [2012]4005); significant special program of Guizhou province (Qian Ke He No. [2012]6013-7); and Youth Innovation Promotion Association from the Chinese Academy of Sciences. The authors are grateful to Dr. Ping Li and Dr. Bo Meng for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Highlights:
• The bioaccumulations of IHg and MeHg in rice were investigated in a greenhouse experiment.
• Rice growing/planting patterns promote residual Hg transforming to organic-bound Hg in soil and promotes MeHg production.
• The BAFs of IHg in rice grains decreased, but MeHg stabilized with soil mercury increasing.
• This is the first study to explore the relationship and risks between soil mercury fractions, especially soluble and exchangeable fraction, and mercury species in rice plants.
Rights and permissions
About this article
Cite this article
Zhou, J., Liu, H., Du, B. et al. Influence of soil mercury concentration and fraction on bioaccumulation process of inorganic mercury and methylmercury in rice (Oryza sativa L.). Environ Sci Pollut Res 22, 6144–6154 (2015). https://doi.org/10.1007/s11356-014-3823-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3823-6