Abstract
Purpose
This study aimed to evaluate the effect of combination of alkyl polyglucoside (APG) and nitrilotriacetic acid (NTA) on improving the efficiency of phytoremediation for pyrene and lead (Pb) co-contaminated soil by Scirpus triqueter.
Materials and methods
Seedlings of S. triqueter with a similar size and biomass (3 g/pot) were grown on 2-month aged soil contaminated with 184.5 mg kg−1of pyrene and 454.3 mg kg−1 of Pb at pH = 8.3. After growth for 10 days, different doses of APG and NTA were added into the soil. After 60 days, the height of plants, Pb concentrations in plants, and pyrene amounts in soil were determined.
Results and discussion
Combined application of NTA and APG with lower dosage (1 + 1 g kg−1 soil and 1 + 2 g kg−1 soil) had no notable negative influence on the growth of S. triqueter. Moreover, significant synergy on Pb accumulation in S. triqueter was achieved with APG and NTA combined application. Besides, the dissipation of pyrene from soil after 60-day planting was increased in APG and NTA treatments when compared with the control treatments. Application of APG alone or combined with NTA had greater effect on enhancing dissipation of pyrene from soil than NTA alone.
Conclusions
This study demonstrated that the remediation of Pb and pyrene co-contaminated soil by S. triqueter can be enhanced by combined application of APG and NTA. Long-term evaluation of this strategy is needed in co-contaminated field sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil and sediment system is considered as an important sink for organic pollutants (OPs) and heavy metals (HMs; Cachada et al. 2012). As a family of OPs, polycyclic aromatic hydrocarbons (PAHs) are ubiquitous in environments (Fabietti et al. 2009). The risk of being exposed to PAHs dramatically increases as the natural balance is being disturbed. In fact, soils contaminated with PAHs usually contain other pollutants, such as HMs (Ehsan et al. 2014). HM contamination of soil and water poses a serious threat to both ecosystems and human health worldwide (Liu et al. 2015). Among heavy metals, lead (Pb) is a poison element, which is known to be a persistent environmental problem (Noll et al. 2014). It has been demonstrated that PAHs coexist with heavy metals, especially with Pb (Cachada et al. 2012). Recently, remediation of heavy metal-PAH co-contaminated soils has drawn much more attention (Chen et al. 2015).
Compared to the physical, chemical, and biological remediation techniques, phytoremediation is becoming more widespread due to its environmental soundness and lower cost (Ehsan et al. 2014). In recent years, many studies on utilizing plants for remedying PAH- or metal-contaminated soils have been performed (Liu et al. 2009; Chigbo and Batty 2013a; Sun et al. 2014). Scirpus triqueter (S. triqueter) is capable of degrading persistent organic compounds (Liu et al. 2011). This plant species has extensive fibrous root system and large specific surface area of root, which make it possible to promote the efficiency of phytoextraction (Liu et al. 2013). In addition, the root exudates secreted by S. triqueter have beneficial effects on soil microorganisms and enzyme activity, thereby improving the PAH degradation (Zhang et al. 2011). Another report has also shown that S. triqueter can take up and translocate Pb and improve the removal rate of PAHs (Liu et al. 2013). Therefore, phytoremediation by S. triqueter is feasible for decontamination of PAHs and HMs in soils.
However, phytoremediation efficiency of PAH- and HM-contaminated soil is always limited by their poor availability, which may result in lower biodegradation and removal, respectively (Lestan et al. 2008). Besides, simultaneous removal of multiple pollutants remains also a major challenge to phytoremediation of co-contaminated soils (Wang et al. 2012). Therefore, increasing the mobility and solubility of PAHs or HMs is the most important for their bioavailability. The potential of adding different surfactants and chelating agents to HM-PAH-contaminated soils have been studied in recent years for their special chemical structure which can increase the availability of the pollutants in the soils. Alkyl polyglucosides (APGs), nonionic surfactants, which are produced from renewable resources such as fatty alcohols and glucose, have low toxicity and high biodegradability (Liu et al. 2013). Some studies indicated that APGs have a strong ability to enhance the phytoremediation effect for OPs (Liu et al. 2011; Zhang et al. 2014). Chelating agents include synthetic chelants such as ethylene diamine tetraacetic acid (EDTA) and natural aminopolycarboxylic acids such as nitrilotriacetic acid (NTA) (Jagetiya and Sharma 2013). In recent years, more and more related studies have been focused on the later because of their biodegradability and lower toxicity for microorganisms and plants (Lan et al. 2013). For example, Quartacci et al. (2007) have reported that the addition of NTA to soil can increase the ability of Brassica carinata to accumulate Pb, and the faster phytoextraction reduces the risk of metals leaching into groundwater. Freitas and do Nascimento (2009) have also found that NTA is highly effective in solubilizing Pb from soil at concentration of 5 mmol kg−1. Phytoremediation assisted with surfactants and chelating agents has been proposed to enhance the efficiency of remediation for PAHs and HMs, respectively, but few focus on their combined application in HM and PAH co-contaminated soil.
The aim of this study was thus to evaluate the effect of combined application of APG and NTA on phytoremeditation for pyrene and Pb by S. triqueter.
2 Materials and methods
2.1 Chemicals
Pyrene (purity 98 %) was purchased from Aladdin Reagent. APG used in the test was C12/14-APG (APG1214) obtained from the China Research Institute of Daily Chemical Industry (Shanxi, China). The other chemicals, analytical grade or better, were bought from Sinopharm.
2.2 Soil preparation
The soil was collected from the top of the soil profile of a field without previous exposure to pyrene and Pb contamination in the east of Shanghai University. The soil was air dried and sieved at 2 mm. The characteristics were pH 8.3, organic matter 19.6 g kg−1, total nitrogen 0.52 g kg−1, clay 7.4 %, silt 60.4 %, and sand 32.2 %.
Mixed contaminated soil with pyrene and Pb was prepared by two sequential steps; 15 kg of air dried soils were firstly mixed with 2 L of [(CH3COO)2Pb] solution (0.015 mol/L). Few days later, 3 g of pyrene dissolved in 2 L of acetone was added into the Pb-contaminated soil (Song et al. 2008). After the solvent evaporated, the mixed contaminated soil was transferred to a box and aged in the dark (25 ± 3 °C) for about 2 months before the experiments. The final concentrations of pyrene and Pb in the soil were measured as 184.5 and 454.3 mg kg−1, respectively.
2.3 Experimental procedures
Based on previous experiments, the pot culture was carried out in a greenhouse at Shanghai University with controlled day/night temperatures of 25/20 °C. S. triqueter used in the experiment was collected from a wetland ecological environment near Huangpu-Yangtze River Estuary, Shanghai, China. After 10-day incubation before the experiment, S. triqueter with a similar size and biomass (3 g/pot) were transplanted into the plastic pots (14.8 cm diameter and 8.8 cm height) containing 500-g contaminated soil. Water content of the soil in each pot was controlled at approximately 60 % of water-holding capacity (WHC) by weighing and adding deionized water every 2 days (Wei et al. 2012). Ten days after transplanting, the test plants were treated with APG and NTA as the following: CK (control), A1 (1 g APG kg−1 soil), A2 (2 g APG kg−1 soil), N1 (1 g NTA kg−1 soil), N2 (2 g NTA kg−1 soil), N1 + A1 (1 g NTA + 1 g APG kg−1 soil), N1 + A2 (1 g NTA + 2 g APG kg−1 soil), N2 + A1 (2 g NTA + 1 g APG kg−1 soil), and N2 + A2 (2 g NTA + 2 g APG kg−1 soil). The mixed solutions of NTA and APG were applied to the soil surface, adjusted to pH 8.3 with NaOH in order to limit soil property modification. The plants were harvested 60 days after planting. Each treatment was replicated three times. Plants of S. triqueter were removed from the pots and carefully rinsed with tap water to remove any soil particles. After measurement of heights, each plant sample was oven-dried (80 °C) to constant weight (dry weight (DW)) by separating root and shoot. Then, the plant samples were placed into polytetrafluoroethylene bags and stored at 4 °C for further analysis. Soil samples sieved through a 2-mm mesh were stored at −20 °C after freeze drying for 24 h.
2.4 Extraction and analysis of pyrene
Pyrene in soil was detected with the method referenced by Sun et al. (2010) with some modification. Two grams of soil with 1 g Na2SO4 was ultrasonically extracted (45 kHz, 300 W) in 10 ml of 1:1 (v/v) solution of dichloromethane and acetone for 30 min, followed by 4000g centrifugation for 20 min. Then, the supernatant was cautiously removed to specimen bottle. This process was repeated two times. The extracts were concentrated using a rotary evaporator (at 40 °C) and exchanged to 2 ml cyclohexane, followed by filtration through 4-g silica gel with 10 ml 1:1 (v/v) mixture of hexane and dichloromethane. The samples were then evaporated and dissolved by hexane with a final volume of 1.0 ml.
Extracts were analyzed using Agilent 7890 gas chromatograph-5975 mass spectrometer (7890GC-5975MS) using helium as carrier gas with velocity of 1 ml/min and a DB-5 capillary column (30 m × 0.25 mm × 0.25 μm). The injection volume was 1 μl and the splitless injection mode was used. The oven temperature was programmed as followed: 100 °C for 2 min, increase at a rate 12 °C/min until 300 °C, and finally maintained at 300 °C for 5 min.
2.5 Determination of Pb
Lead in soil (0.200 g DW) or plant (0.200 g DW) was determined by acid digestion; HNO3 + HClO4 (4:1, v/v) was added for digestion at 220 °C for about 120 min followed by HF + HClO4 (5:1, v/v) for about 120 min. Residues were transferred to 25-ml volumetric flask diluted with 5 % of HNO3. The concentrations of Pb were measured by inductively coupled plasma optical emission spectrometry (ICP-OES) after filtration on a 0.45-μm glass fiber (GF) membrane.
2.6 Calculation of removal of pyrene from soil and Pb bioaccumulation or translocation factors
The removal of pyrene from soil was calculated from the initial and final concentrations of pyrene as the following equation:
where R pyrene is the removal of pyrene, C pyrene is the initial concentration of pyrene in soil, while C′pyrene is the concentration of pyrene in soil after the test.
The Pb bioaccumulation factor (BF) and translocation factor (TF) under different treatments were measured and calculated according to the following equations:
where Pb r and Pb s are the concentrations of Pb in root and shoot, respectively, and DW r and DW s stand for the dry weight of root and shoot, respectively. C soil stands for Pb concentration in soil.
2.7 Statistical methods
All data were analyzed using the SPSS software program (version 17.0 for Windows). The treatment means were analyzed using one-way analysis of variance (ANOVA), and significant differences between the means were determined by the LSD test. The differences were considered statistically significant when P < 0.05.
3 Results and discussion
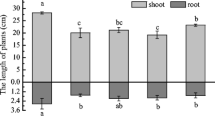
3.1 Growth of S. triqueter with different treatments
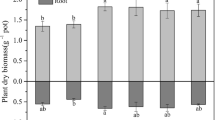
As compared to the control, the single addition of NTA enhanced the length of S. triqueter (Fig. 1) by 11.6 % (N1) and 19.1 % (N2). Also, the biomass of root and shoot were increased significantly by alone application of NTA (Table 1). The increase of plant growth may be due to lower toxicity to S. triqueter or its contribution as a nutrient to plant (Najeeb et al. 2011) or high capacity of S. triqueter to tolerate Pb. However, APG had less effect on increasing the length and biomass of the plant when compared with application of NTA alone. In the treatments of combined application of these two compounds, no notable negative influence on the growth of S. triqueter was found except where APG and NTA were applied at higher concentrations (N2 + A2). Similar phenomenon has been demonstrated in another research (Shi et al. 2009) where the cooperation of high dosage of ethylenediamine disuccinic acid and rhamnolipid has negative effect on ryegrass growth. This reduction of plant growth was probably due to higher concentration of Pb in plant (Table 1), which may exceed the capacity to tolerate toxicity of Pb.
3.2 Accumulation and translocation of Pb
Compared to the single application of APG, NTA had larger capacity to enhance the concentration of Pb in plant tissues (Table 2). For example, Pb root concentration was increased to 400 mg kg−1 DW and 483 mg kg−1 DW with application of NTA (N1 and N2, respectively) but only to 244 mg kg−1 DW and 250 mg kg−1 DW with APG (A1 and A2, respectively). In addition, the values of BF and TF with the treatments of NTA alone were also much higher than with APG. The efficiency of phytoremediation for HMs mainly depends on the amounts of bioavailable metals in soil (Luo et al. 2005; Tandy et al. 2003). As a chelating agent, NTA does help to Pb desorbing out from the soil matrix, then combined it to form complexes (Nowack et al. 2006; Quartacci et al. 2007; Hseu et al. 2013) which can dramatically increase the soluble Pb in soil, while surfactants may not be able to form such complexes with HMs. The slight increase of Pb concentration in root by adding APG might mainly be due to changing the surface tension at the cellular walls of root that increase the uptake of Pb by plant (Di Gregorio et al. 2006; Almeida et al. 2009; Liu et al. 2013).
Although APG had no obvious effect on accumulation or translocation of Pb, the concentration of Pb in plant and BF (or TF) values were extremely increased when NTA was applied combined to APG, which was much higher than in the treatments with NTA alone (Fig. 2). The gap of the observed and theoretical values was used to evaluate the synergistic effect of combined addition of APG and NTA. Figure 2a–d reveals that compared to the theoretical value, there were great promotion and synergy of accumulation and translocation of Pb in plant generally. Two main mechanisms might contribute to this synergy. On one hand, the availability of Pb was greatly increased in the presence of NTA, while surfactants can improve the surface tension at the cellular walls of the root. These changes will happen together with combined application which led more Pb to be accumulated in plant when compared to the single application of NTA. On the other hand, it has been suggested that significant amount of surfactants is adsorbed onto the soil more easily (Almeida et al. 2009; Cao et al. 2013), which may result in competition between surfactants and chelates to be adsorbed on the surface sites of soil particles. So while the NTA and APG were applied simultaneously, there were more soluble NTA in soil to form NTA-Pb chelate so that more Pb was extracted from soil particles. The exception to this situation was found in the treatments of combination of NTA with lower dosage of APG where the observed values of Pb concentration in root were lower than the theoretical ones (N1 + A1 and N2 + A1) and observed value of BF was also lower than the theoretical one (N1 + A1). The potential reason for this situation was that the change caused by lower dosage of APG on surface tension at the cellular walls of the root was not sufficient to lead S. triqueter to accumulate much more Pb in root. However, the values of concentration of Pb in shoot or root and the values of BF or TF were still much higher in this combined application of APG and NTA (BF 0.51 in N1 + A1 and 0.94 in N2 + A1 and TF 0.14 in N1 + A1 and 0.23 in N2 + A1) when compared with the alone application (BF 0.45 in N1 and 0.43 in N2 and TF 0.10 in N1 and 0.19 in N2). However, further researches should be conducted to explain the precise mechanisms of this synergistic effect by the combined application.
Concentration of Pb in plant tissues (a root and b shoot) and BF (c) and TF (d) values with combined application of APG and NTA. The solid and dashed lines denote the observed and theoretical values, respectively, where the theoretical one was calculated concentration of Pb or values of BF (or TF) with single application of NTA plus that in single application of APG
3.3 Dissipation of pyrene from soil
When compared to control, pyrene loss (Fig. 3) both in APG alone and combined with NTA treatments was significantly increased by 63.3 and 72.1 % (42.1 and 42.3 % for NTA). However, based on comparisons of the pyrene loss with application of either APG or NTA, the effect of APG on enhancing removal of pyrene was more significant than NTA. Three mechanisms might explain the phenomenon. Firstly, the special structures of surfactants with hydrophobic and hydrophilic groups lead to the formation of micelles while added in soil. These clusters formed by surfactants can interact with hydrophobic groups of OPs so that the amounts of soluble OPs increase in soil. For this reason, the bioavailability of pyrene was greatly improved in the presence of APG. Secondly, microbial number potentially becomes much higher with APG application, since it has been previously documented that some surfactants like rhamnolipid or saponin have a promoting effect on microbial numbers in the soil (Liao et al. 2015). Finally, adding APG could also change the enzyme activity in the soil. As shown by Shi et al. (2009), a significant increase of polyphenol oxidase, FAD hydrolase, and dehydrogenase activities can enhance biodegradation of PAHs. Additionally, although NTA may not be able to form micelles to improve bioavailability directly just like APG, it may improve it indirectly by changing properties of soil. For example, the removal of HMs with the help of chelates can cause the soil organic matter to become less constrained, thereby increasing the diffusion rate of PAHs (Chigbo and Batty 2013b). In addition, it may also become a carbon source for microorganisms or enhance the enzymatic activities to increase dissipation of pyrene in the soil. More importantly, a little synergistic effect on dissipation of pyrene was found when APG was applied with combination of lower dosage of NTA (N1 + A1 and N1 + A2), where the loss of pyrene was slightly higher than with the application of APG alone. However, this synergistic effect disappeared while the extra NTA (2 g kg−1) was added in the combined system. But, the losses of pyrene (74.4 % in A1 + N2 and 75.0 % in A2 + N2) were still much higher compared with the control (46.2 %) and application of only NTA (65.6 % in N1 and 65.7 % in N2). Effects on bioavailability of pyrene or enzymatic activity potentially happened when both APG and NTA were applied, so the removal of pyrene was increased with combined application compared to the NTA alone. However, surplus NTA in soil may affect the effect of APG to improve bioavailability of pyrene so that removal rate of pyrene in soil was decreased slightly in the treatments of N2 + A1 and N2 + A2 compared to N1 + A1 and N1 + A2. Further research should be carried out to confirm the enhancing mechanisms.
4 Conclusions
The outcome of this investigation has highlighted the effect on assisting phytoremediation for pyrene and Pb from soil by S. triqueter with APG and NTA mixed utilization. The growth of S. triqueter showed no obviously toxic symptoms by application of either APG or NTA. For combined application, only in the treatment of surplus dosage APG and NTA (N2 + A2), plant growth was restricted. The accumulation and translocation of Pb were significantly improved with NTA treatment but not significantly increased with APG application. More interestingly, a synergy and promotion of accumulation and translocation of Pb were achieved with the combined application of APG and NTA. Results also showed that the dissipation ratio of pyrene was increased both by alone application and combined application, which was described as follows: APG ≈ APG + NTA > NTA > CK. Overall, the data in this study indicate that exogenous APG and NTA combined application can increase Pb uptake from soil by S. triqueter and be beneficial in accelerating dissipation of pyrene in soil. Finally, the combined application of APG and NTA represents a feasible approach to enhance phytoremediation of Pb-pyrene co-contaminated soil. However, with the aim of optimizing this combined hardening remediation technology, further studies related to the dosage and ratio of APG and NTA should be conducted to make this approach practical.
References
Almeida CMR, Dias AC, Mucha AP, Bordalo AA, Vasconcelos MTSD (2009) Influence of surfactants on the Cu phytoremediation potential of a salt marsh plant. Chemosphere 75:135–140
Cachada A, Pato P, Rocha-Santos T, da Silva EF, Duarte AC (2012) Levels, sources and potential human health risks of organic pollutants in urban soils. Sci Total Environ 430:184–192
Cao MH, Hu Y, Sun Q, Wang LL, Chen J, Lu XH (2013) Enhanced desorption of PCB and trace metal elements (Pb and Cu) from contaminated soils by saponin and EDDS mixed solution. Environ Pollut 174:93–99
Chen R, Zhou ZR, Liu YX, Jiang J, Li Q, Song HH, Pei DH, Xu H (2015) Mycoremediation potential and tolerance responses of Oudemansiella radicata in cadmium-pyrene co-contaminated soil. J Soils Sediments 15:1083–1093
Chigbo C, Batty L (2013a) Phytoremediation potential of Brassica juncea in Cu-pyrene co-contaminated soil: comparing freshly spiked soil with aged soil. J Environ Manag 129:18–24
Chigbo C, Batty L (2013b) Effect of EDTA and citric acid on phytoremediation of Cr-B[a]P-co-contaminated soil. Environ Sci Pollut Res 20:8955–8963
Di Gregorio S, Barbafieri M, Lampis S, Sanangelantoni AM, Tassi E, Vallini G (2006) Combined application of Triton X-100 and Sinorhizobium sp. Pb002 inoculum for the improvement of lead phytoextraction by Brassica juncea in EDTA amended soil. Chemosphere 63:293–299
Ehsan S, Ali S, Noureen S, Mahmood K, Farid M, Ishaque W, Shakoor MB, Rizwan M (2014) Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol Environ Saf 106:164–172
Fabietti G, Biasioli M, Barberis R, Ajmone-Marsan F (2009) Soil contamination by organic and inorganic pollutants at the regional scale: the case of Piedmont, Italy. J Soils Sediments 10:290–300
Freitas EV, do Nascimento CW (2009) The use of NTA for lead phytoextraction from soil from a battery recycling site. J Hazard Mater 171:833–837
Hseu Z, Jien S, Wang S, Deng H (2013) Using EDDS and NTA for enhanced phytoextraction of Cd by water spinach. J Environ Manag 117:58–64
Jagetiya B, Sharma A (2013) Optimization of chelators to enhance uranium uptake from tailings for phytoremediation. Chemosphere 91:692–696
Lan JC, Zhang SR, Lin HC, Li T, Xu XX, Li Y, Jia YX, Gong GS (2013) Efficiency of biodegradable EDDS, NTA and APAM on enhancing the phytoextraction of cadmium by Siegesbeckia orientalis L. grown in Cd-contaminated soils. Chemosphere 91:1362–1367
Lestan D, Luo CL, Li XD (2008) The use of chelating agents in the remediation of metal-contaminated soils: a review. Environ Pollut 153:3–13
Liao CJ, Liang XJ, Lu GN, Thai T, Xu WD, Dang Z (2015) Effect of surfactant amendment to PAHs-contaminated soil for phytoremediation by maize (Zea mays L.). Ecotoxicol Environ Saf 112:1–6
Liu FH, Zhang XY, Liu XY, Chen XP, Liang X, He CQ, Wei J, Xu G (2013) Alkyl polyglucoside (APG) amendment for improving the phytoremediation of Pb-PAH contaminated soil by the aquatic plant Scirpus triqueter. Soil Sediment Contam 22:1013–1027
Liu WX, Wang QL, Wang BB, Hou JY, Luo YM, Tang CX, Franks A (2015) Plant growth-promoting rhizobacteria enhance the growth and Cd uptake of Sedum plumbizincicola in a Cd-contaminated soil. J Soils Sediments 15:1191–1199
Liu XY, Wang ZZ, Zhang XY, Wang J, Xu G, Cao ZN, Zhong CL, Su PC (2011) Degradation of diesel-originated pollutants in wetlands by Scirpus triqueter and microorganisms. Ecotoxicol Environ Saf 74:1967–1972
Liu ZL, He XY, Chen W, Yuan FH, Yan K, Tao DL (2009) Accumulation and tolerance characteristics of cadmium in a potential hyperaccumulator—Lonicera japonica Thunb. J Hazard Mater 169:170–175
Luo CL, Shen ZG, Li XD (2005) Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 59:1–11
Najeeb U, Jilani G, Ali S, Sarwar M, Xu L, Zhou W (2011) Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater 186:565–574
Noll MR, Almeter K, Pope GG (2014) Distribution of lead in an urban soil: a case study and implications for potential remedial options. Earth Planet Sci Lett 10:353–357
Nowack B, Schulin R, Robinson BH (2006) Critical assessment of chelant-enhanced metal phytoextraction. Environ Sci Technol 40:5225–5232
Quartacci MF, Irtelli B, Baker AJ, Navari-Izzo F (2007) The use of NTA and EDDS for enhanced phytoextraction of metals from a multiply contaminated soil by Brassica carinata. Chemosphere 68:1920–1928
Shi FG, Hao XZ, Zhou DM, Qian Y (2009) Remediation of the combined polluted soil by growing ryegrass enhanced by EDDS/rhamnolipid. J Agro-Environ Sci 28:1818–1823
Song SS, Zhu LZ, Zhou WJ (2008) Simultaneous removal of phenanthrene and cadmium from contaminated soils by saponin, a plant-derived biosurfactant. Environ Pollut 156:1368–1370
Sun L, Liao XY, Yan XL, Zhu GH, Ma D (2014) Evaluation of heavy metal and polycyclic aromatic hydrocarbons accumulation in plants from typical industrial sites: potential candidate in phytoremediation for co-contamination. Environ Sci Pollut Res 21:12494–12504
Sun TR, Cang L, Wang QY, Zhou DM, Cheng JM, Xu H (2010) Roles of abiotic losses, microbes, plant roots, and root exudates on phytoremediation of PAHs in a barren soil. J Hazard Mater 176:919–925
Tandy S, Bossart K, Mueller R, Ritschel J, Hauser L, Schulin R, Nowack B (2003) Extraction of heavy metals from soils using biodegradable chelating agents. Environ Sci Technol 38:937–944
Wang K, Zhang J, Zhu ZQ, Huang HG, Li TQ, He ZL, Yang XE, Alva A (2012) Pig manure vermicompost (PMVC) can improve phytoremediation of Cd and PAHs co-contaminated soil by Sedum alfredii. J Soils Sediments 12:1089–1099
Wei JL, Lai HY, Chen ZS (2012) Chelator effects on bioconcentration and translocation of cadmium by hyperaccumulators, Tagetes patula and Impatiens walleriana. Ecotoxicol Environ Saf 84:173–178
Zhang XY, Liu XY, Liu SS, Liu FH, Chen LS, Xu G, Zhong CL, Su PC, Cao ZN (2011) Responses of Scirpus triqueter, soil enzymes and microbial community during phytoremediation of pyrene contaminated soil in simulated wetland. J Hazard Mater 193:45–51
Zhang XY, Liu XY, Wang Q, Chen XP, Li HB, Wei J, Xu G (2014) Diesel degradation potential of endophytic bacteria isolated from Scirpus triqueter. Int Biodeterior Biodegrad 87:99–105
Acknowledgments
The work was funded by the National Natural Science Foundation of China (No. 41373097) and Program for Innovative Research Team in University (No. IRT13078).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Peter Schroeder
Rights and permissions
About this article
Cite this article
Chen, T., Liu, X., Zhang, X. et al. Enhanced Scirpus triqueter phytoremediation of pyrene and lead co-contaminated soil with alkyl polyglucoside and nitrilotriacetic acid combined application. J Soils Sediments 16, 2090–2096 (2016). https://doi.org/10.1007/s11368-016-1394-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1394-5