Abstract
Age-related impairment of neurovascular coupling (NVC; or “functional hyperemia”) compromises moment-to-moment adjustment of regional cerebral blood flow to increased neuronal activity and thereby contributes to the pathogenesis of vascular cognitive impairment (VCI). Previous studies established a causal link among age-related decline in circulating levels of insulin-like growth factor-1 (IGF-1), neurovascular dysfunction and cognitive impairment. Endothelium-mediated microvascular dilation plays a central role in NVC responses. To determine the functional consequences of impaired IGF-1 input to cerebromicrovascular endothelial cells, endothelium-mediated NVC responses were studied in a novel mouse model of accelerated neurovascular aging: mice with endothelium-specific knockout of IGF1R (VE-Cadherin-CreERT2/Igf1rf/f). Increases in cerebral blood flow in the somatosensory whisker barrel cortex (assessed using laser speckle contrast imaging through a cranial window) in response to contralateral whisker stimulation were significantly attenuated in VE-Cadherin-CreERT2/Igf1rf/f mice as compared to control mice. In VE-Cadherin-CreERT2/Igf1rf/f mice, the effects of the NO synthase inhibitor L-NAME were significantly decreased, suggesting that endothelium-specific disruption of IGF1R signaling impairs the endothelial NO-dependent component of NVC responses. Collectively, these findings provide additional evidence that IGF-1 is critical for cerebromicrovascular endothelial health and maintenance of normal NVC responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related impairment of neurovascular coupling (NVC; or “functional hyperemia”) contributes to the pathogenesis of vascular cognitive impairment (VCI) [1]. Neurovascular dysfunction compromises adjustment of cerebral blood flow to the increased needs of active brain regions, impairing energy and oxygen delivery to the firing neurons and hindering washout of toxic metabolic by-products [1]. Neurovascular coupling depends on a tightly controlled interaction of activated neurons and astrocytes and the release of vasodilator metabolites from the astrocyte end-feet and microvascular endothelial cell, which elicit vasodilation in precapillary arterioles. The cellular mechanisms by which aging impairs neurovascular coupling responses primarily involve a significant reduction in endothelial production/release of nitric oxide (NO) [2,3,4].
Insulin-like growth factor-1 (IGF-1) is an anabolic hormone produced by the liver, which exerts multifaceted vasoprotective and anti-geronic effects [1, 5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Circulating IGF-1 significantly decreases with age in humans and in laboratory animals due to an age-related decline in GH production/release [12, 30, 32,33,34,35]. Importantly, previous studies demonstrate that circulating IGF-1 deficiency in transgenic mouse models impairs neurovascular coupling responses, mimicking the aging phenotype [9, 36]. Each cell type of the neurovascular unit (including neurons, astrocytes, endothelial cells) abundantly express IGF1R, the receptor for IGF-1 and the specific roles of IGF1R signaling in endothelial cells in regulation of NVC responses remains to be determined.
The present study was designed to experimentally test the hypotheses that IGF1R signaling modulates endothelium-dependent NVC responses in the brain and that disruption of IGF1R signaling specifically in endothelial cells impairs functional hyperemia, mimicking aspects of the aging phenotype. To test our hypotheses, we used a novel mouse model with adult-onset, endothelial cells-specific disruption of IGF1R signaling using Cre-lox technology (VE-Cadherin-CreERT2/Igf1rf/f). To assess endothelial NO-mediated NVC responses, increases in cerebral blood flow in the somatosensory whisker barrel cortex in response to contralateral whisker stimulation were measured using laser speckle contrast imaging before and after administration of an NO synthase inhibitor.
Methods
Animals
Igf1rf/f (B6;129-Igf1rtm2Arge/J; loxP sites flanking exon 3) and VE-Cadherin-Cre ERT2 (B6.FVB-Tg(Cdh5-cre)7Mlia/J; Stock No: 006137) mice were obtained from Jackson laboratories. Mice were housed (3–4 per cage) in Allentown XJ cages with Anderson's Enrich-o-cob bedding (Maumee, OH). Igf1rf/f mice were bred in house to generate experimental cohorts. Animals were housed under specific pathogen-free (including helicobacter and parvovirus free) barrier conditions in the Rodent Barrier Facility at University of Oklahoma Health Sciences Center. Mice were bred on a 14-h light/10-h dark cycle and weaned mice were maintained in a 12-h light/12-h dark cycle at 21 °C and were given access to standard irradiated bacteria-free rodent chow (5053 Pico Lab, Purina Mills, Richmond, IN) and reverse osmosis filtered water ad libitum. Male VE-Cadherin-CreERT2 mice were bred with female Igf1rf/f mice to generate VE-Cadherin-CreERT2/Igf1r+/− males, which were bred with Igf1rf/f female mice to obtain the founder colony of VE-Cadherin-CreERT2/Igf1r homozygous floxed mice, following our previously described protocol [18]. These mice were bred with Igf1rf/f mice to generate experimental cohorts of VE-Cadherin-CreERT2/Igf1rf/f and Cre-/Igf1rf/f control mice. Three-month-old mice were injected intraperitoneally with tamoxifen (75 mg/kg body weight; dissolved in corn oil) or vehicle (corn oil) for 5 days. Experiments were conducted after a period of 2 months. All procedures were approved by the Institutional Animal Use and Care Committee of the University of Oklahoma Health Sciences Center.
Measurement of neurovascular coupling responses
On the day of experimentation, mice in each group were anesthetized with isoflurane (4% induction and 1% maintenance), endotracheally intubated and ventilated (MousVent G500; Kent Scientific Co, Torrington, CT). A thermostatic heating pad (Kent Scientific Co, Torrington, CT) was used to maintain rectal temperature at 37 °C [37]. End-tidal CO2 was controlled between 3.2% and 3.7% to keep blood gas values within the physiological range, as described [9, 38, 39]. The right femoral artery was canulated for arterial blood pressure measurement (Living Systems Instrumentations, Burlington, VT) [37]. The blood pressure was within the physiological range throughout the experiments (90–110 mmHg). Mice were immobilized and placed on a stereotaxic frame (Leica Microsystems, Buffalo Grove, IL), the scalp and periosteum were pulled aside and the skull was gently thinned using a dental drill while cooled with dripping buffer. A laser speckle contrast imager (Perimed, Järfälla, Sweden) was placed 10 cm above the thinned skull, and to achieve the highest CBF response the right whiskers were stimulated for 30 s at 5 Hz from side to side as described [40, 41]. Differential perfusion maps of the brain surface were captured. Changes in CBF were assessed above the left barrel cortex in six trials in each group, separated by 5–10 min intervals. To assess the role of NO mediation, CBF responses to whisker stimulation were repeated 15 min after intravenous administration of the nitric oxide synthase inhibitor Nω-Nitro-L-arginine methyl ester (L-NAME). Changes in CBF were averaged and expressed as percent (%) increase from the baseline value [42]. All drugs used in this study were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise indicated.
Statistical analysis
Statistical analysis was carried out by unpaired t test or one-way ANOVA followed by Bonferroni multiple comparison test, as appropriate, using Prism 5.0 for Windows (Graphpad Software, La Jolla, CA). A p value less than 0.05 was considered statistically significant. Data are expressed as mean ± S.E.M.
Results
Endothelium-specific disruption of IGF-1/IGF1R signaling impairs neurovascular coupling
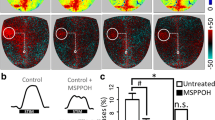
Increases in CBF in the somatosensory whisker barrel cortex in response to contralateral whisker stimulation were significantly attenuated in 6-month-old VE-Cadherin-CreERT2/Igf1rf/f mice (Fig. 1A–C), indicating that endothelium-specific disruption of IGF1R signaling leads to neurovascular dysfunction (n = 6–10 ♂ mice in each group).
Endothelium-specific disruption of IGF-1/IGF1R signaling impairs neurovascular coupling responses in mice. A) Representative pseudocolour laser speckle flowmetry maps of baseline CBF (upper row; shown for orientation purposes) and CBF changes in the whisker barrel field relative to baseline during contralateral whisker stimulation (bottom row, right oval, 30 s, 5 Hz) in control and VE-Cadherin-CreERT2/Igf1rf/f mice before and after administration of the NO synthase inhibitor L-NAME. B shows the time-course of CBF changes after the start of contralateral whisker stimulation (horizontal bars). Summary data are shown in C. Data are mean ± S.E.M. (n = 6–10 ♂ mice in each group), *P < 0.05 vs. Control; #P < 0.05 vs. untreated. n.s.: not significant
Upon activation by neuronal-derived glutamate astrocytes release ATP, which elicits endothelial NO-mediated microvascular dilation in the brain [43]. Endothelial NO mediation is also critical for the upstream conduction and spreading of microvascular dilation [3]. Consistent with this concept we found that in control animals administration of the NO synthase inhibitor L-NAME (Fig. 1B–C) significantly decreased functional hyperemia in the barrel cortex elicited by contralateral whisker stimulation.
In VE-Cadherin-CreERT2/Igf1rf/f mice, the effects of L-NAME (Fig. 1B–C) were significantly decreased, suggesting that endothelium-specific disruption of IGF1R signaling impairs the endothelial NO-dependent component of NVC responses.
Discussion
Endothelial NO mediation plays a critical role both in NVC responses and the upstream conduction and spreading of microvascular dilation [3, 43]. IGF-1 receptors are abundantly expressed on endothelial cells [44]. The present study provides critical evidence that cell-specific disruption of IGF1R signaling in endothelial cells alters their function, impairing NO-mediated NVC responses. These new findings extend the results of our previous studies showing that circulating IGF-1 deficiency also impairs the endothelium-dependent NVC responses [9]. The likely mechanisms by which disruption of endothelial IGF-1/IGF1R signaling impairs NO-mediated NVC responses may include decreased NO bioavailability due to increased production of reactive oxygen species (ROS) [9]. There is strong evidence linking impaired NVC responses to impaired performance on cognitive tasks [1, 38,39,40,41, 45]. Thus, further studies are warranted to determine how the neurovascular phenotype caused by disruption of endothelial IGF-1/IGF1R signaling impacts cognitive function in mice.
Previous studies showed that in addition to regulating vasodilator function IGF-1/IGF1R signaling also modulates many other important aspects of endothelial function, including angiogenesis and barrier function [22, 23, 30, 46,47,48,49,50,51]. There is evidence that disruption of IGF-1/IGF1R signaling may also impact these aspects of cerebromicrovascular endothelial cell function, which may contribute to microvascular rarefaction and blood–brain barrier disruption, exacerbating cognitive impairment associated with IGF-1 deficiency [8, 10, 52, 53]. Circulating insulin at physiological concentrations has low affinity IGF-1R, while under experimental conditions, at supraphysiological levels, it was found that insulin and IGF-1 cross-react with each other’s receptors, albeit at a significantly lower affinity than with their own receptors. Previous studies suggested that IGF1R can regulate insulin sensitivity and NO bioavailability in the endothelium of conduit arteries [54]. Yet, in mice overexpressing human IGF-1R in the endothelium insulin sensitivity is unaffected [55] To better understand the effects of IGF-1/IGF1R signaling on endothelial phenotype, subsequent studies should investigate transcriptional changes in the cerebromicrovascular endothelial cells derived from VE-cadherin-CreERT2/Igf1rf/f mice. While decreasing IGF-1 input to the endothelial cells is clearly detrimental, mice overexpressing human IGF-1R in the endothelium were shown to exhibit unaltered vasorelaxation to endothelium-dependent vasodilators [55].
The aforementioned observations are consistent with the concept that disruption of IGF-1/IGF1R signaling in endothelial cells promotes the acquisition of an accelerated neurovascular aging phenotypes. Accordingly, aging in humans and in laboratory animals results in circulating IGF-1 deficiency [12, 30, 32,33,34], which associates with neurovascular uncoupling, endothelial dysfunction, microvascular rarefaction and disruption of the blood–brain barrier [41, 56,57,58]. Heterochronic parabiosis is a surgical approach for joining the circulatory systems of an aged and a young animal that is used to identify non-cell autonomous mechanisms of aging. We have recently demonstrated that exposure to a young humoral environment rescues endothelial aging phenotypes in mice, including attenuation of oxidative stress and restoration of endothelium-mediated vasodilation [59]. Importantly, transcriptomic analysis identified IGF1R signaling as a likely upstream regulator involved in young blood-mediated vascular rejuvenation [59]. In future studies older VE-Cadherin-CreERT2/Igf1rf/f mice could be used as parabionts to experimentally interrogate the contribution of IGF-1/IGF1R signaling to the vasoprotective effects of young blood transfer.
Taken together, our present findings provide additional support for the concept that deficient IGF-1 input to the cerebromicrovascular endothelial cells compromises the function of the neurovascular unit, impairing NVC responses and likely multiple other aspects of brain health. The findings that disruption of IGF-1/IGF1R signaling results in neurovascular uncoupling and endothelial dysfunction have important translational relevance for the genesis of age-related vascular cognitive impairment and cognitive problems associated with genetic IGF-1 deficiency (e.g. in patients with growth hormone releasing hormone-receptor [GHRH-R] mutations, isolated GH deficiency or GH receptor gene defects [Laron syndrome]). Additionally, multiple IGF1R mutations have been described in children born small for gestational age (SGA) [60, 61], who later exhibit endothelial dysfunction [62] and have decreased levels of intelligence and various cognitive problems [63]. Future studies determining how IGF1R mutations in humans affect endothelial function and NVC responses as well as CBF should be quite revealing. The results of the present study, taken together with the findings of earlier investigations [9, 12, 24,25,26, 53, 64, 65], point to potential multifaceted benefits of various pharmacological, dietary [66, 66] and lifestyle interventions rescuing IGF-1 input to the cerebral microcirculation and the aging brain.
References
Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–8.
Ma J, Ayata C, Huang PL, Fishman MC, Moskowitz MA. Regional cerebral blood flow response to vibrissal stimulation in mice lacking type I NOS gene expression. Am J Physiol. 1996;270:H1085–90.
Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787.
Stobart JL, Lu L, Anderson HD, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2013;110:3149–54.
Higashi Y, Gautam S, Delafontaine P, Sukhanov S. IGF-1 and cardiovascular disease. Growth Horm IGF Res. 2019;45:6–16.
Fulop GA, Ramirez-Perez FI, Kiss T, Tarantini S, Valcarcel Ares MN, Toth P, Yabluchanskiy A, Conley SM, Ballabh P, Martinez-Lemus LA, Ungvari Z, Csiszar A. IGF-1 deficiency promotes pathological remodeling of cerebral arteries: a potential mechanism contributing to the pathogenesis of intracerebral hemorrhages in aging. J Gerontol A Biol Sci Med Sci. 2019;74(4):446–54. https://doi.org/10.1093/gerona/gly144.
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–79.
Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr). 2016;38:273–89.
Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–44.
Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–97.
Dong X, Chang G, Ji XF, Tao DB, Wang YX. The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PLoS One. 2014;9:e94845.
Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27.
Troncoso R, Vicencio JM, Parra V, Nemchenko A, Kawashima Y, Del Campo A, Toro B, Battiprolu PK, Aranguiz P, Chiong M, Yakar S, Gillette TG, Hill JA, Abel ED, Leroith D, Lavandero S. Energy-preserving effects of IGF-1 antagonize starvation-induced cardiac autophagy. Cardiovasc Res. 2012;93:320–9.
Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–39.
von der Thusen JH, Borensztajn KS, Moimas S, van Heiningen S, Teeling P, van Berkel TJ, Biessen EA. IGF-1 has plaque-stabilizing effects in atherosclerosis by altering vascular smooth muscle cell phenotype. Am J Pathol. 2011;178:924–34.
Shai SY, Sukhanov S, Higashi Y, Vaughn C, Rosen CJ, Delafontaine P. Low circulating insulin-like growth factor I increases atherosclerosis in ApoE-deficient mice. Am J Physiol Heart Circ Physiol. 2011;300(5):H1898–906. https://doi.org/10.1152/ajpheart.01081.2010.
Prabhu D, Khan SM, Blackburn K, Marshall JP, Ashpole NM. Loss of insulin-like growth factor-1 signaling in astrocytes disrupts glutamate handling. J Neurochem. 2019;151:689–702.
Logan S, Pharaoh GA, Marlin MC, Masser DR, Matsuzaki S, Wronowski B, Yeganeh A, Parks EE, Premkumar P, Farley JA, Owen DB, Humphries KM, Kinter M, Freeman WM, Szweda LI, Van Remmen H, Sonntag WE. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-beta uptake in astrocytes. Mol Metab. 2018;9:141–55.
Littlejohn EL, Scott D, Saatman KE. Insulin-like growth factor-1 overexpression increases long-term survival of posttrauma-born hippocampal neurons while inhibiting ectopic migration following traumatic brain injury. Acta Neuropathol Commun. 2020;8:46.
Garwood CJ, Ratcliffe LE, Morgan SV, Simpson JE, Owens H, Vazquez-Villasenor I, Heath PR, Romero IA, Ince PG, Wharton SB. Insulin and IGF1 signalling pathways in human astrocytes in vitro and in vivo; characterisation, subcellular localisation and modulation of the receptors. Mol Brain. 2015;8:51.
Pardo J, Uriarte M, Console GM, Reggiani PC, Outeiro TF, Morel GR, Goya RG. Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur J Neurosci. 2016;44:2120–8.
Labandeira-Garcia JL, Costa-Besada MA, Labandeira CM, Villar-Cheda B, Rodriguez-Perez AI. Insulin-Like Growth Factor-1 and Neuroinflammation. Front Aging Neurosci. 2017;9:365.
Okoreeh AK, Bake S, Sohrabji F. Astrocyte-specific insulin-like growth factor-1 gene transfer in aging female rats improves stroke outcomes. Glia. 2017;65:1043–58.
Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46:96–9.
Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225–39.
Muller AP, Fernandez AM, Haas C, Zimmer E, Portela LV, Torres-Aleman I. Reduced brain insulin-like growth factor I function during aging. Mol Cell Neurosci. 2012;49:9–12.
Trueba-Saiz A, Cavada C, Fernandez AM, Leon T, Gonzalez DA, Fortea Ormaechea J, Lleo A, Del Ser T, Nunez A, Torres-Aleman I. Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Transl Psychiatry. 2013;3:e330.
Ascenzi F, Barberi L, Dobrowolny G, Villa Nova Bacurau A, Nicoletti C, Rizzuto E, Rosenthal N, Scicchitano BM, Musaro A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell. 2019;18:e12954.
Williamson TT, Ding B, Zhu X, Frisina RD. Hormone replacement therapy attenuates hearing loss: Mechanisms involving estrogen and the IGF-1 pathway. Aging Cell. 2019;18:e12939.
Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212.
Sanders JL, Guo W, O’Meara ES, Kaplan RC, Pollak MN, Bartz TM, Newman AB, Fried LP, Cappola AR. Trajectories of IGF-I Predict Mortality in Older Adults: The Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2018;73:953–9.
O’Connor KG, Tobin JD, Harman SM, Plato CC, Roy TA, Sherman SS, Blackman MR. Serum levels of insulin-like growth factor-I are related to age and not to body composition in healthy women and men. J Gerontol A Biol Sci Med Sci. 1998;53:M176–82.
Pavlov EP, Harman SM, Merriam GR, Gelato MC, Blackman MR. Responses of growth hormone (GH) and somatomedin-C to GH-releasing hormone in healthy aging men. J Clin Endocrinol Metab. 1986;62:595–600.
Ameri P, Canepa M, Fabbi P, Leoncini G, Milaneschi Y, Mussap M, AlGhatrif M, Balbi M, Viazzi F, Murialdo G, Pontremoli R, Brunelli C, Ferrucci L. Vitamin D modulates the association of circulating insulin-like growth factor-1 with carotid artery intima-media thickness. Atherosclerosis. 2014;236:418–25.
Sherlala RA, Kammerer CM, Kuiper AL, Wojczynski MK, Ukraintseva SV, Feitosa MF, Mengel-From J, Zmuda JM, Minster RL. Relationship between serum IGF-1 and BMI differs by age. J Gerontol A Biol Sci Med Sci. 2021;76(7):1303–8. https://doi.org/10.1093/gerona/glaa282.
Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610.
Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299-308.
Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges E, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–81.
Tarantini S, Balasubramanian P, Yabluchanskiy A, Ashpole NM, Logan S, Kiss T, Ungvari A, Nyul-Toth A, Schwartzman ML, Benyo Z, Sonntag WE, Csiszar A, Ungvari Z. IGF1R signaling regulates astrocyte-mediated neurovascular coupling in mice: implications for brain aging. Geroscience. 2021;43(2):901–11. https://doi.org/10.1007/s11357-021-00350-0.
Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192.
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17(2):e12731. https://doi.org/10.1111/acel.12731.
Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95:1019–26.
Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309:H1837–45.
Chisalita SI, Arnqvist HJ. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab. 2004;286:E896-901.
Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–14.
Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:435–44.
Humpert PM, Djuric Z, Zeuge U, Oikonomou D, Seregin Y, Laine K, Eckstein V, Nawroth PP, Bierhaus A. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor-dependent signaling. Mol Med. 2008;14:301–8.
Norling AM, Gerstenecker AT, Buford TW, Khan B, Oparil S, Lazar RM. The role of exercise in the reversal of IGF-1 deficiencies in microvascular rarefaction and hypertension. Geroscience. 2020;42(1):141–58. https://doi.org/10.1007/s11357-019-00139-2.
Viana IM, de Almeida ME, Lins MP, dos Santos Reis MD, de Araujo Vieira LF, Smaniotto S. Combined effect of insulin-like growth factor-1 and CC chemokine ligand 2 on angiogenic events in endothelial cells. PLoS One. 2015;10:e0121249.
Ko JA, Murata S, Nishida T. Up-regulation of the tight-junction protein ZO-1 by substance P and IGF-1 in A431 cells. Cell Biochem Funct. 2009;27:388–94.
Bake S, Okoreeh A, Khosravian H, Sohrabji F. Insulin-like Growth Factor (IGF)-1 treatment stabilizes the microvascular cytoskeleton under ischemic conditions. Exp Neurol. 2019;311:162–72.
Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–20.
Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–8.
Abbas A, Imrie H, Viswambharan H, Sukumar P, Rajwani A, Cubbon RM, Gage M, Smith J, Galloway S, Yuldeshava N, Kahn M, Xuan S, Grant PJ, Channon KM, Beech DJ, Wheatcroft SB, Kearney MT. The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes. 2011;60:2169–78.
Imrie H, Viswambharan H, Sukumar P, Abbas A, Cubbon RM, Yuldasheva N, Gage M, Smith J, Galloway S, Skromna A, Rashid ST, Futers TS, Xuan S, Gatenby VK, Grant PJ, Channon KM, Beech DJ, Wheatcroft SB, Kearney MT. Novel role of the IGF-1 receptor in endothelial function and repair: studies in endothelium-targeted IGF-1 receptor transgenic mice. Diabetes. 2012;61:2359–68.
Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–20.
Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of Vascular Aging. Circ Res. 2018;123:849–67.
Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:931–41.
Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience. 2020;42(2):727–48. https://doi.org/10.1007/s11357-020-00180-6.
Klammt J, Kiess W, Pfaffle R. IGF1R mutations as cause of SGA. Best Pract Res Clin Endocrinol Metab. 2011;25:191–206.
Ester WA, Hokken-Koelega AC. Polymorphisms in the IGF1 and IGF1R genes and children born small for gestational age: results of large population studies. Best Pract Res Clin Endocrinol Metab. 2008;22:415–31.
Jouret B, Dulac Y, Bassil Eter R, Taktak A, Cristini C, Lounis N, Molinas C, Salles JP, Arnaud C, Acar P, Tauber M. Endothelial function and mechanical arterial properties in children born small for gestational age: comparison with obese children. Horm Res Paediatr. 2011;76:240–7.
Lohaugen GC, Ostgard HF, Andreassen S, Jacobsen GW, Vik T, Brubakk AM, Skranes J, Martinussen M. Small for gestational age and intrauterine growth restriction decreases cognitive function in young adults. J Pediatr. 2013;163:447–53.
Genis L, Davila D, Fernandez S, Pozo-Rodrigalvarez A, Martinez-Murillo R, Torres-Aleman I. Astrocytes require insulin-like growth factor I to protect neurons against oxidative injury. F1000Res. 2014;3:28.
Trejo JL, Piriz J, Llorens-Martin MV, Fernandez AM, Bolos M, LeRoith D, Nunez A, Torres-Aleman I. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry. 2007;12:1118–28.
Balasubramanian P, DelFavero J, Ungvari A, Papp M, Tarantini A, Price N, de Cabo R, Tarantini S. Time-restricted feeding (TRF) for prevention of age-related vascular cognitive impairment and dementia. Ageing Res Rev. 2020;64:101189.
Acknowledgements
This work was supported by grants from the American Heart Association, the American Federation for Aging Research (Irene/Diamond Postdoctoral Transition Award to PB), the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging ((R01-AG055395, R01-AG047879; R01-AG038747; R01-AG072295), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS100782), the National Cancer Institute (NCI;1R01CA255840), the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (U54GM104938, to AY), the Presbyterian Health Foundation and the NKFIH (Nemzeti Szivlabor). The authors acknowledge the support from the NIA-funded Geroscience Training Program in Oklahoma (T32AG052363), the Oklahoma Nathan Shock Center (P30AG050911), the Cellular and Molecular GeroScience CoBRE (1P20GM125528, sub#5337). The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Anna Csiszar serves as Associate Editor for The Journal of Gerontology, Series A: Biological Sciences and Medical Sciences and GeroScience. Dr. Andriy Yabluchanskiy serves as Guest Editor for The American Journal of Physiology-Heart and Circulatory Physiology. Dr. William E. Sonntag serves as Associate Editor for The Journal of Gerontology, Series A: Biological Sciences and GeroScience. Dr. Zoltan Ungvari serves as Associate Editor for The Journal of Gerontology, Series A: Biological Sciences, Editor-in-Chief for GeroScience and as Consulting Editor for The American Journal of Physiology-Heart and Circulatory Physiology.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tarantini, S., Nyúl-Tóth, Á., Yabluchanskiy, A. et al. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. GeroScience 43, 2387–2394 (2021). https://doi.org/10.1007/s11357-021-00405-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-021-00405-2