Abstract
Dual tasking, a defined facet of executive control processes, is subserved, in part, by the prefrontal cortex (PFC). Previous functional near-infrared spectroscopy (fNIRS) studies revealed elevated PFC oxygenated hemoglobin (HbO2) under Dual-Task-Walk (DTW) compared to Single-Task Walk (STW) conditions. Based on the concept of neural inefficiency (i.e., greater activation coupled with similar or worse performance), we hypothesized that decreased cortical thickness across multiple brain regions would be associated with greater HbO2 increases from STW to DTW. Participants were 55 healthy community-dwelling older adults, whose cortical thickness was measured via MRI. HbO2 levels in the PFC, measured via fNIRS, were assessed during active walking under STW and DTW conditions. Statistical analyses were adjusted for demographics and behavioral performance. Linear mixed-effects models revealed that the increase in HbO2 from STW to DTW was moderated by cortical thickness in several regions. Specifically, thinner cortex in specific regions of the frontal, parietal, temporal, and occipital lobes, cingulate cortex, and insula was associated with greater increases in HbO2 levels from single to dual-task walking. In conclusion, participants with thinner cortex in regions implicated in higher order control of walking employed greater neural resources, as measured by increased HbO2, in the PFC during DTW, without demonstrating benefits to behavioral performance. To our knowledge, this is the first study to examine cortical thickness as a marker of neural inefficiency during active walking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gait, a robust measure of health [1, 2], relies on higher order mechanisms of cognitive control and cortical resources [3, 4]. Dual-task designs have been used to examine executive control and allocation of cognitive resources to competing task demands during walking [5,6,7,8,9,10]. Executive control is adversely affected by aging [11] and poor dual-task-walking (DTW) performance has been associated with falls [12], cognitive decline [13], frailty, disability, and mortality [14] in older adults. Functional near-infrared spectroscopy (fNIRS) [15, 16], a noninvasive technique that utilizes near-infrared light to visualize brain activation by quantifying oxygenated (HbO2) and deoxygenated hemoglobin (Hb), has been used to examine brain activation patterns associated with executive control in DTW [17,18,19]. The prefrontal cortex is implicated in cortical control of attention and executive functions including DTW [20], and many studies [21,22,23] including a recent meta-analysis [24] have shown that fNIRS-derived oxygenation levels in the PFC increase from single-task walking (STW) to DTW, due to the increased resources required in the latter condition. Other studies have measured whole brain activity during DTW [25, 26] and used other neuroimaging techniques including EEG to demonstrate the neural costs of DTW [27,28,29].
The examination of neural mechanisms of gait have been limited as typical neuroimaging methods are not conducive to active walking [30]. Nevertheless, many studies have demonstrated relationships between brain morphology and gait characteristics including pace, rhythm, and variability [31]. Specifically, reduced white matter integrity and reduced gray matter volume is associated with worse gait performance in global and specific regions including the frontal lobes, basal ganglia, hippocampus, and cerebellum. Additional research has demonstrated overlapping and distinct neural underpinnings of gait under STW versus DTW conditions [32, 33]. Atrophy of gray matter, which occurs throughout the brain during healthy aging and prominently in the prefrontal cortex [34,35,36,37], may drive, or at least be related to, functional changes in executive functioning [38] and locomotion [30, 31, 33] in older adults. The neural inefficiency hypothesis suggests that more neural resources are used when those needed for a task are limited [39,40,41]. Following this hypothesis, the moderation effect of structural integrity of the brain on activation levels during walking has been examined (i.e., whether task-related brain activation differs based on brain integrity [42, 43]). Reduced whole brain white matter integrity [42] and reduced frontal gray matter volume [43] in older adults were associated with greater increases in activation during DTW compared to STW without an associated improvement in performance. Previous research has found that gray matter volume is more closely related to surface area than to thickness of gray matter, genetically and phenotypically distinct measures [44], and that analyses of cortical thickness may provide a more sensitive measure of age-related brain changes than analyses of gray matter volumetrics [44, 45]. To our knowledge, cortical thickness has not been examined in relation to prefrontal cortex activation during walking in healthy older adults or in any other population.
The current study aimed to examine the moderating effect of regional cortical thickness on the change in HbO2 levels between STW and DTW. To capture neural efficiency, all analyses were adjusted for walking velocity and cognitive task performance during DTW. We predicted that decreased cortical thickness, in brain regions implicated in higher order control of walking, would be associated with a greater STW to DTW increase in the prefrontal cortex HbO2 levels. Based on previous findings, we predicted that this would be seen especially in the prefrontal cortex; however, additional regions of interest have been implicated in volume-based analyses, including parts of the parietal, temporal, and occipital lobes [43]. As gray matter volume and thickness are distinct, we aimed to quantify the relationship between cortical thickness and the STW to DTW change in HbO2. Considering that DTW relies on higher order executive control in addition to sensory and motor activities, we included the entire cortex to examine areas in which cortical thinning may be functionally influential.
Methods
Participants
Recruitment
Participants were recruited from a cohort study of community-dwelling older adults, entitled “Central Control of Mobility in Aging (CCMA).” The procedures for the CCMA study have been described previously [8]. Adults aged 65 years or older were identified as potential participants from population lists in the Westchester county area of New York, USA. These potential participants were mailed a letter, and then contacted by telephone. Verbal consent was obtained, and initial eligibility was determined via telephone interviews, which included assessment of medical history, physical functioning, and screening for dementia [46, 47]. After this initial assessment, eligible individuals were invited to participate in two annual in-person visits, which included psychological, mobility, and neuropsychological assessments. The mobility and dual-task protocol, along with cognitive and psychological data collection, were completed during two visits. The MRI was completed during a separate visit with a subset of the cohort study. Participants in this subset were tested between 2011 and 2016 and had completed the MRI within one year of the dual-task walking protocol. This study was approved by the Institutional Review Board of Albert Einstein College of Medicine (IRB number 2010–224). Written informed consent was obtained from each participant at the beginning of the first study visit. Participants were compensated for each study visit and transportation costs were covered. The work described in this manuscript was carried out in accordance with the Declaration of Helsinki.
Inclusion and exclusion criteria
Adults over the age of 65 were eligible for the study. Exclusion criteria were inability to speak English, inability to ambulate, dementia, significant impairment in vision or hearing, history of a neurological or psychiatric disorder, current medical procedures that would hinder ambulation, and current hemodialysis treatment. Dementia status was diagnosed via multidisciplinary consensus conference based on neuropsychological test scores and self-reported measures of psychological and physical functioning [48]. The subset of the study cohort who completed the MRI protocol included 73 right-handed older adults who were not contraindicated for MRI. Of the 73 participants, a total of 55 who had complete data available for the walking measures were included in the current study.
Measures
Dual-task walking protocol
The walking paradigm consisted of three task conditions: a single-task walk (STW), a single-task alpha (STA), and a dual-task walk (DTW). The STW condition consisted of walking three continuous counterclockwise loops on a 4 × 20-ft electronic walkway, composed of six straight walks connected by five left-handed turns at each end of the walkway. Participants were instructed to walk at their normal pace. Duration of the walking tasks varied between participants as participants completed the three loops at various velocities. The STA condition consisted of standing in place while reciting alternate letters of the alphabet out loud (B, D, F, etc.). The DTW condition required participants to execute the two single tasks concomitantly. Specifically, participants were asked to walk the same three consecutive loops around the walkway while reciting alternate letters of the alphabet out loud. Participants were instructed to pay equal attention to both tasks, to minimize effects of task prioritization. Order of the three tasks was counterbalanced using a Latin square design across participants to limit order effects. These methods have been described and validated previously [7, 8, 22, 49].
Quantitative gait assessment
The participants’ gait was measured by an electronic walkway (Zenometrics, LLC, Peekskill, NY) throughout the DTW protocol. This walkway was connected to ProKinetics Movement Analysis Software (PKMAS) system, which measured footfalls and allows for extraction of gait characteristics including stride velocity and total time walking [50]. Split-half intra-class correlations of the quantitative gait measurements in both STW and DTW conditions were greater than 0.95 revealing excellent internal consistency [22].
Functional near-infrared spectroscopy (fNIRS)
As described and validated previously [16, 22], oxygenated hemoglobin levels (HbO2) were measured in the prefrontal cortex [51] via the fNIRS Imager 1000 (fNIR Devices, LLC, Potomac, MD) during all tasks of the DTW protocol. The fNIRS device measures oxygenated (HbO2) and deoxygenated hemoglobin (Hb) through a sensor that covers the forehead and contains four LED light sources and ten photoreceptors 2.5 cm apart, resulting in 16 channels of data output, at a 2-Hz sampling rate. The placement of the sensor was based on landmarks from the international 10–20 system [52]. HbO2 was utilized for this study as the measure of neural activation in the PFC instead of Hb due to its better reliability and sensitivity to locomotion-related cerebral activity [53, 54].
Preprocessing methods and extraction of hemodynamic response have been described previously [55]. The preprocessing of fNIRS data included visual inspection to identify and eliminate artifacts of saturation, dark current conditions, or extreme noise for all 16 channels. Each light sensor emitted peak wavelengths of 730, 805, and 850 nm. Removal of motion artifacts was applied to the 730 and 850 nm wavelength measurements with the Daubechies 5 (db5) wavelet for spiky noise suppression [56]. The modified Beer–Lambert law was utilized to calculate changes in HbO2 with age and wavelength adjusted differential pathlength factor (DPF) and wavelength and chromophore dependent molar extinction coefficients (ε) by Prahl, as described previously [55, 57, 58]. Spline filtering [59] followed by a finite impulse response low-pass filter with cut-off frequency at 0.08 Hz was applied to remove baseline shifts and physiological artifacts.
The data points were extracted separately for each task and channel. Task-synchronized fNIRS signal extraction, in conjunction with PKMAS data, was conducted with E-Prime 2.0 software (Psychology Software Tools, Inc.). Task-related changes were compared to a baseline measure of HbO2, collected while the participants were instructed to stand still, counting silently, with a fixed gaze [21, 22]. Excellent internal consistency of HbO2 for the STW and DTW conditions was achieved (split-half intra-class correlations ≥ 0.830) [22].
Magnetic resonance imaging
Magnetic resonance imaging was performed in a 3 T Phillips scanner (Achieva TX; Philips Medical Systems, Best, The Netherlands) at the Gruss Magnetic Resonance Research Center of Albert Einstein College of Medicine (Bronx, NY). The scanner was equipped with a 32-channel head coil. Analyses were extracted from a T1-weighted image (MPRAGE − TE/TR/TI = 4.6/9.8/900 ms, voxel size 1 mm isotropic, SENSE acceleration factor 2.6).
The FreeSurfer software package (http://surfer.nmr.mgh.harvard.edu/) was used to extract the cortical thickness measures and cortical segmentation from all study participants [60]. Details of this process have been described previously [61]. Briefly, preprocessing included brain extraction, identification of gray and white matter boundaries, and automatic volume segmentation [62] of cortical regions based on a computed average space and surface-based smoothing at FWHM = 5 mm. FreeSurfer’s cortical parcellation tools identified 68 regions, with 34 in each hemisphere [63]. Cortical parcellation was visually inspected for accuracy by overlaying the segmentation on each subject’s T1 image in FSLeyes [64,65,66]. The cortical thickness values for each region were mean centered prior to input into the statistical model.
Covariates
The participants’ age, sex, global cognitive functioning (Repeatable Battery for the Assessment of Neuropsychological Status; RBANS [67]), Global Health Score, and correct letter generation performance and walking velocity under the DTW condition were entered into the statistical models as covariates of interest. We adjusted for these factors as they might impact dual-task performance, cognition, and neuroanatomy, and for behavior to evaluate inefficiency. Age, sex, and Global Health Score were self-reported by participants. Global Health Score (GHS) was a summary score taken via interview of self-reported dichotomous ratings, indicating presence or absence of ten health conditions: diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson’s disease, chronic obstructive lung disease, angina, and myocardial infarction [48]. Global cognitive function was measured with the total score of the RBANS. To assess cognitive performance under the DTW condition, the rate of correct letter generation was used in order to account for the varied time taken by the participants to complete three loops on the walkway. Rate was calculated by dividing the total number of correct letters generated by the total time walked, extracted from the PKMAS system. As described earlier, stride velocity during the DTW condition was assessed via the PKMAS system.
Statistical analysis
An initial linear mixed model, ignoring the effect of cortical thickness, was run to confirm the main effect of task on HbO2. This model included data from all 16 channels and accounted for correlations across repeated measures within the dual-task paradigm. The outcome was HbO2, with channel and task entered as repeated measures fixed factors. While channels could be treated as random or fixed, we did not find differences in the outcome based on how the channel variable was treated. The analysis adjusted for all covariates noted above.
Sixty-eight regional linear mixed models were carried out across the entire cortex to examine the moderating effect of cortical thickness on the change in HbO2 from STW to DTW. In these models, task and channels served as fixed effects repeated measures, and DTW was the reference condition. The moderating effect of cortical thickness, entered as a covariate, on the change in fNIRS-derived HbO2 across task condition was assessed via two-way interactions of regional brain thickness by task. These models were also adjusted for the covariates mentioned above. False discovery rate was used to correct for multiple comparisons [68]. All analyses were performed utilizing SPSS statistical software (version 26; SPSS, Inc., Chicago, IL).
Results
The final study sample included a total of 55 participants (mean age = 74.84 ± 4.97, 27 females (49.1%)). Participants were excluded for the following reasons: poor quality fNIRS data (n = 7), time between MRI and fNIRS data greater than 1 year (n = 8), and outliers from data exploration (n = 3; e.g., unusually high variance in HbO2 measurements or gait velocity). Participants were relatively healthy, as indicated by a low disease comorbidity score (Global Health Score mean = 1.36 ± 1.08), and demonstrated average overall cognitive function measured by total RBANS score (mean = 92.71 ± 11.28). Complete descriptive statistics of the sample are shown in Table 1. Descriptive statistics of cortical thickness values by region derived from Freesurfer’s cortical parcellation tools are displayed in Table 2.
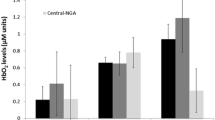
The effect of task on HbO2 was confirmed, showing consistency with previous reports, that HbO2 levels increased from single- to dual-task walking (estimate = − 0.613, p < 0.001, 95% CI [− 0.703, − 0.523]). None of the covariates demonstrated a significant effect on HbO2.
After confirming the main effect of task, full models including the main and moderating effects of cortical thickness were run for each cortical region. Sixty-eight models were run in SPSS, corresponding to the 68 regions extracted by FreeSurfer. After false discovery rate (FDR) correction, 25 models remained significant based on interaction estimates (p < 0.019). Tables 3 and 4 show the main and moderating effects of cortical thickness on task that remained significant. The regions that significantly moderated the STW to DTW change in HbO2 were, by the cortical lobe, as follows: frontal lobe—left rostral middle frontal, left pars orbitalis, right caudal middle frontal, right precentral, and right paracentral; parietal lobe—left inferior parietal and left precuneus; temporal lobe—right superior temporal, right middle temporal, right inferior temporal, right entorhinal, right temporal pole, and left fusiform; occipital lobe—bilateral lateral occipital, bilateral lingual, left cuneus, and bilateral pericalcarine gyrus; cingulate—left posterior, left isthmus, right rostral anterior, and right caudal anterior cingulate; insula—left insula. Estimates of these effects are displayed in Table 3 for the left hemisphere and Table 4 for the right hemisphere. These regions are highlighted in color in Fig. 1 for visualization purposes.
Regions in which cortical thickness significantly moderated the task effect on change in HbO2. FDR-corrected significant regions are highlighted in color. Values of interaction effect estimates are indicated in the color bar. Drawings generated using BrainPainter [69]
In most cortical regions, the main effect of thickness was negative, indicating that increased HbO2 levels in the prefrontal cortex were associated with less thickness. The interaction effects of thickness by task were mostly positive, indicating that HbO2 levels increased more from STW to DTW when individuals had less cortical thickness in those regions. A few regions, however, did not follow these patterns. In the models including the right caudal middle frontal, right entorhinal, right inferior temporal, right middle temporal, and right temporal pole, the main effect of thickness was positive and the interaction of task by thickness was negative. These indicated that greater thickness in those regions was associated with higher HbO2 levels overall and less increase in HbO2 levels from STW to DTW.
Discussion
The current study examined the moderating effect of regional cortical thickness on prefrontal cortex activation during DTW in older adults. The study sample was relatively healthy and demonstrated cognitive ability within the normal range as determined by neuropsychological tests. Results supported the neural inefficiency hypothesis: participants with thinner cortex employed greater neural resources in the PFC during DTW without achieving better behavioral performance. This effect was found in multiple regions, many of which are implicated in higher order control of walking, widespread throughout the cortex. Results suggest that when resources are limited (i.e., thinner cortex) in areas involved in DTW performance, the prefrontal cortex is over-recruited.
The regionally widespread significance of the results is consistent with literature, suggesting that dual-task walking is a complex process, and the regional results may add to our understanding of the relationship between neural structure and function in locomotion. Regions in which the cortical thickness significantly moderated the change in PFC activation from STW to DTW were found in the frontal, parietal, temporal, and occipital lobe, cingulate cortex, and insula.
Cortical regions that moderated the STW-to-DTW change in HbO2
Twenty-five cortical regions showed significant moderation effects on the change in HbO2 from STW to DTW. The following paragraphs summarize the literature that provides theoretical and empirical support for the regional findings and their relation to higher order control of DTW.
Frontal lobe
Consistent with a study examining the moderation effects of gray matter in the PFC on the change in PFC activation in DTW [43], PFC thickness was a significant moderator in the current study in two regions. The left rostral middle frontal gyrus was a significant moderator, indicating that less thickness here led to greater activation during DTW. This provided evidence for the directly measured effects of neural inefficiency. These results are distinct from and less widespread than those for gray matter volume, implying that there may be cellular differences in processing that differentiate functional impacts of gray matter surface area and cortical thickness [70].
The current study aimed to expand upon previous work that focused on the PFC, as DTW relies on processes throughout the brain. Some of the cortical regions which showed significant moderation effect of cortical thickness were novel but not unexpected, such as the right precentral gyrus which contains the right primary motor cortex. Thickness of the right paracentral lobule, which controls motor functioning in the lower limbs [71], was also a significant moderator. Reduced structural integrity in these right hemisphere regions was related to increased PFC involvement. As the current study controlled for gait and cognitive performance, the moderation of PFC activation by cortical thickness in right hemisphere regions that are involved in locomotion points toward compensatory over-activation of the PFC.
Occipital and temporal lobes
Many regions involved in visual processing were highlighted in the results, as thickness of the left and right lateral occipital gyrus, bilateral lingual gyrus, left cuneus, left fusiform, and bilateral pericalcarine cortex were significant moderators of the change in fNIRS-derived HbO2 from STW to DTW. These regions are involved in primary visual processing (pericalcarine cortex) [72], object processing (lateral occipital gyrus, lingual gyrus and cuneus) [73,74,75], and visual processing of letters (left fusiform gyrus) [76]. These gyri span the entire occipital lobe except for the right cuneus and right fusiform gyrus. While visual processing is not typically discussed in the cortical control of gait, similar to implications of motor processing, it is consistent with the neural inefficiency hypothesis that reduced ability to process the visual field would lead to more difficulty in DTW [77]. It is also possible that the cognitive task’s use of the alphabet drove some associations of visual object processing regions, particularly the left fusiform gyrus which is implicated in letter processing and reading in right-handed individuals [78]. Thinner auditory association cortex in the right superior temporal gyrus was also a significant moderator of PFC activation. The auditory association cortex may be related to activity during DTW due to the nature of internal feedback during the cognitive portion (alpha) of the task [79]. The alpha task requires that participants recite alternate letters of the alphabet out loud, during which participants hear themselves produce the letters, process this information, and use it to produce the next letter.
Insula
The results for the left insula, implicated in speech and language processing [80], showed that reduced thickness was related to increased PFC activation in DTW compared to STW conditions. The pars orbitalis is involved in semantic processing and is functionally connected to the PFC [81]. Only thickness of the left pars orbitalis was a significant moderator, which is consistent with current concept of hemispheric specialization wherein the left hemisphere is largely implicated in language processing in right-handed people. As noted earlier, only right-handed people were included in this study.
Parietal lobe and cingulate cortex
The moderation effects in regions implicated in attentional processes and sensory integration are consistent with the underlying theory of the cognitive control required for DTW [9]. Thickness of the left inferior parietal lobule and left precuneus were both significant moderators of change in PFC activation from STW to DTW. The inferior parietal lobule is implicated in the preparation of movement and sensory integration required to perform complex movement [82]. The precuneus is functionally connected to the frontal and parietal cortex and subcortical areas and is involved in spatially guided behavior including movement in space and attention shifting [83]. In addition, widespread regions of the cingulate cortex were implicated in the current study results. The cingulate cortex is involved in functional networks required for attention, motor control, and cognitive control [84, 85]. Each subregion of the cingulate cortex was significant in the current analysis; however, different moderating results are likely operational in the left and right cingulate. Thickness of the cortices of the right rostral anterior, right caudal anterior, left posterior, and left isthmus cingulate were significant moderators of task-related changes in oxygenation levels. The asymmetrical results here may be due to functional connections to other ipsilateral regions involved in DTW control; however, functional implications of subregions of the cingulate cortex are still being actively studied [86].
Thickness in regions related to attenuated activations
Neural inefficiency and compensatory overactivation in the PFC may explain the positive interaction estimates found in 20 of the significant regions; however, in five regions, cortical thickness significantly moderated the change in PFC activation from STW to DTW in the opposite direction. In the right entorhinal cortex, right inferior temporal gyrus, right middle temporal gyrus, right temporal pole, and right caudal middle frontal gyrus, reduced thickness was associated with an attenuated increase in PFC activation from STW to DTW. We hypothesize that this may be attributed to capacity limitations [87]. It is notable that all of these regions, with the exception of the right middle frontal gyrus, are located in the right temporal lobe, which is implicated in visual and verbal processing of objects. Both functions are necessary to recite alternate letters of the alphabet out loud while walking. The entorhinal cortex is involved in spatial processing [88], the inferior temporal gyrus is linked to attention-dependent visual processing [89], the middle temporal gyrus is implicated in semantic processing as well as sensorimotor feedback [90, 91], and the right temporal pole is operational in top-down processing of multisensory information [92]. The right caudal middle frontal gyrus is involved in attentional processes [93]. It is not immediately clear why thinner cortex in these regions is associated with limited neural capacity and results in attenuated increases in PFC activation from STW to DTW conditions, compared to the other regions in which neural inefficiency leads to PFC overactivation. However, statistical analyses assume independence of cortical regions, whereas this is not the case. It is possible that the thickness in these regions is related to PFC activation by another functional process that was not determinable in the present study. It is not uncommon for regional morphology to provide differential effects on outcomes of neural functioning, especially considering the complex networks involved in task-related activation [94].
Study limitations and future directions
The participants were healthy community-dwelling older adults who were dementia-free. This provides good evidence that the implications of cortical thickness in dual-task walking may be generalized to other healthy older adult populations. However, as is common with neuroimaging studies, the sample size was only moderate, and supportive findings from a larger cohort sample would increase confidence in the generalizability of the findings. While mean walking speeds were relatively slow, this was due to entering and exiting the turns within the walking loops [50].
The regions that significantly moderated the change in PFC activation from STW and DTW in this study are implicated in cognitive, sensory, and motor processing. While thinner cortex in these regions may be leading to functional neural differences in these processes during DTW, we only measured neural activity in the PFC. We are thus unable to conclusively determine whether low thickness is associated with different neural activation patterns in areas other than the PFC. Future studies are warranted to examine ways in which structural changes in the brain functionally impact DTW through advanced functional imaging of the entire brain. Future studies may be required to examine the relationship between brain structure and function in the context of walking conditions that manipulate cognitive demands in age-related diseases that impact cognition and locomotion.
Another limitation exists regarding the time interval between fNIRS data acquisition during the DTW paradigm and cortical thickness measurement obtained via MRI. While appointments were aimed to be scheduled as close together as possible, the time window ranged from zero days to 1 year; however, 47 out of 55 participants had both visits within 6 months. It is possible that cortical thickness may have changed within the larger intervals; however, the range of intervals and majority of shorter intervals, coupled with strong significance of results, suggest that the relationships found between cortical thickness and brain activity are meaningful. In a study examining the impact of white-matter integrity on PFC activation during DTW, sensitivity analyses of the acquisition time interval showed that results were strengthened, not reduced, when accounting for larger time intervals [42]. Further, changes in cortical thickness within less than a year have not been reported, and studies of cortical thinning during aging estimate less than 1% change in cortical thickness per year [95].
The fNIRS device measures oxygenated and deoxygenated hemoglobin during active walking. While this allows for obtaining useful indicators of walking performance as opposed to standard neuroimaging that is limited to imagined walking or step machines [30], it is also accompanied by additional artifacts. Recently described processing methods [55] were applied to the fNIRS-derived data in this study, which maximized the signal-to-noise ratio through use of multiple artifact-removal algorithms. These stringent processing methods add confidence to our measurements of brain activity during DTW. In addition, the current fNIRS device does not for allow direct measurement of skin blood flow, which may influence the signal recorded during locomotion [96, 97]. Despite this limitation, the current study examined the moderation effect of cortical thickness on task-related changes in fNIRS signal. Given the counter-balanced tasks with the same walking environment and physical conditions, such potential confounders should not impact the moderating effects of cortical thickness on task-related changes as reported herein.
While either regional or vertex-wise analyses could be used to address our research questions, there was previous evidence of cortical regions in which volume was implicated in the change in PFC activation from STW to DTW [43]. Examining cortical thickness on a regional scale allows for comparisons between the two properties of brain morphology and has allowed us to uncover differences between volume and thickness in relation to PFC activation in DTW. Regional analyses also limit the number of comparisons in our statistical design and allowed us to further correct the type I errors.
Without evidence of neurological disease, we have assumed that thin cortex in these participants is likely due to normal variability and age-related atrophy. However, as we have only measured brain morphology at cross-section, it is unclear whether the thin cortex that moderates PFC activation is indicative of pathological brain atrophy or normal variability in cortical thickness. Longitudinal analysis examining the relationship between gray-matter changes and neural activity during DTW may shed additional light on the impact of aging on these neural mechanisms. Previous research has found that PFC activation under DTW decreased and behavioral performance improved after within-session, repeated learning trials [98]. It would be interesting to examine whether these practice effects will vary due to the structural integrity of the brain, notably cortical thickness, at health and disease. Determining whether older adults with reduced brain integrity can benefit from learning is of clinical significance.
As neurological conditions, including multiple sclerosis and stroke, have been associated with greater increases in PFC activation from STW to DTW [99, 100], it would be of interest to examine whether the neural inefficiency patterns in conjunction with reduced brain integrity may be potentially good biomarkers of neurocognitive or motor outcomes such as cognitive decline, falls, or mobility disability. While this study focused on functional brain measures only in the PFC, utilization of fNIRS and MRI together adds temporal and spatial information which are useful for understanding physiological mechanisms of DTW. It has been suggested that multimodal imaging approaches are useful in understanding neural mechanisms of healthy and diseased aging and may be applied as clinically meaningful biomarkers [101].
Conclusions
This study has provided evidence that thinner cortex in multiple brain regions was related to greater increases in fNIRS-derived activations in dual-task-walking compared to single-task-walking. This supports our hypothesis that low cortical thickness would be related to poor efficiency of the PFC during DTW. The widespread regions that moderated the change in activation patterns across walking conditions that manipulated attention demands provide further evidence for the complicated nature of the cognitive control of DTW. This further suggests that the regions involved in sensory processing, motor control, and cognitive functioning are likely to be operational in the central control of locomotion.
Data availability
Data may be provided on request to the corresponding author.
Code availability
Not applicable.
References
Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–9. https://doi.org/10.1007/s12603-009-0246-z.
Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. https://doi.org/10.1001/jama.2010.1923.
Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–42; quiz 472. https://doi.org/10.1002/mds.21720.
Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein aging study. Neuropsychology. 2006;20(2):215–23. https://doi.org/10.1037/0894-4105.20.2.215.
Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: Part 2. Accounts of psychological refractory-period phenomena. Psychol Rev. 1997;104(4):749–91. https://doi.org/10.1037/0033-295X.104.4.749.
Baddeley AD. Is working memory still working? Eur Psychol. 2002;7(2):85–97. https://doi.org/10.1027/1016-9040.7.2.85.
Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Mot Control. 2012;16(1):64–80. https://doi.org/10.1123/mcj.16.1.64.
Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age (Dordr). 2014;36(1):373–81. https://doi.org/10.1007/s11357-013-9570-7.
Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16(1):1–14.
Leland A, Tavakol K, Scholten J, Mathis D, Maron D, Bakhshi S. The role of dual tasking in the assessment of gait, cognition and community reintegration of veterans with mild traumatic brain injury. Mater Sociomed. 2017;29(4):251–6. https://doi.org/10.5455/msm.2017.29.251-256.
Holtzer R, Stern Y, Rakitin BC. Age-related differences in executive control of working memory. Mem Cognit. 2004;32(8):1333–45. https://doi.org/10.3758/bf03206324.
Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. 2012;60(11):2127–36. https://doi.org/10.1111/j.1532-5415.2012.04209.x.
Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, et al. Association of dual-task gait with incident dementia in mild cognitive impairment: results from the gait and brain study. JAMA Neurol. 2017;74(7):857–65. https://doi.org/10.1001/jamaneurol.2017.0643.
Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012;60(10):1901–5. https://doi.org/10.1111/j.1532-5415.2012.04145.x.
Izzetoglu K, Bunce S, Onaral B, Pourrezaei K, Chance B. Functional optical brain imaging using near-infrared during cognitive tasks. Int J Hum-Comput Interact. 2004;17(2):211–27. https://doi.org/10.1207/s15327590ijhc1702_6.
Bunce SC, Izzetoglu M, Izzetoglu K, Onaral B, Pourrezaei K. Functional near-infrared spectroscopy. IEEE Eng Med Biol Mag. 2006;25(4):54–62.
Gramigna V, Pellegrino G, Cerasa A, Cutini S, Vasta R, Olivadese G, et al. Near-infrared spectroscopy in gait disorders: is it time to begin? Neurorehabil Neural Repair. 2017;31(5):402–12. https://doi.org/10.1177/1545968317693304.
Udina C, Avtzi S, Durduran T, Holtzer R, Rosso AL, Castellano-Tejedor C, et al. Functional near-infrared spectroscopy to study cerebral hemodynamics in older adults during cognitive and motor tasks: a review. Front Aging Neurosci. 2019;11:367. https://doi.org/10.3389/fnagi.2019.00367.
Menant JC, Maidan I, Alcock L, Al-Yahya E, Cerasa A, Clark DJ, et al. A consensus guide to using functional near-infrared spectroscopy in posture and gait research. Gait Posture. 2020;82:254–65. https://doi.org/10.1016/j.gaitpost.2020.09.012.
Agbangla NF, Audiffren M, Albinet CT. Use of near-infrared spectroscopy in the investigation of brain activation during cognitive aging: a systematic review of an emerging area of research. Ageing Res Rev. 2017;38:52–66. https://doi.org/10.1016/j.arr.2017.07.003.
Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011;66(8):879–87. https://doi.org/10.1093/gerona/glr068.
Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage. 2015;112:152–9. https://doi.org/10.1016/j.neuroimage.2015.03.002.
Holtzer R, Schoen C, Demetriou E, Mahoney JR, Izzetoglu M, Wang C, et al. Stress and gender effects on prefrontal cortex oxygenation levels assessed during single and dual-task walking conditions. Eur J Neurosci. 2017;45(5):660–70. https://doi.org/10.1111/ejn.13518.
Bishnoi A, Holtzer R, Hernandez ME. Brain activation changes while walking in adults with and without neurological disease: systematic review and meta-analysis of functional near-infrared spectroscopy studies. Brain Sci. 2021;11(3). https://doi.org/10.3390/brainsci11030291.
Metzger FG, Ehlis AC, Haeussinger FB, Schneeweiss P, Hudak J, Fallgatter AJ, et al. Functional brain imaging of walking while talking – an fNIRS study. Neuroscience. 2017;343:85–93. https://doi.org/10.1016/j.neuroscience.2016.11.032.
Stuart S, Alcock L, Rochester L, Vitorio R, Pantall A. Monitoring multiple cortical regions during walking in young and older adults: dual-task response and comparison challenges. Int J Psychophysiol. 2019;135:63–72. https://doi.org/10.1016/j.ijpsycho.2018.11.006.
De Sanctis P, Butler JS, Malcolm BR, Foxe JJ. Recalibration of inhibitory control systems during walking-related dual-task interference: a mobile brain-body imaging (MOBI) study. Neuroimage. 2014;94:55–64. https://doi.org/10.1016/j.neuroimage.2014.03.016.
Nenna F, Do CT, Protzak J, Gramann K. Alteration of brain dynamics during dual-task overground walking. Eur J Neurosci. 2020. https://doi.org/10.1111/ejn.14956.
Cortney Bradford J, Lukos JR, Passaro A, Ries A, Ferris DP. Effect of locomotor demands on cognitive processing. Sci Rep. 2019;9(1):9234. https://doi.org/10.1038/s41598-019-45396-5.
Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69(11):1375–88. https://doi.org/10.1093/gerona/glu052.
Wilson J, Allcock L, Mc Ardle R, Taylor J-P, Rochester L. The neural correlates of discrete gait characteristics in ageing: a structured review. Neurosci Biobehav Rev. 2019;100:344–69. https://doi.org/10.1016/j.neubiorev.2018.12.017.
Yuan J, Blumen HM, Verghese J, Holtzer R. Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: a resting-state fMRI study. Hum Brain Mapp. 2015;36(4):1484–93. https://doi.org/10.1002/hbm.22717.
Doi T, Blumen HM, Verghese J, Shimada H, Makizako H, Tsutsumimoto K, et al. Gray matter volume and dual-task gait performance in mild cognitive impairment. Brain Imaging Behav. 2017;11(3):887–98. https://doi.org/10.1007/s11682-016-9562-1.
Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–52. https://doi.org/10.1016/j.cger.2013.07.002.
Terribilli D, Schaufelberger MS, Duran FLS, Zanetti MV, Curiati PK, Menezes PR, et al. Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiol Aging. 2011;32(2):354–68. https://doi.org/10.1016/j.neurobiolaging.2009.02.008.
Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, et al. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51(3):943–51. https://doi.org/10.1016/j.neuroimage.2010.03.004.
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–30. https://doi.org/10.1093/cercor/bhh032.
Burzynska AZ, Nagel IE, Preuschhof C, Gluth S, Backman L, Li SC, et al. Cortical thickness is linked to executive functioning in adulthood and aging. Hum Brain Mapp. 2012;33(7):1607–20. https://doi.org/10.1002/hbm.21311.
Haier RJ, Siegel B, Tang C, Abel L, Buchsbaum MS. Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence. 1992;16:415–26.
Haier RJ, Siegel BV, Neucheterlein KH, Hazlett E, Wu JC, Paek J, Browning HL, Buchsbaum MS. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12(2):199–217. https://doi.org/10.1016/0160-2896(88)90016-5.
Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci Biobehav Rev. 2009;33(7):1004–23. https://doi.org/10.1016/j.neubiorev.2009.04.001.
Lucas M, Wagshul ME, Izzetoglu M, Holtzer R. Moderating effect of white matter integrity on brain activation during dual-task walking in older adults. J Gerontol A Biol Sci Med Sci. 2018. https://doi.org/10.1093/gerona/gly131.
Wagshul ME, Lucas M, Ye K, Izzetoglu M, Holtzer R. Multi-modal neuroimaging of dual-task walking: structural MRI and fNIRS analysis reveals prefrontal grey matter volume moderation of brain activation in older adults. Neuroimage. 2019;189:745–54. https://doi.org/10.1016/j.neuroimage.2019.01.045.
Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53(3):1135–46. https://doi.org/10.1016/j.neuroimage.2009.12.028.
Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48(2):371–80. https://doi.org/10.1016/j.neuroimage.2009.06.043.
Lipton RB, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart WF, Verghese J, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51(10):1382–90. https://doi.org/10.1046/j.1532-5415.2003.51455.x.
Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942–8. https://doi.org/10.1212/01.wnl.0000247042.15547.eb.
Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. JAMA. 2008;300(7):823–30. https://doi.org/10.1001/jama.300.7.823.
Holtzer R, Verghese J, Allali G, Izzetoglu M, Wang C, Mahoney JR. Neurological gait abnormalities moderate the functional brain signature of the posture first hypothesis. Brain Topogr. 2016;29(2):334–43. https://doi.org/10.1007/s10548-015-0465-z.
England SE, Verghese J, Mahoney JR, Trantzas C, Holtzer R. Three-level rating of turns while walking. Gait Posture. 2015;41(1):300–3. https://doi.org/10.1016/j.gaitpost.2014.09.010.
Chen M, Blumen HM, Izzetoglu M, Holtzer R. Spatial coregistration of functional near-infrared spectroscopy to brain MRI. J Neuroimaging. 2017;27(5):453–60. https://doi.org/10.1111/jon.12432.
Ayaz H, Izzetoglu M, Platek SM, Bunce S, Izzetoglu K, Pourrezaei K, et al. Registering fNIR data to brain surface image using MRI templates. Conf Proc IEEE Eng Med Biol Soc. 2006;2006:2671–4. https://doi.org/10.1109/iembs.2006.260835.
Leff DR, Orihuela-Espina F, Elwell CE, Athanasiou T, Delpy DT, Darzi AW, et al. Assessment of the cerebral cortex during motor task behaviours in adults: a systematic review of functional near infrared spectroscopy (fNIRS) studies. Neuroimage. 2011;54(4):2922–36. https://doi.org/10.1016/j.neuroimage.2010.10.058.
Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14(5):1186–92. https://doi.org/10.1006/nimg.2001.0905.
Izzetoglu M, Holtzer R. Effects of Processing methods on fNIRS signals assessed during active walking tasks in older adults. IEEE Trans Neural Syst Rehabil Eng. 2020;28(3):699–709. https://doi.org/10.1109/tnsre.2020.2970407.
Molavi B, Dumont GA. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol Meas. 2012;33(2):259–70. https://doi.org/10.1088/0967-3334/33/2/259.
Kim JG, Liu H. Variation of haemoglobin extinction coefficients can cause errors in the determination of haemoglobin concentration measured by near-infrared spectroscopy. Phys Med Biol. 2007;52(20):6295–322. https://doi.org/10.1088/0031-9155/52/20/014.
Scholkmann F, Wolf M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J Biomed Opt. 2013;18(10):105004. https://doi.org/10.1117/1.Jbo.18.10.105004.
Scholkmann F, Spichtig S, Muehlemann T, Wolf M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol Meas. 2010;31(5):649–62. https://doi.org/10.1088/0967-3334/31/5/004.
Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–81. https://doi.org/10.1016/j.neuroimage.2012.01.021.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. https://doi.org/10.1016/s0896-6273(02)00569-x.
Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. https://doi.org/10.1093/cercor/bhg087.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. https://doi.org/10.1016/j.neuroimage.2006.01.021.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. https://doi.org/10.1016/j.neuroimage.2004.07.051.
Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl):S173–86. https://doi.org/10.1016/j.neuroimage.2008.10.055.
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–90. https://doi.org/10.1016/j.neuroimage.2011.09.015.
Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–9. https://doi.org/10.1076/jcen.20.3.310.823.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Methodol). 1995;57(1):289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
Marinescu RV, Eshaghi A, Alexander DC, Golland P. BrainPainter: a software for the visualisation of brain structures, biomarkers and associated pathological processes. Multimodal Br. 2019;11846:112–20. https://doi.org/10.1007/978-3-030-33226-6_13.
Sanabria-Diaz G, Melie-García L, Iturria-Medina Y, Alemán-Gómez Y, Hernández-González G, Valdés-Urrutia L, et al. Surface area and cortical thickness descriptors reveal different attributes of the structural human brain networks. Neuroimage. 2010;50(4):1497–510. https://doi.org/10.1016/j.neuroimage.2010.01.028.
Lim SH, Dinner DS, Pillay PK, Lüders H, Morris HH, Klem G, et al. Functional anatomy of the human supplementary sensorimotor area: results of extraoperative electrical stimulation. Electroencephalogr Clin Neurophysiol. 1994;91(3):179–93. https://doi.org/10.1016/0013-4694(94)90068-x.
Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56(2):366–83. https://doi.org/10.1016/j.neuron.2007.10.012.
Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41(10–11):1409–22. https://doi.org/10.1016/s0042-6989(01)00073-6.
Cant JS, Goodale MA. Attention to form or surface properties modulates different regions of human occipitotemporal cortex. Cereb Cortex. 2007;17(3):713–31. https://doi.org/10.1093/cercor/bhk022.
Parker JG, Zalusky EJ, Kirbas C. Functional MRI mapping of visual function and selective attention for performance assessment and presurgical planning using conjunctive visual search. Brain Behav. 2014;4(2):227–37. https://doi.org/10.1002/brb3.213.
Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(Pt 2):291–307. https://doi.org/10.1093/brain/123.2.291.
Krishnan V, Cho YH, Mohamed O. Role of impaired vision during dual-task walking in young and older adults. Gait Posture. 2017;57:136–40. https://doi.org/10.1016/j.gaitpost.2017.06.006.
Gerrits R, Van der Haegen L, Brysbaert M, Vingerhoets G. Laterality for recognizing written words and faces in the fusiform gyrus covaries with language dominance. Cortex. 2019;117:196–204. https://doi.org/10.1016/j.cortex.2019.03.010.
Houde JF, Nagarajan SS, Sekihara K, Merzenich MM. Modulation of the auditory cortex during speech: an MEG study. J Cogn Neurosci. 2002;14(8):1125–38. https://doi.org/10.1162/089892902760807140.
Oh A, Duerden EG, Pang EW. The role of the insula in speech and language processing. Brain Lang. 2014;135:96–103. https://doi.org/10.1016/j.bandl.2014.06.003.
Belyk M, Brown S, Lim J, Kotz SA. Convergence of semantics and emotional expression within the IFG pars orbitalis. Neuroimage. 2017;156:240–8. https://doi.org/10.1016/j.neuroimage.2017.04.020.
Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123(Pt 11):2306–13. https://doi.org/10.1093/brain/123.11.2306.
Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83. https://doi.org/10.1093/brain/awl004.
Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–7. https://doi.org/10.1126/science.1100301.
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–67. https://doi.org/10.1038/nrn2994.
Rolls ET. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct. 2019;224(9):3001–18. https://doi.org/10.1007/s00429-019-01945-2.
Watanabe K, Funahashi S. Neural mechanisms of dual-task interference and cognitive capacity limitation in the prefrontal cortex. Nat Neurosci. 2014;17(4):601–11. https://doi.org/10.1038/nn.3667.
Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305(5688):1258–64. https://doi.org/10.1126/science.1099901.
Ptak R, Valenza N. The inferior temporal lobe mediates distracter-resistant visual search of patients with spatial neglect. J Cogn Neurosci. 2005;17(5):788–99. https://doi.org/10.1162/0898929053747676.
van Kemenade BM, Arikan BE, Podranski K, Steinsträter O, Kircher T, Straube B. Distinct roles for the cerebellum, angular gyrus, and middle temporal gyrus in action-feedback monitoring. Cereb Cortex. 2019;29(4):1520–31. https://doi.org/10.1093/cercor/bhy048.
Xu J, Wang J, Fan L, Li H, Zhang W, Hu Q, et al. Tractography-based parcellation of the human middle temporal gyrus. Sci Rep. 2015;5:18883. https://doi.org/10.1038/srep18883.
Pehrs C, Zaki J, Schlochtermeier LH, Jacobs AM, Kuchinke L, Koelsch S. The temporal pole top-down modulates the ventral visual stream during social cognition. Cereb Cortex. 2017;27(1):777–92. https://doi.org/10.1093/cercor/bhv226.
Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–15. https://doi.org/10.1038/nrn755.
Habeck C, Stern Y. Neural network approaches and their reproducibility in the study of verbal working memory and Alzheimer’s disease. Clin Neurosci Res. 2007;6(6):381–90. https://doi.org/10.1016/j.cnr.2007.05.004.
Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, et al. Accelerating cortical thinning: unique to dementia or universal in aging? Cereb Cortex. 2014;24(4):919–34. https://doi.org/10.1093/cercor/bhs379.
Kirilina E, Yu N, Jelzow A, Wabnitz H, Jacobs AM, Tachtsidis I. Identifying and quantifying main components of physiological noise in functional near infrared spectroscopy on the prefrontal cortex. Front Hum Neurosci. 2013;7:864. https://doi.org/10.3389/fnhum.2013.00864.
Erdoğan SB, Yücel MA, Akın A. Analysis of task-evoked systemic interference in fNIRS measurements: insights from fMRI. Neuroimage. 2014;87:490–504. https://doi.org/10.1016/j.neuroimage.2013.10.024.
Holtzer R, Izzetoglu M, Chen M, Wang C. Distinct fNIRS-derived HbO2 trajectories during the course and over repeated walking trials under single- and dual-task conditions: implications for within session learning and prefrontal cortex efficiency in older adults. J Gerontol A Biol Sci Med Sci. 2019;74(7):1076–83. https://doi.org/10.1093/gerona/gly181.
Al-Yahya E, Johansen-Berg H, Kischka U, Zarei M, Cockburn J, Dawes H. Prefrontal cortex activation while walking under dual-task conditions in stroke: a multimodal imaging study. Neurorehabil Neural Repair. 2016;30(6):591–9. https://doi.org/10.1177/1545968315613864.
Hernandez ME, Holtzer R, Chaparro G, Jean K, Balto JM, Sandroff BM, et al. Brain activation changes during locomotion in middle-aged to older adults with multiple sclerosis. J Neurol Sci. 2016;370:277–83. https://doi.org/10.1016/j.jns.2016.10.002.
Sui J, Huster R, Yu Q, Segall JM, Calhoun VD. Function–structure associations of the brain: evidence from multimodal connectivity and covariance studies. Neuroimage. 2014;102(Pt 1):11–23. https://doi.org/10.1016/j.neuroimage.2013.09.044.
Funding
This work was supported by the National Institute of Health (R01AG036921, R01AG044007, R01NS109023).
Author information
Authors and Affiliations
Contributions
Conceptualization: D.R., R.H. Methodology: D.R., M.E.W., M.I., R.H. Formal analysis: D.R. Writing—original draft preparation: D.R. Writing—review and editing: M.E.W., M.I., R.H. Funding acquisition: R.H.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments and approved by the institutional review board of Albert Einstein College of Medicine.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflicts of interest
M.I. has a very minor share in the company that manufactures the fNIRS device used in this study. All other authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ross, D., Wagshul, M.E., Izzetoglu, M. et al. Prefrontal cortex activation during dual-task walking in older adults is moderated by thickness of several cortical regions. GeroScience 43, 1959–1974 (2021). https://doi.org/10.1007/s11357-021-00379-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-021-00379-1