Abstract
The posture first hypothesis suggests that under dual-task walking conditions older adults prioritize gait over cognitive task performance. Functional neural confirmation of this hypothesis, however, is lacking. Herein, we determined the functional neural correlates of the posture first hypothesis and hypothesized that the presence of neurological gait abnormalities (NGA) would moderate associations between brain activations, gait and cognitive performance. Using functional near-infrared spectroscopy we assessed changes in oxygenated hemoglobin levels in the pre-frontal cortex (PFC) during normal walk and walk while talk (WWT) conditions in a large cohort of non-demented older adults (n = 236; age = 75.5 ± 6.49 years; female = 51.7 %). NGA were defined as central (due to brain diseases) or peripheral (neuropathic gait) following a standardized neurological examination protocol. Double dissociations between brain activations and behavior emerged as a function of NGA. Higher oxygenation levels during WWT were related to better cognitive performance (estimate = 0.145; p < 0.001) but slower gait velocity (estimate = −6.336, p < 0.05) among normals. In contrast, higher oxygenation levels during WWT among individuals with peripheral NGA were associated with worse cognitive performance (estimate = −0.355; p < 0.001) but faster gait velocity (estimate = 14.855; p < 0.05). Increased activation in the PFC during locomotion may have a compensatory function that is designed to support gait among individuals with peripheral NGA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impairments in locomotion, which are common in aging (Verghese et al. 2006), Mild Cognitive Impairments (Verghese et al. 2008) and dementia (Verghese et al. 2002b), are associated with adverse outcomes (Studenski et al. 2011). Delineating cognitive processes and functional brain networks that subserve locomotion will, therefore, advance knowledge in this area and also offer exciting avenues for risk assessment and intervention procedures for individuals at risk of mobility decline and disability.
Executive functions (EF) have a key role in cognitive control of locomotion (Holtzer et al. 2006). Dual-tasking is a distinct facet of EF (Miyake et al. 2000) that is subserved by the pre-frontal cortex (PFC) (MacDonald et al. 2000). Dual-task paradigms that involve walking while performing a cognitive interference task afford experimental manipulation of attention demands of gait allowing for causal inferences concerning the effect of attention resources on locomotion (Holtzer et al. 2012b). Notably, poor performance on a well-validated walking while talking task (WWT) was associated with increased risk of incident frailty and disability as well as mortality in older adults (Verghese et al. 2012).
The “posture first” hypothesis suggests that older adults are likely to prioritize walking or balance over cognitive task performance under dual-task conditions (Li et al. 2001). Behaviorally, this would translate into relative preservation or reduced dual-task costs in gait compared to the cognitive interference tasks in old compared to young individuals. Such preference can be explained from an evolutionary perspective. Findings with respect to the posture first hypothesis, however, have been mixed (Gerin-Lajoie et al. 2005; Lindenberger et al. 2000). It is noteworthy that failure to demonstrate this preference (Schaefer et al. 2015) may be attributed to limitations of available cognitive resources in older adults (Holtzer et al. 2011). Furthermore, in neurological conditions, such as Parkinson’s disease, patients are reported to use the posture second strategy where cognitive tasks are prioritized over walking, leading to an increase in falls risk (Bloem et al. 2006). Among older adults the presence of neurological gait abnormalities (NGA) increases the risk of incident dementia (Verghese et al. 2002b) and also influences walking performance under dual-task conditions (Holtzer et al. 2014c). NGA are assessed through a structured clinical examination of gait and can be attributed to central or peripheral causes (Verghese et al. 2002a; see methods for details). Notably, the functional brain correlates of the posture first hypothesis, NGA and their possible interactions have not been reported. Shedding light on neural mechanisms of locomotion can yield critical information for risk assessment and interventions of motoric and cognitive outcomes in normal aging and neurodegenerative disorders.
A recent review provided a comprehensive discussion of neuroimaging methods that have been utilized to determine both structural and functional brain correlates of mobility (Holtzer et al. 2014b). Evidence for functional cortical control of gait has been relatively limited, in part, due to methodological limitations that are inherent in traditional neuroimaging methods, specifically the requirements to remain immobile in a supine position during scanning acquisitions. Approaches that were designed to address these limitations include but are not limited to mental imagery of mobility tasks and utilization of radionuclide tracers during simple walking conditions performed outside of the scanner. In this latter method, single photon emission computerized tomography (SPECT) or positron-emission-tomography (PET) that are performed subsequent to the walking task examine task-related distribution patterns of the tracers in the brain. Such methods, however, have significant limitations in terms of subject selectivity, invasiveness of the procedure and costs. Functional near-infrared spectroscopy (fNIRS) studies provide the most compelling evidence for online cortical control of active locomotion (Holtzer et al. 2014b). fNIRS measures changes in cortical oxygenated hemoglobin (HbO2) levels using light–tissue interaction properties of light within the near infrared range (Jobsis 1977). fNIRS has been validated against traditional neuroimaging methods and is better able to handle motion artifacts (Cooper et al. 2012). To date, fNIRS has been the only method successfully implemented to assess changes in activation patterns in the PFC during walking in both single and dual-task conditions (Mirelman et al. 2014). Specifically, our initial study (Holtzer et al. 2011) and recent work (Holtzer et al. 2015) provided the first reproducible evidence for the increased involvement of the PFC in cognitive control of WWT in older adults. These recent findings as well as the established literature supporting the key role EF have in locomotion (Holtzer et al. 2014c, d; Yogev-Seligmann et al. 2008) provide the context and rationale for this investigation.
The current study determined the effect of NGA on the functional neural correlates of locomotion in older adults vis-à-vis the posture first hypothesis. We assessed NGA through structured neurological examination of gait (Verghese et al. 2002a). Peripheral NGA was defined by the presence of neuropathic gait. Central NGA was defined by the presence of gait abnormalities attributed to brain dysfunction. Changes in brain activations in the PFC during active locomotion in normal walk (NW), and walk while talk (WWT) were assessed using fNIRS. We hypothesized that the presence of NGA would influence the change in PFC activation levels across task conditions. Furthermore, we predicted that individuals with NGA would allocate brain resources to support gait whereas individuals without NGA would allocate brain resources to support cognitive performance.
Methods
Participants
Participants, (age ≥ 65 years), were community residing individuals enrolled in “Central Control of Mobility in Aging” (CCMA), a cohort study designed to determine cognitive and brain predictors of mobility. Details concerning CCMA procedures were previously described (Holtzer et al. 2014d). Briefly, a structured telephone interview was administered to obtain verbal assent, assess medical history and mobility function and rule out dementia. Individuals who passed the telephone interview were invited to two in-person study visits at our research center. During these visits participants received comprehensive neuropsychological, cognitive, psychological, and mobility assessments as well as a structured neurological examination. Dementia diagnoses were assigned according to the DSM-IV TR (Association 2000) at consensus diagnostic case conferences (Holtzer et al. 2008). Exclusion criteria were: inability to ambulate independently, current or history of severe neurological or psychiatric disorders, significant loss of vision and/or hearing, and recent or anticipated medical procedures that may affect ambulation. Written informed consents were obtained in-person and approved by the institutional IRB.
Test Procedures and Equipment
Neurological Gait Abnormalities
The presence of NGA (Nutt et al. 1993) was assessed by the study clinician as part of a structured neurological examination that included visual inspection of walking straight as well as during turns, and which also tested cranial nerves, strength, sensation, and deep tendon reflexes (Verghese et al. 2002b). Normal gait was defined by the absence of neurological or non-neurological gait abnormalities. Peripheral NGA was defined by the presence of neuropathic gait, which has been associated with postural instability and falls (Verghese et al. 2010a). Participants diagnosed with neuropathic gait demonstrated unilateral or bilateral foot drop, and other neuropathic signs such as a “stocking”-pattern sensory loss, and an absence of deep-tendon reflexes in the lower limbs. Central NGA status was defined by the presence of gait abnormalities due to brain diseases, and included frontal, ataxic, hemiparetic, unsteady, spastic, Parkinsonian or unsteady subtypes. Individuals with frontal gait demonstrate a wide base, and difficulty in elevating the feet up off the ground. Ataxic gait is a wide-based gait that may also include other features associated with cerebellar disease, such as heel-to-shin incoordination or intention tremor. Hemiparetic gait, typically observed in participants with documented stroke history or the presence of clinical signs, is characterized by a swing of the legs outward in a semicircle from the hip (circumduction). The presence of two or more of the following features was required to diagnose unsteady gait: marked swaying, loss of balance, or falls during normal walk, or while walking in a straight line placing one foot directly in front of the other (tandem gait). Unsteady gait, defined using these criteria, has been shown to increase the risk of developing dementia in older adults supporting its central origins (Verghese et al. 2010a; Verghese et al. 2002b). Test–retest (1 year apart) and inter-rater reliability of the clinical gait assessment using this protocol were excellent (Verghese et al. 2002b).
Experimental Paradigm
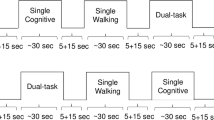
The experimental paradigm included two single task conditions and one dual-task condition. There was one trial per each task condition. The two single tasks were: (1) normal pace walk (NW) and (2) Cognitive (Alpha). In NW participants were asked to walk around the electronic walkway (see Zenometrics system below) at their “normal pace” for three consecutive loops. In Alpha, participants were required to stand still while reciting alternate letters of the alphabet starting with the letter B for 30-s out loud. The dual-task condition, WWT, required the participants to execute the two single tasks concurrently. Specifically, participants were instructed to walk around the walkway for three consecutive loops at their normal pace while reciting alternate letters of the alphabet starting with the letter ‘B’. Participants were instructed to pay equal attention to both tasks (Holtzer et al. 2012b; Holtzer et al. 2014d). The three test conditions were presented in a counterbalanced order using a Latin-square design. Reliability and validity for this walking paradigm have been established (Holtzer et al. 2012a; Verghese et al. 2012). Gait velocity during NW and WWT as well as the number of correct letters and errors generated during Alpha and WWT were used as outcome measures.
Quantitative Gait Assessment
Zenometrics
A 4 × 14 foot Zeno electronic walkway using ProtoKinetics Movement Analysis Software (PKMAS) was utilized to assess quantitative measures of gait (Zenometrics, LLC; Peekskill, NY). The quantitative gait measure used in the current study, stride velocity, was measured based on the location and mathematical parameters between footfalls on the instrumented walkway (i.e., geometric arrangement, spatial and temporal relationship, relative pressures; also see England et al. 2015). Split-half intra-class correlations (ICC) for stride velocity in NW and WWT were greater than 0.95 revealing excellent internal consistency (Holtzer et al. 2015).
fNIRS System
fNIRS measures changes in cortical oxygenated hemoglobin (HbO2) levels using light–tissue interaction properties of light within the near infrared range. fNIRS has been validated against traditional neuroimaging methods and is better able to handle motion artifacts (Cooper et al. 2012). Changes in hemodynamic activity in the PFC were assessed using fNIRS Imager 1000 (fNIRS Devices, LLC, Potomac, MD). The system collects data at a sampling rate of 2 Hz. The fNIRS sensor consists of 4 LED light sources and 10 photodetectors, which cover the forehead using 16 voxels, with a source-detector separation of 2.5 cm. The light sources on the sensor (Epitex Inc. type L4X730/4X805/4X850-40Q96-I) contain three built-in LEDs having peak wavelengths at 730, 805, and 850 nm, with an overall outer diameter of 9.2 ± 0.2 mm. The photodetectors (Bur Brown, type OPT101) are monolithic photodiodes with a single supply transimpedance amplifier. We follow a standard sensor placement procedure (Holtzer et al. 2015). The sensor is placed on the forehead so that the horizontal symmetry axis central coincides with symmetry axis of the head, (i.e. in between the eyes). On the vertical axis, the sensor is positioned right above the eyebrows in relation to the international 10–20 system so that FP1 and FP2 marker locations are positioned approximately on the bottom channel row level (Ayaz et al. 2006).
Preprocessing and Hemodynamic Signal Extraction
Raw data were inspected to identify and remove raw intensity measurements at 730 and 850 nm that reached saturation or dark current conditions. To eliminate possible respiration, heart rate signals and unwanted high frequency noise raw intensity measurements at 730 and 850 nm were low-pass filtered with a finite impulse response filter of cut-off frequency at 0.14 Hz (Izzetoglu et al. 2010). There were no adverse events, and trials were not rejected due to participants’ failure to comply with task demands. Noise (saturation or dark current conditions) was observed in 4 % of the data that were subsequently excluded. Oxygenated hemoglobin (HbO2), deoxygenated hemoglobin (Hb), oxygenation or oxygen index (HbO2-Hb) and total hemoglobin (HbO2 + Hb) signals can be calculated from the artifact-removed raw intensity measurements at 730 and 850 nm using modified Beer-Lambert law (Izzetoglu et al. 2010). HbO2 is more reliable and sensitive to locomotion-related changes in cerebral oxygenation (Harada et al. 2009) and was thus used in the current study. Previous fNIRS studies reported proximal baseline conditions that ranged from 5 to 15 s (Izzetoglu et al. 2007). Consistent with prior work (Holtzer et al. 2011, 2015) relative changes in the concentrations of HbO2 in each experimental condition were obtained using the most proximal standing 10-s baseline. During the baseline procedure participants were asked to remain still, fixate on the wall directly in front of them, and count silently in their head at a rate of about one number per second. A separate baseline was administered immediately prior to the start of each task. For each experimental condition (NW, Alpha and WWT), the baseline levels for the 10-s period were adjusted to a zero mean HbO2 value. Hence, the changes in HbO2 levels in each of the three task conditions were normalized to the same level of the individualized baseline condition.
Epoch and Feature Extraction
Individual mean HbO2 data were extracted separately for each channel for each task condition. For Alpha, mean HbO2 values for each channel, which were based on the entire 30 s task duration, were averaged and used for feature extraction and comparison. We implemented additional steps that were designed to optimize the acquisition and extraction of task related HbO2 in NW and WWT by synchronizing fNIRS and gait events. A central “hub” computer with E-Prime 2.0 software sent synchronized triggers to both the fNIRS system and PKMAS. The fNIRS acquisition software received numerical triggers from E-prime that were each indicative of a unique condition and represented the beginning or end of either a baseline or test condition. A second level post-processing time synchronization method was implemented using the first recorded foot contact with the walkway as a time stamp. The recording of fNIRS was terminated at the end of the 6th and final straight walk. This end point was determined algorithmically by PKMAS as previously described (England et al. 2015). HbO2 data in each of the fNIRS channels in NW and WWT within these time points were extracted, averaged and used for comparisons between task conditions. Internal consistency of HbO2 measurements, determined by split-half intra-class correlations within each task, was excellent for NW (0.830), Alpha (0.864) and WWT (0.849) (Holtzer et al. 2015).
Additional measures
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was used to assess level of cognitive function (Duff et al. 2008).
A disease comorbidity summary score (range 0–10) was used to characterize overall levels of health (Holtzer et al. 2006, 2008).
Statistical Analysis
Descriptive statistics (mean ± SD) were provided for continuous variables for the entire cohort and also stratified by group. The level of statistical significance was set at α = 0.05. A linear mixed effects model was used to determined the effect of dual-tasking (NW vs. WWT), NGA and their interaction on gait velocity. GEE (generalized estimating equation) Poisson models for count of correct letters and errors were used to determine the effect of dual-tasking (Alpha vs. WWT), NGA and their interaction on the rate of correct letters and errors with the logarithm of time as the offset term. A separate linear mixed effect model was used to compare HbO2 levels in WWT to NW and Alpha. The moderating effect of NGA on task related changes in HbO2 levels was examined via interactions between task and NGA. Linear regression and Poisson models were used to determine the neural correlates of the posture first hypothesis. Specifically, linear regression examined whether NGA status moderated associations between HbO2 levels (predictor) and gait velocity (outcome variable) in WWT. The moderating effect of NGA on the association between HbO2 levels and gait velocity was assessed via interaction terms of NGA status × HbO2 levels. Poisson model assessed the association between HbO2 levels and the rate of correct letter generation in WWT. The moderating effect of NGA on the association between HbO2 levels and the rate of letter generation was examined via interactions of HbO2 levels × NGA status. Analyses controlled for gender, age, education, disease comorbidity, and RBANS total index score. Statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, N.C.).
Results
A total of 236 non-demented participants (mean ± SD age = 75.5 ± 6.49 years; mean ± SD education = 14.54 ± 3.15 years; female = 51.7 %) were included. The low mean disease comorbidity score 1.52(± 1.10) confirmed the relatively healthy nature of the sample. The mean RBANS total index score (91.95 ± 12.92) was indicative of average cognitive function. Of the total sample, 167 participants had no gait abnormalities, 40 were diagnosed with peripheral NGA, and 29 with central NGA. Of the 29 central NGA cases 2 were hemiparetic, 1 frontal, 2 Parkinsonian, 3 ataxic, 19 unsteady, and 2 spastic. All three groups were mutually exclusive; there was no overlap between the central and peripheral NGA groups or among the different central gait subtypes. The demographic characteristics of the sample were further stratified by NGA status (Table 1).
Mean gait velocity in WWT compared to NW was significantly reduced (estimate = −16.413; p < 0.001) among normals. Peripheral (estimate = −11.892; p < 0.001) and Central (estimate = −8.454; p = 0.011) NGA were associated with slower gait velocity compared to normals under the single task. The interactions of NGA status × task were not significant.
The rate of correct letter generation was not significantly reduced in WWT compared to Alpha (estimate of log of rate ratio = −0.02; p = 0.568) among normals. However, the error rate was significantly increased in WWT compared to Alpha (estimate of log of rate ratio = 0.600; p < 0.001) among normals. The main effects and interactions of NGA status with task were not significant.
A Linear mixed effects model examined the effect and interactions of NGA status and task on HbO2 levels in WWT compared to NW and Alpha (Table 2; Fig. 1).
As shown in Table 2, HbO2 levels were significantly higher in WWT compared to NW (estimate = −0.506; p = 0.007) among normals. Central NGA influenced the change in HbO2 levels in WWT compared to NW relative to normals (estimate = 0.395; p < 0.001) and the peripheral NGA (estimate = −0.596; p < 0.001) group. Central NGA also influenced the change in HbO2 levels in WWT compared to Alpha relative to normals (estimate = 0.441; p = 0.018) and the peripheral NGA (estimate = −0.660; p = 0.010) group. In all four moderation effects central NGA was associated with significantly attenuated changes in HbO2 levels in WWT compared to the single tasks.
Linear regression was used to determine whether NGA moderated associations between HbO2 levels (median split) and gait velocity during WWT (Table 3 panel A). The moderating effects of NGA status on the association between HbO2 levels (median split) and the rate of letter generation during WWT were assessed via Poisson model (Table 3, panel B). Results revealed that, in normals, higher HbO2 levels were associated with slower gait velocity (estimate = −6.336; p = 0.031), but higher rate of correct letter generation (estimate = 0.145; p < 0.001). As expected, peripheral NGA (estimate = −17.498; p < 0.001) and central NGA (estimate = −8.454; p < 0.001) were both associated with slower gait velocity during WWT compared to the normals among those with low HbO2 levels. The association between HbO2 levels and gait velocity was different between peripheral NGA and normals (estimate = 14.855; p = 0.032). In contrast to normals, the association between HbO2 levels and gait velocity was positive in peripheral NGA (high compared to low HbO2 levels were associated with an increase of 8.815 velocity units). Differential associations between HbO2 levels and the rate of letter generation between peripheral NGA and normals were also observed (difference in log of rate ratio = −0.355; p < 0.001). In contrast to normals, the association between HbO2 levels and the rate of letter generation was negative in peripheral NGA. Specifically, in this group high compared to low HbO2 levels were associated with slower rate of letter generation, (log of the rate ratio of letter generation = −0.21, p = 0.0085). The difference in the association between HbO2 levels and gait velocity between central NGA and normals was not significant, though these associations were in opposite directions (−6.337 among normal vs 3.573 among central NGA; difference = 9.910; p = 0.173). The difference in the association between HbO2 levels and the rate of letter generation between central NGA and normals was significant (log of rate ratio in high versus low HbO2 level is −0.029 among central NGA, compared to 0.146 among normals; difference in log of rate ratio = −0.174,p < 0.042). In contrast to normals, the association between HbO2 levels and the rate of letter generation was negative and not significant in central NGA.
Discussion
Determining the functional brain correlates of gait during active locomotion, especially under attention demanding conditions has been a major challenge (Holtzer et al. 2014b). Using fNIRS, we have reported, initially in a small sample, (Holtzer et al. 2011) and confirmed more recently in a larger cohort (Holtzer et al. 2015) the pivotal role of the PFC in monitoring and allocation attention resources to support gait performance, notably when cognitive demands are increased under dual-task conditions. In the current study, we determined the effect of NGA on the functional cortical correlates of locomotion vis-à-vis the posture first hypothesis. As expected, peripheral and central NGA were both associated with slower gait compared to normals. Central NGA was associated with attenuated changes in PFC HbO2 levels during WWT compared to the single tasks relative to the peripheral NGA and normal groups. This attenuation is consistent with limited capacity models predicting age-related reductions in brain activations in response to cognitively challenging tasks (Reuter-Lorenz et al. 2000). In contrast, individuals with peripheral NGA showed the greatest elevations in HbO2 levels during WWT, possibly to compensate for peripheral physiological limitations that hinder walking.
The posture first hypothesis suggests that older adults show a preference to walking over talking because it’s evolutionarily adaptive (Li et al. 2001). Accordingly, higher HbO2 levels during WWT would be expected to predict faster gait but not better cognitive performance. We observed double dissociations between brain activation patterns and behavioral performance as a function of NGA status. Higher HbO2 levels among normals predicted better cognitive performance but slower gait. The absence of NGA was likely associated with greater confidence in one’s locomotive capabilities allowing healthy individuals to direct brain resources to support the cognitive task while walking. Compared to normals, individuals with peripheral NGA displayed diametrically opposite response patterns wherein higher oxygenation levels were associated with faster gait velocity but worse cognitive performance. Hence, neural confirmation of the posture first hypothesis emerged among older adults whose postural and locomotive abilities were compromised. Consequently, under attention-demanding conditions such as WWT they directed cognitive resources to support gait, which is evolutionarily adaptive. This finding is also consistent with compensatory reallocation models (Cabeza 2002) suggesting that in older adults increased involvement of the PFC supports task performance compensating for reduced efficiency of other brain regions (Stern 2009). It is important to note that NGA status was determined independently by the study physician who did not conduct the quantitative gait assessment and was blind to its outcome.
Study Limitations and Future Directions
The current study examined task-related changes in brain activations during actual locomotion focusing on the PFC. Existing models (Drew et al. 2004) and neuroimaging studies that assess gray matter volume and white matter integrity (see Rosso et al. 2013; Holtzer et al. 2014a for reviews) implicate additional cortical and sub-cortical brain regions in higher order control of gait. Hence, the findings reported herein provide a limited window into brain control of locomotion. The focus on the PFC, however, is justified theoretically and is supported by empirical findings (Holtzer et al. 2012b). In resting state fMRI of actual locomotion (Yuan et al. 2014) and fMRI of imagined locomotion (Blumen et al. 2014) studies, we identified latent brain patterns that were related to gait under single and dual-task conditions. In both studies, the PFC was uniquely associated with actual and imagined WWT performance providing converging evidence to the key role this brain region plays in cognitive control of locomotion, notably under attention-demanding conditions. While NGA may be multifactorial, we used mutually exclusive groups to compare our findings. Whereas the presence of neuropathic gait served as the sole criterion for peripheral NGA several gait subtypes defined central NGA. The heterogeneity of the central NGA group, however, is mitigated by established diagnostic reliability of the different gait subtypes, which have all been individually linked to brain dysfunction and dementia risk. The low prevalence of frontal and Parkinsonian gait subtypes is explained by the relatively healthy status of this sample. Furthermore, previous diagnosis of Parkinson’s disease or dementia was an exclusion criterion. The high prevalence of unsteady gait is consistent with prior work demonstrating it’s utility in predicting dementia and supporting its central origins (Verghese et al. 2002b; 2007). The relatively small number of participants in the central NGA group and distribution of gait disorder types precluded meaningful stratified analysis but visual inspection of the data did not suggest that specific NGA should be excluded. We further note that the heterogeneity of the central NGA group could have resulted in greater within group differences and thus underestimation the reported effects. The mean velocity under NW was relatively low but normative gait velocity is typically reported for straight walks. In this study, participants were required to turn for a total of five times in each walking condition on a relatively short instrumented walkway resulting in reduced gait velocity. Furthermore, gait velocities, which were assessed separately in PKMAS under the current protocol and in GAITRite (the latter was used to establish normative gait data Oh-Park et al. 2011) were very highly correlated (r = 0.84, p < 0.0001) providing strong evidence to the ecological validity and generalizability of the walking velocity reported herein. The fNIRS device used in the current study offers critical advantages in terms of portability, weight and utilization in mobility protocols having minimal to no impact on gait performance. The limitations of this device, however, preclude direct assessment of factors such as differences in skull thickness, skin response or frontal sinuses that may influence cortical changes in activation patterns during locomotion as assessed with fNIRS. These important issues have been discussed elsewhere (Erdogan et al. 2014; Kirilina et al. 2012). We emphasize, however, that these factors, while important, were not likely to influence the moderating effects of NGA on the task related changes in PFC activation levels and double dissociations reported herein between activation levels, cognitive and gait performance.
Clinical Implications
Clinical assessment of gait can be conducted in the context of neurological examinations in clinical and research settings. It can be accomplished in a time efficient manner (approximately two minutes), in various environmental conditions and without reliance on sophisticated equipment. The clinical utility of clinical gait assessment in predicting incident dementia and falls has already been shown (Verghese et al. 2002b). The findings of the current study extend previous work revealing that data from such brief assessments is informative with respect to the differential involvement of the PFC in cognitive motor tasks in non-demented older adults. These findings have direct implications for treatment and risk assessment of impaired locomotion and cognitive dysfunction. Dual-task paradigms afford a rehabilitative training framework and a window into the effect of cognitive remediation on mobility in normal (Verghese et al. 2010b) as well as disease populations (Schwenk et al. 2010; Yogev-Seligmann et al. 2012). We suggest that NGA have an effect on the cognitive strategy individuals use to negotiate the demands of gait under dual-task conditions. This knowledge can, therefore, be used to guide interventions that are tailored to individual characteristics. Whether or not the attenuated brain response observed in central NGA during WWT represents an upper ceiling imposed by capacity limitations or a more flexible threshold that is amenable for treatment, perhaps with greater number of repetitions, remains to be evaluated in future research. We note that the severity of NGA may have an effect on both brain activations during WWT and brain responsiveness to cognitive motor rehabilitation of attention-demanding locomotion. Performance on WWT predicts adverse outcomes including falls (Ayers et al. 2014) as well as frailty disability and mortality (Verghese et al. 2012). Identifying factors that influence how individuals with known clinical presentations approach and execute this task may have important implications with respect to risk assessment of falls and other mobility impairments.
In summary, the current study provided first evidence for the functional brain signature of the posture first hypothesis. Brain signature, which was defined by task-related changes in HbO2 levels in the PFC, varied as a function of NGA status. Increased activation in the PFC during attention-demanding walking conditions has a compensatory function that is designed to support gait in individuals with peripheral NGA.
References
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders (text revision ed.). American Psychiatric Press, Washington, DC
Ayaz H, Izzetoglu M, Platek SM, Bunce S, Izzetoglu K, Pourrezaei K, Onaral B (2006) Registering fNIR data to brain surface image using MRI templates. Conf Proc IEEE Eng Med Biol Soc 1:2671–2674. doi:10.1109/IEMBS.2006.260835
Ayers EI, Tow AC, Holtzer R, Verghese J (2014) Walking while talking and falls in aging. Gerontology 60(2):108–113. doi:10.1159/000355119
Bloem BR, Grimbergen YA, van Dijk JG, Munneke M (2006) The “posture second” strategy: a review of wrong priorities in Parkinson’s disease. J Neurol Sci 248(1–2):196–204. doi:10.1016/j.jns.2006.05.010
Blumen HM, Holtzer R, Brown LL, Gazes Y, Verghese J (2014) Behavioral and neural correlates of imagined walking and walking-while-talking in the elderly. Hum Brain Mapp. doi:10.1002/hbm.22461
Cabeza R (2002). Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17(1), 85–100. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11931290
Cooper RJ, Selb J, Gagnon L, Phillip D, Schytz HW, Iversen HK, Boas DA (2012) A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Front Neurosci 6:147. doi:10.3389/fnins.2012.00147
Drew T, Prentice S, Schepens B (2004) Cortical and brainstem control of locomotion. Prog Brain Res 143:251–261. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14653170
Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, Sutker PB (2008) Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol 23(5):603–612. doi:10.1016/j.acn.2008.06.004
England SE, Verghese J, Mahoney JR, Trantzas C, Holtzer R (2015) Three-level rating of turns while walking. Gait Posture 41(1):300–303. doi:10.1016/j.gaitpost.2014.09.010
Erdogan SB, Yucel MA, Akin A (2014) Analysis of task-evoked systemic interference in fNIRS measurements: insights from fMRI. Neuroimage 87:490–504. doi:10.1016/j.neuroimage.2013.10.024
Gerin-Lajoie M, Richards CL, McFadyen BJ (2005) The negotiation of stationary and moving obstructions during walking: anticipatory locomotor adaptations and preservation of personal space. Motor Control 9(3):242–269
Harada T, Miyai I, Suzuki M, Kubota K (2009) Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res 193(3):445–454. doi:10.1007/s00221-008-1643-y
Holtzer R, Verghese J, Xue X, Lipton RB (2006) Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology 20(2):215–223. doi:10.1037/0894-4105.20.2.215
Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB (2008) Within-person across-neuropsychological test variability and incident dementia. JAMA 300(7):823–830. doi:10.1001/jama.300.7.823
Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J (2011) fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 66(8):879–887. doi:10.1093/gerona/glr068
Holtzer R, Wang C, Lipton R, Verghese J (2012a) The protective effects of executive functions and episodic memory on gait speed decline in aging defined in the context of cognitive reserve. J Am Geriatr Soc 60(11):2093–2098. doi:10.1111/j.1532-5415.2012.04193.x
Holtzer R, Wang C, Verghese J (2012b) The relationship between attention and gait in aging: facts and fallacies. Motor Control 16(1):64–80. http://www.ncbi.nlm.nih.gov/pubmed/22402221
Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM (2014a) Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci 69(11):1375–1388. doi:10.1093/gerona/glu052
Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM (2014b) Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glu052
Holtzer R, Mahoney J, Verghese J (2014c) Intraindividual variability in executive functions but not speed of processing or conflict resolution predicts performance differences in gait speed in older adults. J Gerontol A Biol Sci Med Sci 69(8):980–986. doi:10.1093/gerona/glt180
Holtzer R, Wang C, Verghese J (2014d) Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age (Dordr) 36(1):373–381. doi:10.1007/s11357-013-9570-7
Holtzer R, Mahoney CJ, Wang C, England SE, Verghese J (2015) Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage 112:152–159
Izzetoglu M, Bunce SC, Izzetoglu K, Onaral B, Pourrezaei K (2007) Functional brain imaging using near-infrared technology. IEEE Eng Med Biol Mag 26(4):38–46. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17672230
Izzetoglu M, Chitrapu P, Bunce S, Onaral B (2010) Motion artifact cancellation in NIR spectroscopy using discrete Kalman filtering. Biomed Eng Online 9:16. doi:10.1186/1475-925X-9-16
Jobsis FF (1977) Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198(4323):1264–1267. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=929199
Kirilina E, Jelzow A, Heine A, Niessing M, Wabnitz H, Bruhl R, Tachtsidis I (2012) The physiological origin of task-evoked systemic artefacts in functional near infrared spectroscopy. Neuroimage 61(1):70–81. doi:10.1016/j.neuroimage.2012.02.074
Li KZH, Lindenberger U, Freund AM, Baltes PB (2001) Walking while memorizing: age-related differences in compensatory behavior. Psychol Sci 12(3):230–237
Lindenberger U, Marsiske M, Baltes PB (2000) Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging 15(3):417–436
MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS (2000) Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288(5472):1835–1838
Mirelman A, Maidan I, Bernad-Elazari H, Nieuwhof F, Reelick M, Giladi N, Hausdorff JM (2014) Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J Neuroeng Rehabil 12(11):85. doi:10.1186/1743-0003-11-85
Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000) The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit Psychol 41(1):49–100. doi:10.1006/cogp.1999.0734
Nutt JG, Marsden CD, Thompson PD (1993) Human walking and higher-level gait disorders, particularly in the elderly. Neurology 43(2):268–279. http://www.ncbi.nlm.nih.gov/pubmed/8437689
Oh-Park M, Xue X, Holtzer R, Verghese J (2011) Transient versus persistent fear of falling in community-dwelling older adults: incidence and risk factors. J Am Geriatr Soc 59(7):1225–1231. doi:10.1111/j.1532-5415.2011.03475.x
Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA (2000) Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci 12(1):174–187. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10769314
Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, Rosano C (2013) Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci 68(11):1379–1386. doi:10.1093/gerona/glt089
Schaefer S, Schellenbach M, Lindenberger U, Woollacott M (2015) Walking in high-risk settings: do older adults still prioritize gait when distracted by a cognitive task? Exp Brain Res 233(1):79–88. doi:10.1007/s00221-014-4093-8
Schwenk M, Zieschang T, Oster P, Hauer K (2010) Dual-task performances can be improved in patients with dementia: a randomized controlled trial. Neurology 74(24):1961–1968. doi:10.1212/WNL.0b013e3181e39696
Stern Y (2009) Cognitive reserve. Neuropsychologia 47(10):2015–2028. doi:10.1016/j.neuropsychologia.2009.03.004
Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Guralnik J (2011) Gait speed and survival in older adults. JAMA 305(1):50–58. doi:10.1001/jama.2010.1923
Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H (2002) Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med 347(22):1761–1768. doi:10.1056/NEJMoa020441
Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB (2006) Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc 54(2):255–261. doi:10.1111/j.1532-5415.2005.00580.x
Verghese J, Derby C, Katz MJ, Lipton RB (2007) High risk neurological gait syndrome and vascular dementia. J Neural Transm 114(10):1249–1252. doi:10.1007/s00702-007-0762-0
Verghese J, Robbins M, Holtzer R, Zimmerman M, Wang C, Xue X, Lipton RB (2008) Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc 56(7):1244–1251. doi:10.1111/j.1532-5415.2008.01758.x
Verghese J, Ambrose AF, Lipton RB, Wang C (2010a) Neurological gait abnormalities and risk of falls in older adults. J Neurol 257(3):392–398. doi:10.1007/s00415-009-5332-y
Verghese J, Mahoney J, Ambrose AF, Wang C, Holtzer R (2010b) Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci 65(12):1338–1343. doi:10.1093/gerona/glq127
Verghese J, Holtzer R, Lipton RB, Wang C (2012) Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc 60(10):1901–1905. doi:10.1111/j.1532-5415.2012.04145.x
Yogev-Seligmann G, Hausdorff JM, Giladi N (2008) The role of executive function and attention in gait. Mov Disord 23(3):329–342. doi:10.1002/mds.21720
Yogev-Seligmann G, Giladi N, Brozgol M, Hausdorff JM (2012) A training program to improve gait while dual tasking in patients with Parkinson’s disease: a pilot study. Arch Phys Med Rehabil 93(1):176–181. doi:10.1016/j.apmr.2011.06.005
Yuan J, Blumen HM, Verghese J, Holtzer R (2014) Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: a resting-state fMRI study. Hum Brain Mapp. doi:10.1002/hbm.22717
Acknowledgments
This research was supported by the National Institutes on Aging R01AG036921 (PI: R. Holtzer) and R01AG044007 (PI: J. Verghese).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Dr. Izzetoglu has a very minor share in the company that manufactures the fNIRS device used in this study. All other authors have no conflicts of interest to report in relation to the current article.
Rights and permissions
About this article
Cite this article
Holtzer, R., Verghese, J., Allali, G. et al. Neurological Gait Abnormalities Moderate the Functional Brain Signature of the Posture First Hypothesis. Brain Topogr 29, 334–343 (2016). https://doi.org/10.1007/s10548-015-0465-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-015-0465-z