Abstract
Purpose
To contrast older and younger adults’ prefrontal cortex (PFC) neural activity (through changes in oxygenated hemoglobin) during single and dual tasks, and to compare decrements in task performance.
Methods
Changes in oxygenated hemoglobin of dorsolateral PFC were monitored using functional near-infrared spectroscopy during single tasks of spelling backwards (cognitive task) and 30 m preferred paced walk; and a dual task combining both. Gait velocity was measured by a pressure sensitive mat.
Results
Twenty sex-matched younger (27.6 ± 3.5 years) and 17 older adults (71.2 ± 4.9 years) were recruited. The left PFC oxygenated hemoglobin decreased from start (1st quintile) to the end (5th quintile) of the walking task in younger adults ( – 0.03 ± 0.03 to – 0.72 ± 0.20 µM; p < .05) unlike the non-significant change in older adults (0.03 ± 0.06 to – 0.41 ± 0.32 µM, p > .05). Overall, oxygenation increased bilaterally during dual versus single walk task in older adults (Left PFC: 0.22 ± 0.16 vs. – 0.23 ± 0.21 µM, respectively; Right PFC: 0.17 ± 0.18 vs. – 0.33 ± 0.22 µM, respectively), but only in right PFC in younger adults ( – 0.02 ± 0.15 vs. – 0.47 ± 0.13 µM). Older adults exhibited lower velocity during the dual task compared to younger adults (1.03 ± 0.16 vs. 1.20 ± 0.17 m/s, respectively). Older age was associated with dual task cost on velocity during walking after adjusting for confounding variables.
Conclusions
Age-related cognitive decline in older adults may increase neural activity for cognitive tasks and diminish walking automaticity that may lead to decrements during dual tasking; the greater PFC increases in the oxygenated hemoglobin and lower velocity may be due to increased cognitive load and limited attentional resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In healthy adults, routine tasks such as walking are automatized. Movement automaticity refers to the ability of central nervous system to enable walking with minimal use of cognitive resources (e.g., attention) (Clark 2015). Healthy older people walk more slowly, with a shorter step length and greater stance width; all of these gait changes appear to be directed towards improving walking stability and may indicate a loss of automaticity (Guimaraes and Isaacs 1980). Consequently, the inability of maintaining task automatization is postulated to contribute to dual task decrements, poor balance and even falls (Silsupadol et al. 2009). A decline in automaticity in older adults may be improved by dual task training that has shown to improve balance and cognitive measures (Silsupadol et al. 2009); however, literature on the associated mechanisms is lacking.
The prefrontal cortex (PFC) is responsible for executive functions involved in walking and cognitively challenging tasks (Nóbrega-Sousa et al. 2020). These cognitive functions decline with age, leading to cognitive impairment and increased fall risk (Herman et al. 2010). Difficult tasks involving high attentional demands, working memory and motor planning can result in loss of automaticity and lead to increased activation of PFC (MacDonald et al. 2000; Holtzer et al. 2014; Salzman et al. 2021). In contrast, a decrease in PFC activity at the end of the task, relative to the beginning would suggest automaticity (Clark 2015; Stuart et al. 2019). A greater decrease suggests a greater level of automaticity (Vandenbossche et al. 2013; Hermand et al. 2020). However, age-related cognitive decline may negate these changes and alternatively be counterbalanced by neural mechanisms such as compensation (Cabeza et al. 2018).
Compensation refers to the increased recruitment and interaction between PFC and other brain regions when a particular neural region has declined performance as a result of aging or a neurological condition. This increased activity in the brain is postulated to improve performance of the task at hand. Compensation has been documented in older compared to younger adults during tasks with relatively low cognitive demand (e.g., memorization), a marker of cognitive frailty (Cabeza et al. 2002). Cognitive frailty as manifested by cognitive impairment and physical frailty (Bu et al. 2021) can be accentuated during dual tasking resulting in decrement in performance of one or both tasks (cognitive and/or motor). This is termed dual task cost. Decrements commonly occur when attentional demand of performing two tasks concurrently exceeds an individual’s attentional capacity, which increase with age and is a sign of cognitive frailty (Al-Yahya et al. 2011). The capacity sharing model explains this phenomenon of limited attentional capacity and suggests that pools of processing resources are divided when multitasking. When one task is prioritized during dual tasking, more cognitive resources are allocated towards it, which may result in a decline of performance in the second task (Tombu and Jolicoeur, 2003). Therefore, automaticity of a task that is not prioritized during dual task performance may decline if attentional resources are not directed towards it.
The neurovascular coupling associated with cognitive and motor tasks can be evaluated using functional near-infrared spectroscopy (fNIRS) that measures relative changes in oxygenated hemoglobin (∆O2Hb) to infer neuronal activity (Holtzer et al. 2014; Maidan et al. 2016; Hawkins et al. 2018). fNIRS has several distinct qualities that are advantageous to provide a measure of neural activity by quantifying ΔO2Hb (Piper et al. 2014). A more conventional neuroimaging method is functional magnetic resonance imaging (fMRI) that indirectly measures neuronal activity using blood-oxygen-level dependent (BOLD) imaging. However, fMRI cannot be used to monitor neuronal activity during physical movements required for daily activities such as walking. Further, access is limited, it is expensive and it is limited by claustrophobia in some participants. In contrast, fNIRS provides real-time measurements, allows measurement during movement, is relatively unobtrusive and is inexpensive (Clark 2015). Examining dual task cost while monitoring dorsolateral PFC ΔO2Hb may provide insights into the cognitive load of single versus dual task as well as whether automaticity occurs during walking in younger and older adults (Clark 2015; Stuart et al. 2019; St-Amant et al. 2020; Salzman et al. 2021).
Previous studies have analyzed the PFC ∆O2Hb in younger adults (Techayusukcharoen et al. 2019), and older adults (Harada et al. 2009; Chen et al. 2017; Corp et al. 2018; Ross et al. 2021), during a cognitive task and/or walking. Dual tasking involving usual paced walking has shown to increase PFC O2Hb in both older and younger adults (Holtzer et al. 2011, 2015; Fraser et al. 2016) and worsen gait performance especially in older adults, but the findings of mental tracking tasks as they relate to gait that involve manipulating information in the working memory are inconsistent (Al-Yahya et al. 2011; Pelicioni et al. 2019). Moreover, studies have also shown mixed results regarding which of the two groups has greater PFC ∆O2Hb during dual tasking. Holtzer et al. (2011) reported greater increase in younger adults, while Beurskens et al. (2014) reported a decrease in older adults. In contrast, higher PFC ∆O2Hb has also been reported in older compared to younger adults (Fraser et al. 2016; Mirelman et al. 2017). To date, evaluation of both left and right dorsolateral PFC ∆O2Hb to assess automaticity comparing younger and older adults during walking in dual task studies has not been reported. Comparison of left and right dorsolateral PFC ∆O2Hb is important to elucidate the impact of aging on unilateral versus bilateral activity differences in younger and older adults.
In order to elucidate neural correlates of walking performance and its automaticity, the objectives of this study were to (1) compare dorsolateral PFC ΔO2Hb and changes in cognitive and gait performance between single and dual tasks within and between healthy younger and older adults; (2) compare PFC ΔO2Hb at the beginning and end of the tasks to assess automaticity; (3) determine the contribution of age to dorsolateral PFC ΔO2Hb, and dual task cost on velocity and spelling backwards accuracy while controlling for potential confounders.
Materials and methods
Participants
Healthy younger (n = 20) and older participants (n = 17) were recruited through emails and flyers from the University of Toronto, University Health Network associated hospitals and community centers in downtown Toronto, Canada. The sample size calculation based on 80% power to detect a standardized effect size of 0.80 at p < 0.05 required 20 participants per group for between group differences and 11–12 for within group differences. Inclusion criteria were healthy men and women aged between 18 and 35 years (younger group) and ≥ 65 years (older group). Exclusion criteria were: current smokers; acute illness within 3 months; unstable cardiovascular, neurological or musculoskeletal conditions that interfere with independent ambulation or ability to stand on one leg; and cognitive impairment or lack of English fluency that may interfere with providing informed consent or following study instructions. All participants provided written informed consent before participation. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Research Ethics Board of University of Toronto (protocol ID: 33,466).
Procedure
In this cross-sectional study, participants’ safety during exercise was screened with the American College of Sports Medicine questionnaire (Thompson et al. 2010). Anthropometric measures (height and weight) and hand and leg dominance were recorded. Questionnaires were then administered: (1) Digit Span Forward and Backward Tests (Wechsler 1997) were administered up to 5 digit sequences. Participants recalled 2–5 digit sequences in the same or reverse-order until two sequences with the same number of digits could not be recalled (Wechsler 1997); (2) Montreal Cognitive Assessment (MoCA) a valid and reliable screening tool for cognitive dysfunction (Nasreddine et al. 2005) evaluated attention and concentration, executive functions, memory, language, visuo-constructional skills, conceptual thinking, calculations, and orientation; (3) Medication and Comorbidities Questionnaire was used to document participants’ medications and health conditions (Deyo et al. 1992); (4) Single Leg Stance, a balance test, asked participants to stand on their dominant leg for as long as they could, while bending the non-dominant leg at 90° angle with hands on hips (Friden et al. 1989). The BIOPAC wireless fNIRS device model FNIR100W-1 (fNIR Devices LLC, Potomac, MD) was secured over the participants’ forehead (McKendrick et al. 2017) to record dorsolateral PFC ∆O2Hb during all subsequent measures. Before and after each single and dual task, the participants completed a baseline task that involved spelling words forward from flashcards for 1 min. Two single tasks were performed in random order: (1) preferred paced walk; and (2) spelling backwards as the cognitive task. Subsequently, a dual task was performed, which paired tasks 1 and 2.
A BIOPAC wireless fNIRS device with 2 emitters and 4 detectors was secured over the forehead; the emitters were vertically aligned with the participants’ iris (Fig. 1). Light intensity data at 730 nm and 850 nm were obtained using Cognitive Optical Brain Imaging (COBI) Studio at a frequency of 4 Hz and processed in fnirSoft (Drexel University, Philadelphia, PA) (Ayaz 2010). LED current and detector gain settings were adjusted to prevent low or saturated light signals for each participant. A low-pass, finite impulse response, filter with hamming order 57 and cutoff frequency of 0.05 was applied to attenuate physiological artifacts of respiration and pulse. Data were inspected for motion artifacts visually and through the Sliding Motion Artifact Rejection algorithm. ΔO2Hb were calculated using the modified Beer-Lambert law (Artinis Medical Systems 2000).
fNIRS device (posterior aspect) showing 2 emitters and 4 detectors on its inner surface (upper image). The device was positioned over the forehead (lower image) and the emitters were aligned vertically with the iris (Ayaz 2010). The interoptode distance between the emitters and the detectors was 2.5 cm
Single and dual tasks
Participants performed two single tasks in random order and then in combination as a dual task: (1) Spelling Backwards, is classified as a mental tracking task. Participants were asked to spell 5 letter words backwards (Hollman et al. 2010) that were read out loud by the investigator from a list of 100 unique words for 1 min. Spelling accuracy and number of words attempted were recorded. This task was considered relevant to daily activities that require multitasking, and holding information in mind while performing a mental task (i.e., making purchasing decisions while shopping). (2) Walking Tasks (Bonetti et al. 2019) involved preferred (usual pace) paced walking for 30 m (6 passes) over a 5 × 0.88 m pressure sensitive Zeno Walkway (ProtoKinetics LLC, Havertown, PA) that contained a grid of 13,824 sensors. Gait velocity was calculated using the ProtoKinetics Movement Analysis Software (ProtoKinetics LLC, Havertown, PA). Preferred paced walking task was chosen to mimic the more usual pace of walking during activities of daily living.
Statistical analysis
Two-way mixed analysis of variance (ANOVA) tested differences in mean ΔO2Hb data of entire tasks’ duration between tasks and groups for: (1) left and (2) right PFC. Post hoc tests used t tests. One-way repeated measures ANOVA compared ΔO2Hb during both walking tasks between left and right PFC in each group with Bonferroni post hoc test. Paired sample t tests compared velocity between tasks, within each group, and Wilcoxon signed rank test for ordinal data (words spelled backwards) (Manor et al. 2016). Decrements of spelling backwards accuracy and gait velocity were calculated as follows:
Using two-way mixed ANOVA, automaticity of walking was assessed by examining the PFC ΔO2Hb during the first quintile (Q1) compared to the fifth quintile (Q5) of walk duration and contrasted between groups. Although not hypothesized to decrease, a similar analysis (two-way mixed ANOVA) examined differences of spelling backwards PFC ΔO2Hb between time points (Q1 vs Q5) and groups. Differences in participant group characteristics were compared using unpaired t tests and proportions were tested using Chi-squared (i.e., sex, handedness). Multiple linear regression was used to determine the contribution of age to outcome variables—dorsolateral PFC ∆O2Hb and dual task cost on spelling backwards accuracy and gait velocity—while adjusting for confounding variables: Montreal Cognitive Assessment scores and single leg stance duration. Statistical tests were performed in SPSS (version 27).
Results
Participants’ characteristics
The older group was similar to the younger group in several attributes but differed in age (mean 27.6 versus 71.2 years), had a 13% higher BMI, had more comorbidities, took more medications and had a single leg stance duration that was 26.3% of the duration shown by younger adults (Table 1). Within the younger adults group, 11/20 (55%) participants had a normal BMI (18.5–24.9 kg/m2), 6/20 (30%) were overweight (25.0–29.9 kg/m2), while 1 (5%) was underweight (< 18.5 kg/m2) and 2 (10%) were obese (> 30.0 kg/m2). On the other hand, 5 (29.4%), 5 (29.4%) and 7 (41.2%) of 17 older participants were of normal weight, overweight and obese according to BMI cut-offs guidelines (WHO, 2020). Using MoCA cut-off score of < 26 (Nasreddine et al. 2005), 16/20 participants in the younger and 10/17 in the older adults group were above the threshold for not having cognitive impairment.
PFC neural activity—O2Hb
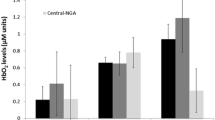
In older adults, O2Hb increased bilaterally (p < 0.050) during the preferred paced walking dual versus single task (left PFC: t(16), 1.87, p = 0.040, d = 0.45; right PFC: t(16) = 2.39, p = 0.015, d = 0.58) (Fig. 2); in contrast, O2Hb only increased during preferred paced walk dual compared to single task in the right PFC (t(19) = 2.36, p = 0.015, d = 0.53) in younger adults. No significant interactions were found between groups and two walking tasks for ∆O2Hb using mixed ANOVA. Moreover, no significant differences were found between any of the four tasks in left or right PFC (p > 0.050).
Dorsolateral prefrontal cortex changes in oxygenated hemoglobin (PFC ∆O2Hb) during single and dual tasks (mean ± standard error). In older adults, O2Hb increased during preferred paced walk dual (PPW + SB) versus single task (PPW) in the left and right PFC, but only in the left PFC in younger adults. *indicates differences at p < .05
The increase in left and right PFC O2Hb during spelling backwards from Q1 to Q5 tended to be higher in older compared to younger adults (left PFC: t(35) = 1.68, p = 0.051, d = 0.555; right PFC: t(35) = 1.50, p = 0.072, d = 0.494, respectively). Both groups increased in left and right PFC O2Hb during spelling backwards from Q1 to Q5 (p ≤ 0.003) (Fig. 3). During preferred paced walk, older adults only showed a significant decrease in right PFC O2Hb (t(16) = 1.939, p = 0.035, d = 0.470) whereas younger adults showed significant decreases in PFC O2Hb bilaterally from Q1 to Q5 (left PFC: t(18) = 3.422, p < 0.002, d = 0.785; right PFC: t(19) = 3.471, p < 0.002, d = 0.776, respectively) (Fig. 3).
Changes in left and right dorsolateral prefrontal cortex (dlPFC) ∆O2Hb during the first (Q1) and last quintile (Q5) of the single tasks: preferred paced walking (upper panel) and spelling backwards (lower panel). Means and standard errors are shown. *Fifth quintile different than first quintile at p = 0.035; **difference at p ≤ 0.002.Decreased automaticity contributes to dual task decrements in older compared to younger adults
Dual task cost on gait velocity and cognitive task accuracy
Although both groups had a similar single task preferred paced walk velocity, dual task velocity in young adults did not change. In contrast, dual task velocity in older adults decreased compared to single preferred paced walk task (p < 0.003), which was significantly lower than the younger groups’ dual task velocity (p = 0.002). Moreover, older adults showed a greater dual task decrement compared to the younger group (p = 0.001).
Older adults compared to younger adults spelled fewer words during the single spelling backwards task (p = 0.039) and were less accurate (p = 0.049) (Table 2). Both groups attempted fewer words during the dual task compared to single spelling backwards task (p < 0.003) (Table 2). Compared to the single spelling backwards task, the younger adults had reduced accuracy during the dual task unlike older adults (Table 2).
Effect of age on outcome variables
Linear regression indicated that older age was significantly associated with dual task cost on velocity during preferred paced walking (F(3,33) = 5.87, p = 0.002, adjusted R2 = 0.289) after adjusting for single leg stance duration and Montreal Cognitive Assessment scores (Table 3). Age explained 29% of the variation in preferred paced walk velocity (R2 = 0.29). Age was not significantly associated with the right or left dorsolateral PFC ∆O2Hb or dual task cost on spelling backwards accuracy during the dual task.
Adjusted R2 = 0.29, SE of the estimate = 10.88, effect size = 0.41, power = 0.88
Discussion
Comparison of results to literature
The aging-related neurophysiological changes associated with the interaction of cognitive impairment and physical frailty (e.g., diminished walking automaticity) are not well understood. The unique findings of this study were that the overall O2Hb increased bilaterally in the dorsal PFC during preferred paced walking dual versus single task in older adults, but only in the right PFC in younger adults. In contrast, the younger group showed signs of automaticity bilaterally in the PFC during single task walking and lower increases during the single task backwards spelling. As expected, both groups did not show signs of automaticity during the spelling backwards task. The combined absolute higher levels of O2Hb in older adults during spelling backwards and walking, may have contributed to the unilateral decrease in PFC O2Hb during walking unlike the bilateral decrease in the younger group. Of clinical importance, walking velocity decreased during dual compared to single task in older adults in contrast to younger adults. The impact of age on velocity was confirmed using regression analysis adjusted for MoCA scores (assessment of cognitive impairment) and single leg stance duration (assessment of balance).
Higher ∆O2Hb bilaterally during dual versus single tasking in older, in contrast to younger adults, may be explained by hemispheric asymmetry reduction in older adults (HAROLD) model, which attributes bilateral activity to declining unilateral neural efficiency and an inability to recruit required neural regions (Li et al. 2009). The increased PFC neural activity during dual tasking may result from less efficient neural processing (Cabeza et al. 2018; Li et al. 2018) and greater cognitive demands (i.e., compensation) for intentional movement control and goal oriented preparation for upcoming sequential gait steps in older adults (Pochon et al. 2001; Harada et al. 2009). In particular, the increases in left PFC O2Hb during dual tasking in older adults may be reflective of divided attention and the need for language processing, whereas increases in the right PFC may be indicative of sustained attention and episodic memory retrieval (Cabeza and Nyberg 2000). Mental tracking tasks that involve manipulating information in working memory (e.g., backwards spelling) are linked to bilateral PFC activation (Petrides 1995). These higher PFC demands are consistent with early signs of cognitive impairment in older adults.
Time series analysis of PFC ∆O2Hb was used to evaluate automaticity (Vandenbossche et al. 2013; Hawkins et al. 2018; Hermand et al. 2020). Backwards spelling is a cognitively challenging task containing all unique words, thus making it very difficult to become automatized. Thus, as expected, both young and old adults failed to show automaticity and exhibited higher PFC ∆O2Hb at the end relative to beginning of the task. On the other hand, automaticity was more apparent during walking. Moreover, bilateral versus unilateral evidence and larger effect sizes were observed in younger compared to older adults during walking. Similar walking velocity was observed during this routine task in both groups but the greater dual task cost in older adults is consistent with a decline in automaticity and calls for attention to cognitive-motor rehabilitation interventions to restore walking automaticity.
The dual task cost on velocity during preferred paced walking was found to be dependent on age. This is corroborated by a meta-analysis on dual tasking (Al-Yahya et al. 2011). In the present study, age explained 29% of the variance in dual task cost during preferred paced walking. The decrements observed during dual tasks might be due to higher attentional and executive functions demands for maintaining dynamic balance during gait, (Patel et al. 2014) as executive functions and the associated neural networks walking and spelling backwards may be intertwined (Smith et al. 2016). Therefore, diversion of limited and diminished cognitive resources from more demanding task of maintaining balance during the dual task may explain the reduction in velocity in the older group and their increased risk of falls during activities of daily living.
Decrements in velocity and spelling backwards accuracy may be explained by capacity sharing model (Tombu and Jolicoeur, 2003; Patel et al. 2014) and task (Yogev-Seligmann et al. 2012). The capacity sharing model purports that pools of processing resources need to be divided amongst tasks during multitasking (Pashler and Johnston 1998; Tombu and Jolicoeur 2003). Prioritizing and allocating more cognitive resources towards one task leaves less cognitive capacity for the other task. This can lead to decrements in both tasks due to divided resources, but greater decrement in the one with less resources. Moreover, crosstalk between overlapping neural networks and executive functions required for walking and spelling backwards may also explain the decrements in both tasks during dual tasking (Pashler and Johnston 1998). In this study, decrements in velocity during dual task only occurred in older adults, suggesting that younger adults were better able to manage attentional demands required by the tasks unlike older adults. This finding may also be explained by task prioritization as an age-related difference between individuals. Literature suggested that older individuals utilize posture-first strategy, where they prioritize gait over cognitive task performance to prevent loss of balance (Berger and Bernard-Demanze 2011). However, there have also been reports of failing to following the posture-first strategy (Lindenberger et al. 2000; Corp et al. 2018), which is indicative of limitations of the cognitive resources.
Study limitations
This study has some limitations. The fNIRS used in the present study provides data on relative, rather than absolute, ∆O2Hb unlike the frequency-and time-domain fNIRS. Moreover, the MoCA scores only showed a trend towards difference between younger and older adults indicating that the mean sample data didn’t reflect significant cognitive impairment in older adults (Dale et al. 2018). A larger sample size and broader spread may provide the power to show the influence of this measure. Moreover, potential visual or auditory impairment may have influenced PFC ∆O2Hb and impacted participants’ performance during spelling and walking, thereby introducing bias in R. A future study may utilize an fNIRS device that enables measurements from a greater surface area of the head to provide insight into the activity of other neural regions (e.g., supplementary motor area, posterior occipital temporal areas) and networks (Mirelman et al. 2017; Papegaaij et al. 2017) in the cognitively impaired individuals during cognitive-motor tasks. Such data may then be utilized to devise appropriate rehabilitation interventions with focus on enhancing automaticity of walking.
Conclusions
Older adults had greater neural activity and greater dual task deficits in walking velocity than younger adults. Reduced dual task walking velocity and higher PFC ∆O2Hb may indicate limited attentional resources with aging and suggest diminished automaticity. Individuals should be assessed at an earlier age to pre-habilitate and prevent functional decline that could increase risk of falls and other limitations in activities of daily living.
Availability of data and material
The datasets of the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- ∆O2Hb:
-
Changes in oxygenated hemoglobin
- BMI:
-
Body mass index
- fNIRS:
-
Functional near-infrared spectroscopy
- PFC:
-
Prefrontal cortex
- PPW:
-
Preferred paced walk
- Q1:
-
First quintile
- Q5:
-
Fifth quintile
- SB:
-
Spelling backwards
References
Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J (2011) Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev 35:715–728. https://doi.org/10.1016/j.neubiorev.2010.08.008
Artinis medical systems (2000) The theory of Near Infrared Spectroscopy. Artinis Medical Systems | fNIRS and NIRS devices. Available at: https://www.artinis.com/theory-of-nirs [Accessed June 7, 2021]
Ayaz H (2010) Functional near infrared spectroscopy based brain computer interface
Berger L, Bernard-Demanze L (2011) Age-related effects of a memorizing spatial task in the adults and elderly postural control. Gait Posture 33:300–302. https://doi.org/10.1016/j.gaitpost.2010.10.082
Beurskens R, Helmich I, Rein R, Bock O (2014) Age-related changes in prefrontal activity during walking in dual-task situations: a fNIRS study. Int J Psychophysiol 92:122–128. https://doi.org/10.1016/j.ijpsycho.2014.03.005
Bonetti LV, Hassan SA, Kasawara KT, Reid WD (2019) The effect of mental tracking task on spatiotemporal gait parameters in healthy younger and middle- and older aged participants during dual tasking. Exp Brain Res 237:3123–3132. https://doi.org/10.1007/s00221-019-05659-z
Bu Z, Huang A, Xue M, Li Q, Bai Y, Xu G (2021) Cognitive frailty as a predictor of adverse outcomes among older adults: A systematic review and meta-analysis. Brain and Behavior 11:e01926. https://doi.org/10.1002/brb3.1926
Cabeza R, Nyberg L (2000) Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12:1–47
Cabeza R, Anderson ND, Locantore JK, McIntosh AR (2002) Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 17:1394–1402. https://doi.org/10.1006/nimg.2002.1280
Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL et al (2018) Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci 19:701–710. https://doi.org/10.1038/s41583-018-0068-2
Chen M, Pillemer S, England S, Izzetoglu M, Mahoney JR, Holtzer R (2017) Neural correlates of obstacle negotiation in older adults: An fNIRS study. Gait Posture 58:130–135. https://doi.org/10.1016/j.gaitpost.2017.07.043
Clark DJ (2015) Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2015.00246
Corp DT, Youssef GJ, Clark RA, Gomes-Osman J, Yücel MA, Oldham SJ et al (2018) Reduced motor cortex inhibition and a ‘cognitive-first’ prioritisation strategy for older adults during dual-tasking. Exp Gerontol 113:95–105. https://doi.org/10.1016/j.exger.2018.09.018
Dale W, Kotwal AA, Shega JW, Schumm LP, Kern DW, Pinto J et al (2018) Cognitive function and its risk factors among older US adults living at home. Alzheimer Dis Assoc Disord 32:207–213. https://doi.org/10.1097/WAD.0000000000000241
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619
Fraser SA, Dupuy O, Pouliot P, Lesage F, Bherer L (2016) Comparable cerebral oxygenation patterns in younger and older adults during dual-task walking with increasing load. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2016.00240
Friden T, Zätterström R, Lindstrand A, Moritz U (1989) A stabilometric technique for evaluation of lower limb instabilities. Am J Sports Med 17:118–122. https://doi.org/10.1177/036354658901700120
Guimaraes RM, Isaacs B (1980) Characteristics of the gait in old people who fall. Int Rehabil Med 2:177–180. https://doi.org/10.3109/09638288009163984
Harada T, Miyai I, Suzuki M, Kubota K (2009) Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res 193:445–454. https://doi.org/10.1007/s00221-008-1643-y
Hawkins KA, Fox EJ, Daly JJ, Rose DK, Christou EA, McGuirk TE et al (2018) Prefrontal over-activation during walking in people with mobility deficits: Interpretation and functional implications. Hum Mov Sci 59:46–55. https://doi.org/10.1016/j.humov.2018.03.010
Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM (2010) Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci 65:1086–1092. https://doi.org/10.1093/gerona/glq077
Hermand E, Compagnat M, Dupuy O, Salle J-Y, Daviet J-C, Perrochon A (2020) Functional status is associated with prefrontal cortex activation in gait in subacute stroke patients: a functional near-infrared spectroscopy study. Front Neurol 11:1298. https://doi.org/10.3389/fneur.2020.559227
Hollman JH, Childs KB, McNeil ML, Mueller AC, Quilter CM, Youdas JW (2010) Number of strides required for reliable measurements of pace, rhythm and variability parameters of gait during normal and dual task walking in older individuals. Gait Posture 32:23–28. https://doi.org/10.1016/j.gaitpost.2010.02.017
Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J (2011) fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 66:879–887. https://doi.org/10.1093/gerona/glr068
Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM (2014) Neuroimaging of mobility in aging: a targeted review. J Gerontol Series A 69:1375–1388. https://doi.org/10.1093/gerona/glu052
Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J (2015) Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage 112:152–159. https://doi.org/10.1016/j.neuroimage.2015.03.002
Li Z, Moore AB, Tyner C, Hu X (2009) Asymmetric Connectivity Reduction and its Relationship to “HAROLD” in Aging Brain. Brain Res 1295:149–158. https://doi.org/10.1016/j.brainres.2009.08.004
Li KZH, Bherer L, Mirelman A, Maidan I, Hausdorff JM (2018) Cognitive involvement in balance, gait and dual-tasking in aging: a focused review from a neuroscience of aging perspective. Front Neurol 9:913. https://doi.org/10.3389/fneur.2018.00913
Lindenberger U, Marsiske M, Baltes PB (2000) Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging 15:417–436
MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000) Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288:1835–1838
Maidan I, Nieuwhof F, Bernad-Elazari H, Reelick MF, Bloem BR, Giladi N et al (2016) The role of the frontal lobe in complex walking among patients with Parkinson’s disease and healthy older adults: an fNIRS study. Neurorehabil Neural Repair 30:963–971. https://doi.org/10.1177/1545968316650426
Manor B, Zhou J, Jor’dan A, Zhang J, Fang J, Pascual-Leone A (2016) Reduction of dual-task costs by noninvasive modulation of prefrontal activity in healthy elders. J Cognit Neurosci 28:275–281. https://doi.org/10.1162/jocn_a_00897
McKendrick R, Mehta R, Ayaz H, Scheldrup M, Parasuraman R (2017) Prefrontal hemodynamics of physical activity and environmental complexity during cognitive work. Hum Factors 59:147–162. https://doi.org/10.1177/0018720816675053
Mirelman A, Maidan I, Bernad-Elazari H, Shustack S, Giladi N, Hausdorff JM (2017) Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn 115:41–46. https://doi.org/10.1016/j.bandc.2017.04.002
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I et al (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Nóbrega-Sousa P, Gobbi LTB, Orcioli-Silva D, da Conceição NR, Beretta VS, Vitório R (2020) Prefrontal cortex activity during walking: effects of aging and associations with gait and executive function. Neurorehabil Neural Repair 34:915–924. https://doi.org/10.1177/1545968320953824
Papegaaij S, Hortobágyi T, Godde B, Kaan WA, Erhard P, Voelcker-Rehage C (2017) Neural correlates of motor-cognitive dual-tasking in young and old adults. PLoS ONE 12:e0189025. https://doi.org/10.1371/journal.pone.0189025
Pashler H, Johnston JC (1998) “Attentional limitations in dual-task performance,” in Attention (Hove, England: Psychology Press/Erlbaum (UK) Taylor & Francis), 155–189
Patel P, Lamar M, Bhatt T (2014) Effect of type of cognitive task and walking speed on cognitive-motor interference during dual-task walking. Neuroscience 260:140–148. https://doi.org/10.1016/j.neuroscience.2013.12.016
Pelicioni PHS, Tijsma M, Lord SR, Menant J (2019) Prefrontal cortical activation measured by fNIRS during walking: effects of age, disease and secondary task. PeerJ 7:e6833. https://doi.org/10.7717/peerj.6833
Petrides M (1995) Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann N Y Acad Sci 769:85–96
Piper SK, Krueger A, Koch SP, Mehnert J, Habermehl C, Steinbrink J et al (2014) A wearable multi-channel fNIRS system for brain imaging in freely moving subjects. Neuroimage. https://doi.org/10.1016/j.neuroimage.2013.06.062
Pochon JB, Levy R, Poline JB, Crozier S, Lehéricy S, Pillon B et al (2001) The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cereb Cortex 11:260–266
Ross D, Wagshul ME, Izzetoglu M, Holtzer R (2021) Prefrontal cortex activation during dual-task walking in older adults is moderated by thickness of several cortical regions. GeroScience 43:1959–1974. https://doi.org/10.1007/s11357-021-00379-1
Salzman T, Tobón Vallejo D, Polskaia N, Michaud L, St-Amant G, Lajoie Y et al (2021) Hemodynamic and behavioral changes in older adults during cognitively demanding dual tasks. Brain Behavior 11:e02021. https://doi.org/10.1002/brb3.2021
Silsupadol P, Lugade V, Shumway-Cook A, van Donkelaar P, Chou L-S, Mayr U et al (2009) Training-related changes in dual-task walking performance of elderly persons with balance impairment: a double-blind, randomized controlled trial. Gait Posture 29:634–639. https://doi.org/10.1016/j.gaitpost.2009.01.006
Smith E, Cusack T, Blake C (2016) The effect of a dual task on gait speed in community dwelling older adults: A systematic review and meta-analysis. Gait Posture 44:250–258. https://doi.org/10.1016/j.gaitpost.2015.12.017
St-Amant G, Rahman T, Polskaia N, Fraser S, Lajoie Y (2020) Unveilling the cerebral and sensory contributions to automatic postural control during dual-task standing. Hum Mov Sci 70:102587. https://doi.org/10.1016/j.humov.2020.102587
Stuart S, Belluscio V, Quinn JF, Mancini M (2019) Pre-frontal cortical activity during walking and turning is reliable and differentiates across young, older adults and people with Parkinson’s disease. Front Neurol 10:536. https://doi.org/10.3389/fneur.2019.00536
Techayusukcharoen R, Iida S, Aoki C (2019) Observing brain function via functional near-infrared spectroscopy during cognitive program training (dual task) in young people. J Phys Ther Sci 31:550–555. https://doi.org/10.1589/jpts.31.550
Thompson WR, Gordon NF, Pescatello Li, S. (eds) (2010) American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription, 8th edn. Lippincott Williams & Wilkins/Wolter Kluwer, Philadelphia
Tombu M, Jolicoeur P (2003) A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform 29:3–18. https://doi.org/10.1037//0096-1523.29.1.3
Vandenbossche J, Deroost N, Soetens E, Coomans D, Spildooren J, Vercruysse S et al (2013) Freezing of gait in Parkinson’s disease: disturbances in automaticity and control. Front Hum Neurosci 6:356. https://doi.org/10.3389/fnhum.2012.00356
Wechsler D (1997) WAIS-III: Administration and scoring manual: Wechsler adult intelligence scale--third edition. 3rd edition. Psychological Corporation
WHO (2020) Obesity and overweight. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [Accessed March 1, 2021]
Yogev-Seligmann G, Hausdorff JM, Giladi N (2012) Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov Disord 27:765–770. https://doi.org/10.1002/mds.24963
Acknowledgements
The authors would like to acknowledge Dr. Masatoshi Hanada for his assistance with data collection and Dr. Hasan Ayaz for his technical support with fNIRS data.
Funding
This work was funded by the Research and Innovation Ontario, Grant number 501683, Canada Foundation for Innovation, Grant number 501682, Ontario Respiratory Care Society, Grant number 503659, the Department of Physical Therapy 208294 and Rehabilitation Sciences Institute 100771 at the University of Toronto. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: WDR and LVB; methodology: WDR, LVB and DR; software, SAH; validation: all authors; formal analysis: SAH. WDR, DR; investigation: SAH, LVB and KTK; resources: WDR; data curation: SAH, KTK and WDR; writing—original draft preparation: SAH; writing—review and editing: all authors; visualization: SAH and WDR; supervision: WDR; project administration: all authors; funding acquisition: WDR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval
The study protocol was approved by the Research Ethics Board of University of Toronto (protocol ID: 33,466).
Consent to participate
All participants provided written informed consent before participation.
Consent for publication
All participants consented to having their data published and/or used in presentations.
Additional information
Communicated by Lori Ann Vallis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassan, S.A., Bonetti, L.V., Kasawara, K.T. et al. Decreased automaticity contributes to dual task decrements in older compared to younger adults. Eur J Appl Physiol 122, 965–974 (2022). https://doi.org/10.1007/s00421-022-04891-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-04891-w