Abstract
The anti-hyperglycemic medication metformin has potential to be the first drug tested to slow aging in humans. While the Targeting Aging with Metformin (TAME) proposal and other small-scale clinical trials have the potential to support aging as a treatment indication, we propose that the goals of the TAME trial might not be entirely consistent with the Geroscience goal of extending healthspan. There is expanding epidemiological support for the health benefits of metformin in individuals already diagnosed with overt chronic disease. However, it remains to be understood if these protective effects extend to those free of chronic disease. Within this editorial, we seek to highlight critical gaps in knowledge that should be considered when testing metformin as a treatment to target aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a primary risk factor for nearly every chronic disease. Identifying strategies that target the shared biological mechanisms of aging could lead to interventions that postpone the onset of most debilitating age-related chronic diseases. The World Health Organization recently added “old age” to the 2018 International Classification of Disease (ICD), but the Food and Drug Administration (FDA) does not currently recognize aging as a treatment indication. The Targeting Aging with Metformin (TAME) proposal could be the first clinical trial to examine an intervention to slow aging rather than to treat a specific age-related chronic disease in humans (Barzilai et al. 2016). An additional overarching goal of this effort is to create a regulatory framework that recognizes aging as an indication for treatment. Clinical trials that aim to postpone the onset of age-related morbidities have potential to provide paradigm-shifting evidence to support aging as a future treatment indication. We are strong supporters of targeting aging as a condition. However, in this editorial, we introduce new perspectives about metformin as the first intervention for these goals.
An effective treatment that targets aging prevents chronic disease
Matt Kaeberlein recently summarized the “health” of the healthspan concept (Kaeberlein 2018). In this editorial, healthspan was defined as “the period of life spent in good health, free from the chronic diseases and disabilities of aging” (Kaeberlein 2018). By this definition, which we subscribe to, lifespan is divided into a period free of disease (healthspan) and a period marked by the accumulation of age-associated disease and disability (Kaeberlein 2018; Seals et al. 2016). Importantly, these two periods are distinct from each other with the period free of disease—the healthspan—preceding the onset of one or more age-related diseases. If the goal of a treatment is to extend healthspan, the treatment must start before any chronic diseases are present, thereby delaying the onset of the first age-related chronic disease. It is worth noting that the National Institute on Aging (NIA) Interventions Testing Program (ITP) uses treatments throughout a lifespan while investigating the potential of treatments to promote healthy aging (Nadon et al. 2008).
Although the term healthspan is associated with the TAME proposal (Barzilai et al. 2016; Justice et al. 2018), it does not appear that the goal of TAME is to extend healthspan. The TAME proposal seeks to determine if metformin can target aging by slowing the sequelae of existing age-related morbidity. The proposed trial will test if metformin can delay the time that individuals already burdened with a chronic disease develop new, additional age-related conditions. This approach is likely an effort to accomplish the trial within a realistic time-table (5–10 years), sufficient sample size (n = 3000), and commensurate budget ($50 million). However, in line with current Geroscience initiatives, and the concept of healthspan, we believe that it is also critical to evaluate the efficacy of metformin to extend healthspan in individuals who are currently free of chronic disease. A potential first step before investing in a large, expensive, multi-center clinical trial is to identify if metformin can improve hallmarks of healthy aging in individuals without overt disease, and to determine the characteristics of the individuals who do or do not reap the health benefits of metformin. Within the population of disease-free individuals, some individuals are at greater risk for developing chronic disease because of the range of metabolic health and prevalence of risk factors. Some individuals may, for example, be more amenable to the health benefits of metformin while others are not. Therefore, there is still a critical need for studies to understand how metformin may extend healthspan in subjects without disease, but with varying degrees of risk for age-related comorbidities.
Mechanisms of action are not well understood
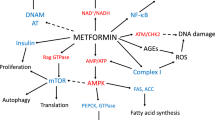
One ongoing difficulty with determining the efficacy of metformin is that its mechanism of action is still not completely understood. The primary target tissue of metformin is believed to be the liver, while evidence suggests metformin can also be detected and influence metabolic processes in the skeletal muscle, adipose tissue, intestine, brain, and cardiovascular tissues (Buse et al. 2016; Gormsen et al. 2016; Kane et al. 2010; Konopka et al. 2016, 2018; Kulkarni et al. 2018; Lee and Ko 2014; Long et al. 2017; Madiraju et al. 2014, 2018; Wang et al. 2012). The ability of metformin to influence multiple tissues is an attractive characteristic of a treatment to target aging in the whole organism.
Several studies in animals and cells have shown that supraphysiologic doses of metformin can inhibit complex I of the electron transport system (ETS) leading to decreased mitochondrial respiration and/or H2O2 emissions in the skeletal muscle, fibroblasts, liver, heart, and cancer cells (Bridges et al. 2014; Brunmair et al. 2004; Kane et al. 2010; Martin-Montalvo et al. 2013; Owen et al. 2000; Wessels et al. 2014; Wheaton et al. 2014). Complex I is the first complex within the ETS and operates by oxidizing NADH, transferring electrons via iron-sulfur clusters, and reducing ubiquinone. Emerging evidence suggests that metformin non-competitively and reversibly binds to a step that is coupled to ubiquinone reduction to inhibit complex I (Bridges et al. 2014). Studies in submitochondrial particles also suggest that metformin inhibits complex I before but not after the initiation of respiration (Bridges et al. 2014). However, it is important to highlight that not all studies demonstrate that metformin inhibits complex I (Kristensen et al. 2013; Larsen et al. 2011; Madiraju et al. 2014, 2018). These equivocal findings vary depending on the metformin dose, mitochondrial preparation and protocol, and subject characteristics. Incompletely defined mechanisms of action for metformin lead to uncertainties about its efficacy in a diverse population.
The positive effects of metformin are not universal
Although there is growing evidence to support the health benefits of metformin, to date, no studies have demonstrated health or lifespan-extending effects of metformin in humans. Metformin is an attractive potential aging treatment due to 60 years of documented safety, high adherence rates, low cost, and benefits in those with metabolic disease. However, the health benefits of metformin are not universal. Evidence supporting the use of metformin to increase lifespan and health outcomes originates from lower order model organisms (Cabreiro et al. 2013; De Haes et al. 2014; Onken and Driscoll 2010), whereas rodent models have been more mixed (Anisimov et al. 2008, 2011; Dhahbi et al. 2005; Martin-Montalvo et al. 2013; Smith et al. 2010; Strong et al. 2016). Although the majority of health outcomes improve in rodent models, lifespan either increases (Anisimov et al. 2008, 2011; Dhahbi et al. 2005; Martin-Montalvo et al. 2013) or remains unchanged with metformin treatment (Smith et al. 2010; Strong et al. 2016). One study not supporting lifespan extension is notable because it was part of the NIA Interventions Testing Program (ITP) using heterogeneous mouse models that are designed to mimic the genetic diversity of humans (Strong et al. 2016). In humans, even the effectiveness of metformin for T2D is variable. When genome-wide association studies (GWAS) have attempted to predict genetic determinants of metformin treatment efficacy for T2D, only 20–34% (depending on physiological outcome) of variability was accounted for (Zhou et al. 2014). Therefore, there are several important questions related to treatment efficacy of metformin for healthspan that remain to be definitively answered.
Is metformin effective in individuals without type 2 diabetes?
In human clinical trials, retrospective data and trials in populations with type 2 diabetes (T2D) show improved overall survival with metformin (Bannister et al. 2014), as well as decreased risk of all-cause mortality and cardiovascular disease (UKPDS Group 1998), cancer incidence (Gandini et al. 2014; Wu et al. 2014), and cognitive decline (Cheng et al. 2014; Ng et al. 2014). A recent meta-analysis of clinical trials in human subjects also demonstrated that that diabetics taking metformin have lower all-cause mortality and/or cancer incidence than other diabetics and the general population (Campbell et al. 2017). Although these trials in human subjects build a strong rationale that metformin has potential health and lifespan-extending effects, the completed studies were in subjects with T2D and/or other comorbidities, and none were in subjects absent of disease.
In non-diabetic individuals, the benefits of metformin are less clear. Treating non-diabetic patients with 4 months of metformin (1000 mg/day) after acute myocardial infarction did not improve left ventricular ejection fraction (Lexis et al. 2014) nor confer long-term benefits of reducing the onset of new diabetes or major adverse cardiac events (Hartman et al. 2017). Eighteen months of metformin treatment (1700 mg/day) in older (63 years), non-diabetic patients with heart disease improved several indices of glycemia including HbA1c, fasting glucose, fasting insulin, and HOMA-IR but did not influence carotid intima-media thickness (Preiss et al. 2014). The landmark Diabetes Prevention Program examined the progression to T2D in 3234 subjects at risk for diabetes (Knowler et al. 2002). Over the 3 years of study, the metformin treatment group (1700 mg/day) demonstrated a 31% lower progression to T2D and the lifestyle intervention group demonstrated a 58% lower progression to T2D compared to the placebo control (Knowler et al. 2002). However, the effect of metformin was minimal in those with a lower BMI (< 30 kg/m2) and lower fasting glucose (< 110 mg/dL), a pattern that was not observed in the lifestyle intervention. These data suggest that within a group of individuals at risk for T2D, the healthier subjects did not reap the same benefits from metformin as their relatively less healthy counterparts. In further support, a small study of 20 insulin-resistant individuals found that metformin (1700 mg/day) improved indices of insulin sensitivity in subjects that had T2D or a family history of T2D, but actually worsened insulin sensitivity in obese subjects without T2D or family history of T2D (Iannello et al. 2004). Therefore, there remains a lack of support for metformin extending healthspan in human subjects, which requires further resolution of how individuals with low, moderate, and high risk may benefit from metformin treatment.
Are the health benefits of metformin secondary to glucose and/or insulin lowering effects?
It is currently unknown if the benefits of metformin on healthspan are primarily due to positive effects on glucose control. Within the UK Prospective Diabetes Study (UKPDS), metformin, sulfonylurea, and insulin had similar benefits on median fasting plasma glucose and HbA1c over the 10-year follow-up of individuals with T2D (UKPDS Group 1998). Despite similar glucose control, the metformin treatment group (median dose of 2550 mg/day) had lower risk of developing micro- or macrovascular complications, diabetes-related death, and all-cause mortality compared to insulin and sulfonylurea treatment (UKPDS Group 1998). Similarly, a recent meta-analysis of clinical trials in human subjects also demonstrated that metformin had decreased cardiovascular disease compared to diabetics on other glucose-lowering therapies (Bannister et al. 2014). These data would suggest that the improved health outcomes in patients prescribed metformin cannot be completely explained by glucose-lowering effects. However, these findings do not rule out the impact of increased exogenous or endogenous insulin on cardiovascular complications or death in patients within the comparator insulin or sulfonylurea treatment groups. Although some studies suggest that the glucose-lowering effects may not completely explain the full protective benefits of metformin compared to other glucose lowering medications, it remains to be determined if the cardiovascular and mortality benefits in T2D or potentially other populations are a direct result of metformin or secondary to lowered glucose and/or insulin.
Is metformin effective in older adults?
Antagonistic pleiotropy is a well-known concept that something may have opposing effects dependent on stage of life (Williams 1957). Therefore, there may be a need to start healthspan-extending treatments later in life, but prior to the onset of chronic disease. In regard to metformin, a study examined metformin treatment starting at 3, 9, and 15 months of age in female outbred SHR mice (Anisimov et al. 2011). Metformin extended lifespan when started at 3 and 9 months in mice with and without tumors. However, when started later in life, metformin did not influence lifespan in mice with tumors and decreased lifespan in mice without tumors (Anisimov et al. 2011). In humans, similar glycemic control can be achieved by intensive treatment in young and old individuals with T2D (ADVANCE Collaborative Group et al. 2008; Miller et al. 2014a), but these studies included a cocktail of glucose-lowering agents and not specifically metformin. No studies have directly tested if metformin can maintain its primary indication of lowering glucose or other purported health-related benefits in older versus middle aged or younger adults. In ~ 3000 participants enrolled in the Diabetes Prevention Study, 20% of the subjects were older (≥ 60 years). Although the study was not designed or powered a priori to detect significant differences between subgroups such as age, secondary analysis suggests a trend for differences between age groups in response to metformin (Crandall and Barzilai 2003; Diabetes Prevention Program Research Group et al. 2006; Knowler et al. 2002). Metformin delayed the development of T2D in the youngest cohort by 44% (21 to 60 95% CI) but did not affect the incidence of T2D in adults ≥ 60 years old (11%; − 33 to 41 95% CI) (Knowler et al. 2002). Additionally, based on the confidence intervals, there appears to be nearly twice the variability in older compared to younger groups, with some older participants ending up worse with metformin treatment than the placebo group. These data suggest that age may influence the effectiveness and the variability in response to metformin. In support of these findings, older adults (62 years) consuming metformin during aerobic exercise training had nearly twice the variability in the change in insulin sensitivity compared to the placebo group with nearly half of the participants ending up with worse insulin sensitivity after treatment (Konopka et al. 2018). If metformin is to be recommended to aging adults as a therapy to extend healthspan, studies are needed to directly test the influence of age on the effectiveness of metformin therapy.
Is metformin effective in combination with other geroprotective therapies?
As a practical issue, it is important to understand if metformin is effective when combined with other established approaches to improve health. Caloric restriction without malnutrition and rapamycin are two of the most well studied interventions to extend lifespan in multiple animal models. When metformin is combined with caloric restriction in a diabetic rat model, there appears to be a greater effect on lowering postprandial glucose and proteins involved with hepatic lipogenesis than either treatment alone (Linden et al. 2015). Although metformin alone did not extend lifespan in the ITP trial, the addition of metformin to rapamycin extended median lifespan by 23% in male and female heterogeneous mice. Compared to historical trials of rapamycin alone (Miller et al. 2011, 2014b), the addition of metformin to rapamycin extends lifespan to a similar extent in females and perhaps a greater extent in males (Strong et al. 2016). The potential for a greater lifespan extension in males when combining metformin and rapamycin does not appear to be related to the ability of metformin to alleviate glucose intolerance caused by rapamycin at a young age (Weiss et al. 2018).
Although informative, rapamycin and caloric restriction are not yet considered treatment strategies for humans. On the other hand, mounting evidence indicates that long-term exercise training is a bona fide treatment to extend human healthspan (Blair et al. 1989; Cartee et al. 2016; Physical Activity Committee 2018; Zampieri et al. 2015; Williams 2001). Moderate-to-vigorous intensity physical activity is inversely related to premature mortality, cardiometabolic disease, immobility, and several cancers (Physical Activity Committee 2018). Exercise training induces multiple adaptations, including increased cardiorespiratory fitness (CRF), insulin sensitivity, skeletal muscle size, and function. In apparently healthy individuals, each 3.5 ml kg−1 min−1 increase in CRF was associated with an 11%, 16%, and 14% reduction in all-cause, cardiovascular disease, and cancer mortality (Imboden et al. 2018). Insulin resistance is associated with a myriad of age-related chronic conditions, including T2D, CVD, and frailty (Barzilay et al. 2007; Bonora et al. 2002; Facchini et al. 2001; Petersen et al. 2003). Age-related loss of muscle mass is also associated with the decline of CRF, insulin action, dependence, and mobility (Goodpaster et al. 2001, 2006; Reed et al. 1991; Reid and Fielding 2012; Reid et al. 2008). How then does metformin interact with exercise training to influence CRF, insulin sensitivity, and skeletal muscle mass?
We recently completed a randomized double-blind aerobic exercise training study in individuals that had one risk factor for T2D but were otherwise healthy. Half of the individuals (n = 27) received 1500–2000 mg of metformin with the exercise training, while the other half (n = 26) received a placebo (Konopka et al. 2018). When metformin was added to exercise training, the exercise-induced improvement in skeletal muscle mitochondrial respiration, CRF, and whole-body insulin sensitivity were attenuated (Konopka et al. 2018). In addition, some individuals actually had decrements in whole-body insulin sensitivity. Our results are similar to other studies that show metformin attenuated the exercise-induced increase in CRF and prevented the increase in insulin sensitivity in non-diabetic individuals (Malin et al. 2012; Sharoff et al. 2010). These effects do not seem to be unique to aerobic exercise training. Preliminary indications from a double-blind placebo-control clinical trial in the elderly (Long et al. 2017) show that metformin may also blunt the hypertrophic response to resistance exercise training measured by DXA, and muscle density measured by computed tomography (personal communication).
Due to the close relationship of CRF, insulin action, and skeletal muscle mass to mortality, morbidity, and quality of life, the antagonistic effect of metformin on CRF, insulin sensitivity, and skeletal muscle mass in non-diabetic young (Braun et al. 2008; Sharoff et al. 2010), middle-aged (Malin et al. 2012), and older (Konopka et al. 2018; Peterson et al. 2018) adults raises important questions about the efficacy of metformin to extend healthspan. Indeed, several important points need to be further examined to understand: are the inhibitory effects of clinical doses of metformin on some important physiological adaptations only apparent when challenged with exercise, does the range of metabolic health—even within individuals free of chronic disease—influence the positive or negative impact of metformin treatment, and what are the long-term implications of metformin treatment on healthspan when started prior to the onset of chronic disease.
Conclusion
For this perspective, we have provided a different outlook about using metformin as the first treatment to target aging. We have taken the viewpoint that a clinical trial demonstrating an increase in healthspan is critically important for Geroscience. We acknowledge that the TAME proposal may not be targeting healthspan but rather the prevention of further accumulation of chronic disease. While this goal may indicate aging as an underlying mechanism for chronic disease, this approach may not be totally consistent with current Geroscience goals. We believe that an intervention designed to extend healthspan should, by definition, begin before the accumulation of age-related comorbidities. In addition, the intervention should be effective in delaying the onset of disease even when started later in life. Finally, the intervention should not be detrimental in those that are disease free, or when used in combination with other healthspan-extending treatments such as exercise. We are at an exciting and pivotal time where the TAME proposal and other small-scale clinical trials could provide the necessary evidence to demonstrate that the biology of aging in humans can be modulated to extend healthy lifespan.
References
ADVANCE Collaborative Group, Patel A, Macmahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572
Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV (2008) Metformin slows down aging and extends life span of female SHR mice. Cell Cycle Georget Tex 7:2769–2773
Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, Poroshina TE (2011) If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging 3:148–157
Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ (2014) Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab 16:1165–1173
Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA (2016) Metformin as a tool to target aging. Cell Metab 23:1060–1065
Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP (2007) Insulin resistance and inflammation as precursors of frailty: the cardiovascular health study. Arch Intern Med 167:635–641
Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW (1989) Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262:2395–2401
Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Cacciatori V, Santi L, Targher G, Bonadonna R, Muggeo M (2002) HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care 25:1135–1141
Braun B, Eze P, Stephens BR, Hagobian TA, Sharoff CG, Chipkin SR, Goldstein B (2008) Impact of metformin on peak aerobic capacity. Appl Physiol Nutr Metab 33:61–67
Bridges HR, Jones AJY, Pollak MN, Hirst J (2014) Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J 462:475–487
Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhäusl W, Fürnsinn C (2004) Thiazolidinediones, like metformin, inhibit respiratory complex I a common mechanism contributing to their antidiabetic actions? Diabetes 53:1052–1059
Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M (2016) The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies. Diabetes Care 39:198–205
Cabreiro F, Au C, Leung K-Y, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene NDE, Gems D (2013) Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153:228–239
Campbell JM, Bellman SM, Stephenson MD, Lisy K (2017) Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev 40:31–44
Cartee GD, Hepple RT, Bamman MM, Zierath JR (2016) Exercise promotes healthy aging of skeletal muscle. Cell Metab 23:1034–1047
Cheng C, Lin C-H, Tsai Y-W, Tsai C-J, Chou P-H, Lan T-H (2014) Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol A Biol Sci Med Sci 69:1299–1305
Crandall J, Barzilai N (2003) Treatment of diabetes mellitus in older people: oral therapy options. J Am Geriatr Soc 51:272–274
De Haes W, Frooninckx L, Van Assche R, Smolders A, Depuydt G, Billen J, Braeckman BP, Schoofs L, Temmerman L (2014) Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci U S A 111:E2501–E2509
Dhahbi JM, Mote PL, Fahy GM, Spindler SR (2005) Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics 23:343–350
Diabetes Prevention Program Research Group, Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett-Connor E, Fowler S, Dagogo-Jack S, Andres R (2006) The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci 61:1075–1081
Facchini FS, Hua N, Abbasi F, Reaven GM (2001) Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 86:3574–3578
Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E (2014) Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res Phila 7:867–885
Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB (2001) Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol 90:2157–2165
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61:1059–1064
Gormsen LC, Sundelin EI, Jensen JB, Vendelbo MH, Jakobsen S, Munk OL, Christensen MMH, Brøsen K, Frøkiær J, Jessen N (2016) In vivo imaging of human 11C-metformin in peripheral organs: dosimetry, biodistribution, and kinetic analyses. J Nucl Med 57:1920–1926
Hartman MHT, Prins JKB, Schurer RAJ, Lipsic E, Lexis CPH, van der Horst-Schrivers ANA, van Veldhuisen DJ, van der Horst ICC, van der Harst P (2017) Two-year follow-up of 4 months metformin treatment vs. placebo in ST-elevation myocardial infarction: data from the GIPS-III RCT. Clin Res Cardiol 106:939–946
Iannello S, Camuto M, Cavaleri A, Milazzo P, Pisano MG, Bellomia D, Belfiore F (2004) Effects of short-term metformin treatment on insulin sensitivity of blood glucose and free fatty acids. Diabetes Obes Metab 6:8–15
Imboden MT, Harber MP, Whaley MH, Finch WH, Bishop DL, Kaminsky LA (2018) Cardiorespiratory fitness and mortality in healthy men and women. J Am Coll Cardiol 72:2283–2292
Justice JN, Ferrucci L, Newman AB, Aroda VR, Bahnson JL, Divers J, Espeland MA, Marcovina S, Pollak MN, Kritchevsky SB, Barzilai N, Kuchel GA (2018) A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. GeroScience 40:419–436
Kaeberlein M (2018) How healthy is the healthspan concept? GeroScience 40:361–364
Kane DA, Anderson EJ, Price JW, Woodlief TL, Lin C-T, Bikman BT, Cortright RN, Neufer PD (2010) Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic Biol Med 49:1082–1087
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403
Konopka AR, Esponda RR, Robinson MM, Johnson ML, Carter RE, Schiavon M, Cobelli C, Wondisford FE, Lanza IR, Nair KS (2016) Hyperglucagonemia mitigates the effect of metformin on glucose production in prediabetes. Cell Rep 15:1394–1400
Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, Musci RV, Safairad OD, Linden MA, Biela LM, Bailey SM, Hamilton KL, Miller BF (2018) Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell e12880
Kristensen JM, Larsen S, Helge JW, Dela F, Wojtaszewski JFP (2013) Two weeks of metformin treatment enhances mitochondrial respiration in skeletal muscle of AMPK kinase dead but not wild type mice. PLoS One 8:e53533
Kulkarni AS, Brutsaert EF, Anghel V, Zhang K, Bloomgarden N, Pollak M, Mar JC, Hawkins M, Crandall JP & Barzilai N (2018) Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell 17
Larsen S, Rabøl R, Hansen CN, Madsbad S, Helge JW, Dela F (2011) Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia 55:443–449
Lee H, Ko G (2014) Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol 80:5935–5943
Lexis CPH, van der Horst ICC, Lipsic E, Wieringa WG, de Boer RA, van den Heuvel AFM, van der Werf HW, Schurer RAJ, Pundziute G, Tan ES, Nieuwland W, Willemsen HM, Dorhout B, Molmans BHW, van der Horst-Schrivers ANA, Wolffenbuttel BHR, ter Horst GJ, van Rossum AC, Tijssen JGP, Hillege HL, de Smet BJGL, van der Harst P, van Veldhuisen DJ, Investigators GIPS-III (2014) Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: the GIPS-III randomized clinical trial. JAMA 311:1526–1535
Linden MA, Lopez KT, Fletcher JA, Morris EM, Meers GM, Siddique S, Laughlin MH, Sowers JR, Thyfault JP, Ibdah JA, Rector RS (2015) Combining metformin therapy with caloric restriction for the management of type 2 diabetes and nonalcoholic fatty liver disease in obese rats. Appl Physiol Nutr Metab Physiol Appl Nutr Metab 40:1038–1047
Long DE, Peck BD, Martz JL, Tuggle SC, Bush HM, McGwin G, Kern PA, Bamman MM & Peterson CA (2017) Metformin to Augment Strength Training Effective Response in Seniors (MASTERS): study protocol for a randomized controlled trial. Trials 18. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5405504/ [Accessed January 25, 2019]
Madiraju AK, Erion DM, Rahimi Y, Zhang X-M, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez J-P, Lee H-Y, Cline GW, Samuel VT, Kibbey RG, Shulman GI (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510:542–546
Madiraju AK, Qiu Y, Perry RJ, Rahimi Y, Zhang X-M, Zhang D, Camporez J-PG, Cline GW, Butrico GM, Kemp BE, Casals G, Steinberg GR, Vatner DF, Petersen KF, Shulman GI (2018) Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med Available at: http://www.nature.com/articles/s41591-018-0125-4 [Accessed August 22, 2018] 24:1384–1394
Malin SK, Gerber R, Chipkin SR, Braun B (2012) Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care 35:131–136
Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin M-J, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R (2013) Metformin improves healthspan and lifespan in mice. Nat Commun 4:2192
Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R (2011) Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66:191–201
Miller ME, Williamson JD, Gerstein HC, Byington RP, Cushman WC, Ginsberg HN, Ambrosius WT, Lovato L, Applegate WB (2014a) Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial. Diabetes Care 37:634–643
Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R (2014b) Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13:468–477
Nadon NL, Strong R, Miller RA, Nelson J, Javors M, Sharp ZD, Peralba JM, Harrison DE (2008) Design of aging intervention studies: the NIA interventions testing program. Age Dordr Neth 30:187–199
Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B (2014) Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41:61–68
Onken B, Driscoll M (2010) Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS One 5:e8758
Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348:607–614
Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142
Peterson C, Grace Walton R, Craig Tuggle S, Kulkarni A (2018) Metformin to augment strength training effective response in seniors: the masters trial. Innov Aging 2:544–545
Physical Activity Committee (2018) Scientific Report - 2018 Physical Activity Guidelines - health.gov. Available at: https://health.gov/paguidelines/second-edition/report/
Preiss D, Lloyd SM, Ford I, McMurray JJ, Holman RR, Welsh P, Fisher M, Packard CJ, Sattar N (2014) Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol 2:116–124
Reed RL, Pearlmutter L, Yochum K, Meredith KE, Mooradian AD (1991) The relationship between muscle mass and muscle strength in the elderly. J Am Geriatr Soc 39:555–561
Reid KF, Fielding RA (2012) Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40:4–12
Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA (2008) Lower extremity muscle mass predicts functional performance in mobility-limited elders. J Nutr Health Aging 12:493–498
Seals DR, Justice JN, LaRocca TJ (2016) Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol 594:2001–2024
Sharoff CG, Hagobian TA, Malin SK, Chipkin SR, Yu H, Hirshman MF, Goodyear LJ, Braun B (2010) Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol-Endocrinol Metab 298:E815–E823
Smith DL, Elam CF, Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB (2010) Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci 65:468–474
Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Marinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE (2016) Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15:872–884
UKPDS Group (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet Lond Engl 352:854–865
Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD (2012) Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 11:23–35
Weiss R, Fernandez E, Liu Y, Strong R, Salmon AB (2018) Metformin reduces glucose intolerance caused by rapamycin treatment in genetically heterogeneous female mice. Aging 10:386–401
Wessels B, Ciapaite J, van den Broek NMA, Nicolay K & Prompers JJ (2014) Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a dose-dependent manner. PLoS One 9. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4065055/ [Accessed April 15, 2015]
Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, Chandel NS (2014) Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3:e02242
Williams GC (1957) Pleiotropy, natural selection, and the evolution of senescence. Evolution 11(4):398–411. http://doi.org/jstor.org/stable/2406060
Williams PT (2001) Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc 33:754–761
Wu JW, Boudreau DM, Park Y, Simonds NI, Freedman AN (2014) Commonly used diabetes and cardiovascular medications and cancer recurrence and cancer-specific mortality: a review of the literature. Expert Opin Drug Saf 13:1071–1099
Zampieri S, Pietrangelo L, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, Pond A, Grim-Stieger M, Cvecka J, Sedliak M, Tirpáková V, Mayr W, Sarabon N, Rossini K, Barberi L, De Rossi M, Romanello V, Boncompagni S, Musarò A, Sandri M, Protasi F, Carraro U, Kern H (2015) Lifelong physical exercise delays age-associated skeletal muscle decline. J Gerontol A Biol Sci Med Sci 70:163–173
Zhou K, Donnelly L, Yang J, Li M, Deshmukh H, Van Zuydam N, Ahlqvist E, Wellcome Trust Case Control Consortium 2, Spencer CC, Groop L, Morris AD, Colhoun HM, Sham PC, McCarthy MI, Palmer CNA, Pearson ER (2014) Heritability of variation in glycaemic response to metformin: a genome-wide complex trait analysis. Lancet Diabetes Endocrinol 2:481–487
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Konopka, A.R., Miller, B.F. Taming expectations of metformin as a treatment to extend healthspan. GeroScience 41, 101–108 (2019). https://doi.org/10.1007/s11357-019-00057-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-019-00057-3