Abstract
Recent advances indicate that biological aging is a potentially modifiable driver of late-life function and chronic disease and have led to the development of geroscience-guided therapeutic trials such as TAME (Targeting Aging with MEtformin). TAME is a proposed randomized clinical trial using metformin to affect molecular aging pathways to slow the incidence of age-related multi-morbidity and functional decline. In trials focusing on clinical end-points (e.g., disease diagnosis or death), biomarkers help show that the intervention is affecting the underlying aging biology before sufficient clinical events have accumulated to test the study hypothesis. Since there is no standard set of biomarkers of aging for clinical trials, an expert panel was convened and comprehensive literature reviews conducted to identify 258 initial candidate biomarkers of aging and age-related disease. Next selection criteria were derived and applied to refine this set emphasizing: (1) measurement reliability and feasibility; (2) relevance to aging; (3) robust and consistent ability to predict all-cause mortality, clinical and functional outcomes; and (4) responsiveness to intervention. Application of these selection criteria to the current literature resulted in a short list of blood-based biomarkers proposed for TAME: IL-6, TNFα-receptor I or II, CRP, GDF15, insulin, IGF1, cystatin C, NT-proBNP, and hemoglobin A1c. The present report provides a conceptual framework for the selection of blood-based biomarkers for use in geroscience-guided clinical trials. This work also revealed the scarcity of well-vetted biomarkers for human studies that reflect underlying biologic aging hallmarks, and the need to leverage proposed trials for future biomarker discovery and validation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The geroscience hypothesis holds that a specific set of shared biological mechanisms of aging increases the susceptibility of aged individuals to several chronic diseases and loss of function and that therapies developed to target such shared “drivers” have the potential to delay the onset and progression of multiple chronic diseases and functional decline. The inter-related cellular biologic processes that drive the biology of aging are known as “pillars” or “hallmarks” of aging, and accumulating evidence from mammalian animal models supports the premise that geroscience-guided interventions targeting these processes can extend healthspan and lifespan (Barzilai et al. 2016; Burch et al. 2014; Espeland et al. 2017; Kennedy et al. 2014; Longo et al. 2015; Sierra 2016a). A new generation of clinical trials is being designed to test the geroscience hypothesis in humans (Justice et al. 2016; Newman et al. 2016b; Sierra 2016b). One example is the proposed multicenter clinical trial, Targeting Aging with MEtformin (TAME), which will evaluate whether metformin, a commonly used diabetes drug that also targets the biology of aging, can (1) prevent or delay the incidence of multiple age-related chronic diseases, (2) help maintain function, and (3) influence biological markers of aging in older persons (Barzilai et al. 2016). To accomplish this latter aim, a strategy to select the most appropriate biomarkers of aging to be included in geroscience-guided clinical trials is needed.
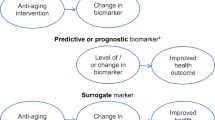
Hypothetically, a biomarker of aging should reflect the underlying biology, and a change in biomarker levels should have parallel changes that occur in the susceptibility to disease and loss of function. Thus, interventions targeting aging should result in changes in biomarkers that will eventually delay the incidence, accumulation, clinical evolution, and functional consequences of chronic age-related diseases. One of the critical roles played by biomarkers could be that of surrogate endpoints reflective of risk and progression of several major diseases. In support of this role, the US Food and Drug Administration (FDA) and the Institute of Medicine highlighted the importance of biomarkers as surrogate measures in drug development and trials involving chronic diseases such as cancer and heart disease; however, few existing biomarkers have sufficient clinical evidence of association with the rate of development of aging phenotypes and multi-morbidity ((IOM) 2010; Biomarkers Definitions Working 2001). The FDA currently does not consider aging an indication for drug development or labeling, which may also impede the emergence of knowledge supporting discovery and validation of geroscience-relevant biomarkers. As a result, there are at this time no approved or commonly accepted biomarkers of aging for clinical trials, nor is there a consensus set of validated biomarkers of the biologic pillars or hallmarks of aging that would be applicable to clinical research. This poses a major translational gap that must be bridged in order to facilitate scientific progress.
The purpose of this report is to outline a conceptual framework and evidence-based approach to the prioritization and selection of a panel of blood biomarkers for use in randomized controlled clinical trials of a geroscience therapy. A multi-disciplinary Biomarkers Workgroup convened for a planning workshop and met weekly by phone over an 8-month period. The Biomarkers Workgroup defined biomarker criteria, prioritized selection parameters, and provided guidance for resource development and inclusion of discovery-based platforms. The development of the TAME trial served as an opportunity to apply the selection criteria to putative blood-based biomarkers identified through an exhaustive literature review. The result is an evidence-based short list of proposed biomarkers for the TAME trial, and a framework that could be applied to next-generation clinical trials targeting aging.
Conceptual framework
Biomarker definitions
According to consensus definitions by Baker and Sprott (Baker III and Sprott 1988; Sprott 1988, 2010), and the American Federation for Aging Research (AFAR), biomarkers of aging are measures of a biological parameter that, either alone or as a multivariate composite, monitor a biological process underlying aging rather than effects of a specific disease; predict the rate of aging and mortality better than chronological age; can be safely tested across repeat measures in the same organism; and work in humans and in laboratory animals such as mice. The Biomarkers Workgroup adapted these definitions to develop selection criteria tailored to the context of geroscience-guided randomized clinical trials:
-
1.
Measurement reliability and feasibility. The biomarker should be feasible to measure in a clinical trial without incurring undue risk to human subjects, and meet trial specific reliability requirements (see “Trial Context” below), with standardized measurement.
-
2.
Represent biologic aging processes. The biomarker should have face validity such that it represents a process or processes relevant to biologic aging hallmarks, and changes in a measurable and consistent manner with chronological age.
-
3.
Robust and consistent association with risk of death, and clinical/functional trial endpoints. Association with risk. The biomarker should be consistently associated with increased risk of clinical and functional endpoints including all-cause mortality even when controlling for chronological age. Ideally, the biomarker would be involved in the causal pathway, such that direct manipulation of the biomarker changes the associated risk, but at minimum, the biomarker level would move in a direction that predicts the clinical or functional outcome. Robust. The changes in biomarker level with age and associations with risk should be robust across species, datasets, or populations.
-
4.
Responsive to intervention. A biomarker of aging for geroscience-guided trials should be responsive to interventions that affect the biology of aging ideally over a relatively short period of time. A quick response to intervention would allow for shorter trials for fully vetted biomarkers.
The criteria above were developed for research studies in humans. Foundational efforts to identify and categorize the cellular and molecular “hallmarks” of aging introduced a host of potential biomarkers for mammalian aging based on evidence in mouse models and some simpler organisms such as Caenorhabditis elegans and Drosophila melanogaster. Clinical translation of these biomarkers can be problematic, with barriers such as access to tissues, environmental or genetic control, and use of resource and assays that are not feasible in clinical research. However, reports often mix evidence from animal models and humans indiscriminately, and aside from a few thoughtful reviews and studies, relatively, little work has emerged on measures of the biologic “hallmarks” of aging specifically for human research (Burkle et al. 2015; Khan et al. 2017; Rochon et al. 2011). Accordingly, the present framework presented focuses solely on markers that can be measured in humans. Though focused on human research, analogous frameworks to reverse translate for preclinical testing in mammalian species such as rodent, dog, and nonhuman primate can be envisioned.
Trial context
This framework should be tailored to the specific clinical trial design or investigational drug being used. It is broad enough to include biochemical assays, clinical measures, imaging, and physiological tests, such as gait speed, grip strength, cognitive assessments, spirometry, and blood pressure. Specific biomarkers selected or types of biomarkers considered depend primarily on the context of the trial. Importantly, the determination of the biomarker must be feasible for the population, size, duration, budget, and logistical constraints of the clinical trial. Trial context dictates:
-
Feasibility within trial design:
-
Acceptable additional risk to participants for biomarker determination
-
Inclusion of proposed measures within clinical visits and resource availability
-
-
Reliability of assays:
-
Accuracy of assay and agreement across technical replicates
-
Assay short-term test-retest reliability (correlation ≥ 0.7)
-
Reliability and reproducibility of measurement across trial sites or laboratories
-
-
Sensitivity to detect change:
-
Assay detection limits in specific study population
-
Within-subject variation over study duration (e.g., stability over months, years)
-
Estimated intervention effects on biomarker measure.
-
Changes in biomarker levels should be consistently related to changes in risk of mortality, disease, or functional outcomes and should be reasonably robust to confounders and common medical maneuvers in those recruited. This requires careful consideration of the specific population, including age and sex specific biomarker reference ranges, effects of comorbid conditions, and concurrent use of common medications. An example is low-density lipoprotein cholesterol (LDL-C), which is a prominent biomarker of atherosclerotic heart disease, and in middle-aged adults, elevated levels of LDL-C are associated with greater risk of cardiovascular related events and mortality. However, at advanced ages, the converse is true, and low levels of LDL-C may be related to higher risk. Moreover, commonly prescribed medications to control lipid levels could result in a change in LDL-C and related biomarkers that are independent of the investigational drug and not reflective of a change in underlying aging biology (High and Kritchevsky 2015).
Biomarker categories
The Biomarkers Workgroup identified three primary biomarker categories consistent with models proposed by NIH Biomarker Definitions Working Groups and FDA guidance, markers of the (A) investigational drug, (B) underlying biology, and (C) clinical disease outcomes. Biomarkers of the investigational drug can contribute knowledge about clinical pharmacology and inter-individual variation in responses to treatments, and can include circulating measures of levels of the drug or its relevant metabolites, or known drug-specific effects that could mediate effects on trial outcomes. Markers of underlying biology provide proof of concept and mechanistic insight, and may suggest future therapeutic candidates. Biomarkers of the clinical disease (s) being targeted or population studied may provide early indicators of drug effects on clinical disease and could serve as surrogate trial endpoints. The final selection of biomarkers addressing effects of the investigational drug and clinical outcomes should be matched to the unique features of each individual trial, yet overarching features of biomarkers linking the underlying biology to clinical outcomes should have a degree of consistency across all proposed geroscience-guided clinical trials. The present report is focused on those features of blood-based biomarkers of biologic aging or age-related diseases that may be generalizable to other geroscience-guided trials.
Case study: Targeting Aging with MEtformin (TAME)
TAME is a proposed 6-year double blind placebo-controlled randomized trial of metformin involving 3000 nondiabetic men and women aged 65–80 years to be recruited across 14 US-based sites. Metformin was selected based on its effects on biological hallmarks of aging in cells and animal models: metformin inhibits the mitochondrial complex I in the electron transport chain and reduces endogenous production of reactive oxygen species (ROS) (Batandier et al. 2006; Bridges et al. 2014; Kickstein et al. 2010); activates of AMP-activated kinase (AMPK) (Cho et al. 2015; Duca et al. 2015; Zheng et al. 2012), decreases insulin/insulin-like growth factor-1 (IGF-1) signaling (Barzilai et al. 2016; Nair et al. 2014) (Foretz et al. 2010, 2014), reduces DNA damage (Liu et al. 2011); inflammation and the senescence associated secretory phenotype (Lu et al. 2015; Moiseeva et al. 2013; Saisho 2015). When administered in vivo in rodents, the lifespan effects of metformin alone are either not observed (Smith Jr. et al. 2010; Strong et al. 2016), or relatively modest (~ 4–6% extension of median lifespan) (Martin-Montalvo et al. 2013). However, the effects on health are substantial, with improvements on tests of physical and cognitive function, cataracts, oral glucose, and insulin tolerance improved by up to 30% (Allard et al. 2015; Martin-Montalvo et al. 2013). These findings are coupled by observation that in persons with diabetes, the use of metformin is associated with lower rates of cancer (Landman et al. 2010; Lee et al. 2011; Libby et al. 2009), cardiovascular risk factors and events (Abualsuod et al. 2015; Kooy et al. 2009), dementia (Luchsinger et al. 2016; Ng et al. 2014), and all-cause mortality (Bannister et al. 2014; Johnson et al. 2005; Roussel et al. 2010; Schramm et al. 2011). TAME was conceptualized as a prototype geroscience-guided trial using metformin to target clinical outcomes of aging. Main trial outcomes are the incidence of (1) death or any new age-related chronic disease (myocardial infarction, stroke, hospitalized heart failure, cancer, dementia or mild cognitive impairment, multimorbidity) and (2) major age-related functional outcomes (major decline in mobility or cognitive function, or onset of activities of daily living limitation). Biomarkers of aging comprise an exploratory trial outcome, and we hypothesize that metformin’s beneficial effects, if observed, will be associated with markers of biologic aging.
As there is currently no consensus on what biomarkers of aging should be preferentially addressed in geroscience-guided trials, a Biomarkers Workgroup was convened for a planning workshop (NIA; Baltimore, MD) and met weekly by phone for 8 months (Oct 2017–May 2018). The workgroup consisted of experts in the basic biology of aging, metformin pharmacology, gerontology, biostatistics, epidemiology, endocrinology, and geriatric medicine (see author list for participating workgroup members). The workgroup led defined biomarker parameters and conducted an exhaustive search to pre-specify biomarkers and rigorously apply the trial biomarker criteria. An overview of the process of biomarker identification, prioritization and selection, and proposed list of biomarkers is shown in Fig. 1.
Candidate biomarker identification
A total of 258 potential biomarkers of aging were identified by input from individual members of the Biomarkers Workgroup. Additionally, literature was reviewed to identify a set of biomarkers of biological aging: published multi-assay composites (Belsky et al. 2015; Belsky et al. 2017a; Fried et al. 2001; Howlett et al. 2014; Li et al. 2015; Mitnitski et al. 2013, 2015; Mitnitski and Rockwood 2015; Sanders et al. 2014; Sebastiani et al. 2017), consensus-derived panels (Burkle et al. 2015; Engelfriet et al. 2013; Jylhava et al. 2017; Khan et al. 2017; Lara et al. 2015; Wagner et al. 2016; Xia et al. 2017), and large aging studies (Martin-Ruiz et al. 2011; Rochon et al. 2011) were consulted and 229 candidate biomarkers identified. An additional 29 recognized biomarkers of TAME’s clinical cardiovascular, cancer, and cognitive outcomes were identified (Supplement Material 1).
Exclusions and prioritization
Sixty-seven biomarkers of aging that were not blood-based (e.g., imaging, physiologic) were omitted from consideration as several functional measures were already considered for determination of secondary trial outcome. Thirty-nine markers were excluded based on participant or resource burden, low feasibility, or assay reliability concerns (Supplemental Material 2). For example, measures that require access to cells derived from standard blood draw (e.g., CD4/CD8 T cell ratio, T cell p16INK4a expression, mitochondrial respirometry) may be ideal biomarkers of aging, but have low feasibility for large trials due to significant resource burden, need of skilled laboratory personnel and specialized equipment that may not be readily available or easily standardized across clinical trial sites. Other biomarkers demonstrate uncertain assay reliability or validity. These include circulating growth/differentiating factor 11 (GDF11) assays using antisera with cross reactivity issues (Rodgers 2016; Rodgers and Eldridge 2015) or require a highly specific immunoplexed LC-MS/MS assay which is reliable but may not be feasible for large-scale trials such as TAME (Schafer et al. 2016). Additionally, several cytokines (e.g., interleukin-2, interleukin-1β, interferon-γ) demonstrate inconsistent detectability resulting from analyte degradation in long-term storage, or low assay sensitivity (McKay et al. 2017).
The remaining 86 candidate biomarkers were ranked based on frequency of appearance in the literature and weighted by strength of expert opinion and utility in monitoring disease outcomes (see Supplemental Material 1).
-
1.
Frequency of use: appearance in 17 consulted publications was tallied.
-
2.
Utility in diagnosing or monitoring disease: 48 biomarkers of clinical importance for clinical disease evaluation were noted from FDA guidance documents or disease association statements (e.g., American Heart Association). For the CVD endpoints MI, stroke, and CHF, 33 total biomarkers were identified (18 common to aging biomarkers list, 15 new) (Chow et al. 2017; Jickling and Sharp 2015; Thygesen et al. 2012). Two FDA-recognized markers for early AD or MCI were identified (Administration 2018), and 11 for cancer prognosis, staging, or disease monitoring.
-
3.
Expert opinion: markers that appeared in the literature were weighted based on strength of Biomarker Workgroup expert suggestion: suggested exclusion (−), unmentioned (), consideration (+), recommended (++), and strongly recommended (+++).
Biomarker selection
The top 20 ranked candidate biomarkers were evaluated according to the Biomarker Workgroup-identified criteria. The Biomarker Workgroup evaluated face validity of biomarker, and considerations related to feasibility and potential confound by common medical conditions or treatments. Literature reviews using Pubmed were conducted for each biomarker to evaluate association with risk, robustness, and responsiveness to relevant interventions (overview Table 1, and full listings in Supplemental Material) with filters for (1) age (≥ 45 years), (2) prospective studies, and (3) human or clinical research. Direction of associations and magnitude of effects across publications, datasets, and populations were tracked.
-
Association with risk of clinical disease or functional decline/disability onset: PubMed search strategies were used to evaluate association of each individual candidate biomarker with risk of clinical events, disease-related mortality, disability, or functional declines and all-cause mortality (Supplemental Material 3). In addition, separate searches for biomarker with each clinical disease (CVD, MCI/AD, cancer) and functional outcome (mobility, disability, frailty) were also conducted and evidence of associations noted. Studies in populations with acute or severe diseases were excluded. Given wide differences in outcomes, populations, and model adjustments, the estimated effects sizes were not pooled or systematically summarized.
-
Associations with risk of death: PubMed searches used: <selected biomarker> AND “mortality” OR “all-cause” OR “death” OR “lifespan.” Publication reference lists and the website MortalityPredictors.org were consulted to identify additional relevant publications. A detailed listing of studies is included in Supplemental Material 3. Estimated effect sizes of biomarker’s association with all-cause death were summarized as age-adjusted hazard ratios (HR), with range HRs, and number of studies age-adjusted models considered listed (Table 1).
-
Responsiveness to interventions: PubMed search and published data from the Diabetes Prevention Program (DPP) were consulted to determine whether the biomarker of interest was sensitive to change in less than 6 years when exposed to interventions of interest. Geroscience-identified interventions were searched (e.g., metformin, caloric restriction). If data were available, the percent change in the candidate biomarker with metformin treatment was evaluated compared to reference group or placebo control (Supplemental Material 4).
Based on this systematic process, 8 of the 258 pre-specified blood-based biomarkers remained as candidate markers to use as an exploratory outcome:
-
Inflammation: interleukin-6 (IL-6), tumor necrosis factor α receptor II (TNFRII), high sensitivity c-reactive protein (CRP)
-
Stress response and mitochondria: growth differentiating factor 15 (GDF15)
-
Nutrient signaling: fasting insulin, insulin-like growth factor 1 (IGF-1)
-
Kidney aging: cystatin C
-
Cardiovascular: N-terminal B-type natriuretic peptides (NT-proBNP)
-
Metabolic aging: hemoglobin A1c
Each biomarker and its role are briefly explained a graphical table (Fig. 2). A few specific comments on selections are provided here: TNFα and TNF receptors (I, II) were examined; however, TNFα receptors (e.g., TNFRII) were workgroup recommended to minimize analytic variability, given the fact that serum TNFα serum levels tend to be low and unstable with storage at − 80 °C (Barron et al. 2015; Cesari et al. 2003; Marti et al. 2014). Moreover, IL-6, CRP, and TNFα are commonly used and are independently associated with mortality risk (Bruunsgaard et al. 2003; Lio et al. 2003; Penninx et al. 2004; Reuben et al. 2002; Roubenoff et al. 2003; Stork et al. 2006), but the combination of IL-6 and TNF receptor levels has been shown to perform particularly well when combined as a pro-inflammatory cytokine score predicting the risk of mortality and mobility disability is elevated (Varadhan et al. 2014). While the biomarkers above generally meet the workgroup-derived criteria, IGF-1 does not: the relationship between IGF-1 and mortality/frailty is U-shaped with both high and low levels associated with all-cause mortality and adverse health outcomes (Andreassen et al. 2009; Burgers et al. 2011; Cappola et al. 2003; Doi et al. 2016; Friedrich et al. 2009; Hu et al. 2009; Kaplan et al. 2007; Laughlin et al. 2004; Leng et al. 2009; Maggio et al. 2007; Roubenoff et al. 2003; Saydah et al. 2007; van der Spoel et al. 2015), which could complicate the interpretation of change with intervention. However, ample evidence indicates its importance to biological aging, including use as a target for intervention to improve healthspan and lifespan in mice (Mao et al. 2018). Hemoglobin A1c (HbA1c, HGBA1c) was selected as a marker of metformin’s potential glycemic effects in TAME and therefore excluded from consideration as a biomarker of aging. In TAME, diabetes was excluded as an inclusion criterion or outcome measure, and even in a nondiabetic population, the effects of metformin on glucose metabolism may be difficult to separate from those on biological aging. Nevertheless, HbA1c is a prominent biomarker of aging and generally meets workgroup-identified criteria; therefore, in other studies, its use should be considered as a marker of metabolic aging (Dubowitz et al. 2014; Palta et al. 2017; Pani et al. 2008). Innovative “omics”-based approaches and epigenetic markers did not meet selection criteria, but the Biomarkers Workgroup noted unique advantages as biomarkers of aging and represent an area ripe for development for future trials. For example, the stress response and mitochondrial marker GDF15 did not appear in literature searchers for biomarkers of aging, but was instead identified by experts on the workgroup based on proteomic analyses, and ample evidence as a marker of metformin (Gerstein et al. 2017), association with risk of mortality (Wiklund et al. 2010), cardiovascular disease, and heart failure, and biology of aging as a senescence-associated secretory protein and marker of systemic energy homeostasis (Chung et al. 2017; Kim et al. 2018). This example underscores the importance of sustained vigilance for developments from various platforms for proteomics, metabolics, and other techniques and store biologic samples for future assays work in this area.
Graphical table. IL-6, CRP, TNFRII: Giovannini et al. 2011; Kennedy et al. 2014; Lopez-Otin et al. 2013; Michaud et al. 2013; Cameron et al. 2016; Fontana et al. 2006; Harvie et al. 2011; Lettieri-Barbato et al. 2016. GDF15: Bauskin et al. 2005; Brown et al. 2002, 2006; Jiang et al. 2016; Kempf et al. 2007; Lankeit et al. 2008; Welsh et al. 2003; Wiklund et al. 2010. IGF-1, Insulin: Bartke et al. 2001; Barzilai et al. 1998; Breese et al. 1991; Brown-Borg and Bartke 2012; Fontana et al. 2010; Kenyon 2001; Lee et al. 2001; Longo and Finch 2003; Masternak and Bartke 2012; Rincon et al. 2005; Fontana et al. 2006; Harvie et al. 2011; Pijl et al. 2001; Guevara-Aguirre et al. 2011; Steuerman et al. 2011. Cystatin C: Odden et al. 2010; Sebastiani et al. 2016; Barron et al. 2015; Foster et al. 2013; Shlipak et al. 2006; Svensson-Farbom et al. 2014; Wu et al. 2011; Hart et al. 2013, 2017; Newman et al. 2016a; Sarnak et al. 2008. NT-proBNP: Braunwald 2008; Masson et al. 2006; Omland et al. 2007; Sanders et al. 2018. Hemoglobin A1c: Dubowitz et al. 2014; Palta et al. 2017; Pani et al. 2008
Discussion
Geroscience research has shown that aging has a distinct biology and that this biology can be targeted in order to extend health and longevity in animal models. As a result, geroscience-guided clinical trials such as TAME designed to test the geroscience hypothesis in humans are being planned. This report presents a conceptual framework for inclusion of biomarkers in such clinical trials and uses TAME as the test case to apply this framework. Exhaustive efforts were undertaken to arrive at a concise list of well-justified biomarkers for use in a clinical trial (Fig. 2), including markers of inflammation (IL-6, TNFRII, CRP), stress response and mitochondrial health (GDF15), nutrient signaling (insulin, IGF-1), multisystem disease markers of kidney aging (cystatin C), cardiovascular health (NT-proBNP), and metabolic aging (hemoglobin A1c). The present review and discussion highlight the paucity of markers reflecting the biologic hallmarks of aging validated for use in clinical research, and the importance of including data-intensive approaches to drive biomarker discovery and uncover new potential therapeutic targets for next-generation geroscience trials.
Notable biomarker exclusions
The Biomarker Workgroup initially proposed to include a biomarker from each of the 7 to 9 geroscience-identified biologic “hallmarks” or “pillars” of aging, but soon concluded that this approach was not practical or feasible at this time. Many markers require access to tissues or resources that are not feasible for large clinical trials. Relatively, few markers can be directly measured from samples obtained from blood draw, or have validated and reliable blood-based surrogates. Several other putative biomarkers of aging were not proposed as candidate biomarkers due to failure to meet workgroup-identified selection criteria. For example, telomere length is frequently used as a biomarker of aging, with in vitro and in vivo research implicating telomere length in cellular senescence and oxidative stress (Blackburn 2000; Blasco 2007; Lopez-Otin et al. 2013; von Zglinicki 2002). However, telomere length is inconsistently or not associated at all with clinical or functional outcomes (Sanders and Newman 2013).
The epigenetic clock, a biomarker of aging strongly associated with chronological age, consistently predicts risk of mortality and key clinical outcomes (Chen et al. 2016; Horvath 2013; Horvath et al. 2016; Levine et al. 2015; Marioni et al. 2015a; Marioni et al. 2015b). Preclinical evidence suggest epigenetic aging signatures in liver may be altered by interventions such as caloric restriction and rapamycin (Wang et al. 2017), which lends promise of epigenetic clocks for clinical trials in the future. Though epigenetic clocks may detect cross-sectional differences in diet and lifestyle, currently responsiveness to an intervention like metformin is not well supported in epidemiologic literature (Kim et al. 2017; Quach et al. 2017). This coupled with relative expense to quantify, tipped the balance of cost-benefit. This may change in the future: costs will likely decline, and ongoing efforts are underway calibrate epigenetic clocks on physiologic age scores that may prove particularly useful as a biomarker for geroscience-guided trials (Levine et al. 2018). Continued refinement and validation of these clocks in human clinical trials are needed, and geroscience-guided trials provide excellent validation tools for biomarker development. Likewise, hypothesis-free, data-intensive molecular approaches do not meet criteria for pre-specified trial biomarkers but represent an important area for scientific development. The molecular signatures derived from interdependent processes provide insight into the complex and multifactorial processes underlying biologic aging and responses to intervention. These platforms are increasingly more attractive for clinical trials as new technologies emerge and assay costs decrease. Such biomarker discovery requires carefully planned collections of sample materials and investigator engagement for ancillary studies using new techniques to evaluate novel, putative biomarkers.
Analytic considerations
This review proposes a variety of a priori suggestions for biomarkers for consideration in the design of randomized controlled geroscience-guided clinical trials, yet the analytic plan is intentionally minimal. This open approach is in accord with recent FDA guidance for clinical trials on age-related diseases without consensus biomarkers (e.g., early Alzheimer’s disease). When inadequate information exists for hierarchical structuring of a series of biomarkers as a supporting outcome, FDA encourages trial sponsors to “analyze the results of these biomarkers independently, though in a prespecified fashion, with the understanding that these findings will be interpreted in the context of the state of the scientific evidence” (Administration 2018). This is particularly relevant to TAME, which was designed in a collaborative and consensus building process with FDA guidance. This open analytic strategy is also intended to encourage use of the biomarker data to develop, refine, and validate multi-assay biomarker composites for next-generation geroscience-guided trials. Ultimately, the validation of biomarkers of biological aging requires longitudinal data because, owing to compensatory and resilience mechanism; there is a temporal gap between the changes that occur in biomarkers and the changes in clinical outcomes, either disease related of functionally related. Implementation of multiple biomarkers of the “pillars” or “hallmarks” of aging is a tantamount strategy to develop the best set of measure to use in clinical trials.
Composites can be developed using combinatorial techniques such as factor analysis or principal component analysis (Karasik et al. 2012; Sebastiani et al. 2017) or weighted and summed based each markers’ prediction of death (Sanders et al. 2018). Such composites should be demonstrated to predict aging outcomes, including death and disability, independently of chronologic age and should also be superior to age itself in the strength of the association with these outcomes (Sanders et al. 2012a). Moreover, multi-assay composites and deficit accumulation indices are likely to outperform single-biomarkers as an index of biological age (Belsky et al. 2017b; Cohen et al. 2017; Evans et al. 2014; Li et al. 2015; Mitnitski et al. 2002; Sanders et al. 2014, 2018; Sebastiani et al. 2016; Sebastiani et al. 2017). Limitations of individual clinical biomarkers in epidemiological research are well known, including risk of conditional associations, misidentification of aging biomarkers, oversimplification of the basic biology/physiology of the aging organism, and poor generalizability and reproducibility (Cohen et al. 2017). Of interest, a systematic evaluation of 11 markers of aging, including telomere length, epigenetic clocks, and clinical biomarker composites, confirms that biomarker composite measures were most consistently related to physical and cognitive function compared with any one biomarker (Belsky et al. 2017b; Newman et al. 2008). Additionally, multi-assay biomarker indices are responsive to interventions targeting the biology of aging, such as caloric restriction (Belsky et al. 2017a). For example, the Healthy Aging Index (HAI), a composite score of physiologic aging that predicts mortality (O'Connell et al. 2018; Sanders et al. 2014, 2018; Wu et al. 2017), cardiovascular disease (McCabe et al. 2016), and disability (Sanders et al. 2012b), is improved with weight loss by caloric restriction in older adults (Shaver et al. 2018). The effect of caloric restriction on the HAI was greater than any individual component or candidate biomarker, and reflects a meaningful difference: the net reduction of HAI by 0.63 points translates to an approximate 9% reduction in mortality risk. In sum, accumulating evidence supports the utility of multicomponent biomarker scores and deficit accumulation indices for future geroscience-guided clinical trials.
Biomarker discovery and resource development
A panel of a priori defined biomarkers has inherent utility for geroscience-guided trials but may not capture the complex and multifactorial processes underlying aging. This gap can be addressed by high-throughput “omics” technologies. Technologies used in sequencing are dramatically reshaping research and drug development, and powerful discovery and screening technologies permit the assessment of biological parameters to permit the molecular and cellular basis for variation in response to therapy and to explain the clinical response to intervention in clinical trials (Biomarkers Definitions Working 2001). Data-intensive technologies for biomarker discovery are increasingly common and include combinatorial chemistry, mass spectrometry, high-throughput screening, cell- and tissue-based microarray, proteomic, and microbiome platforms. Biomarker strategies for geroscience-guided clinical trials are recommended to leverage such technologies as part of the overall biomarker plan. Given the cost, specialization, and invasiveness often required, it may not be feasible to include within large multicenter or multi-year trials, but opportunities for smaller sub-studies or ancillary investigations involving biomarker discovery and cell/tissue collections could be conducted at specialized sites or with lower collection frequency.
Rigorous procedures for biospecimen collections and repository curation are central to future biomarker discovery and resource development for next-generation geroscience-guided trials. The specifics of the plan are almost entirely dependent on the context of the trial, but at a minimum serum and plasma (EDTA and citrated) should be aliquoted and archived, and DNA/RNA isolated if resources warrant. To gain mechanistic insight, isolated and cryopreserved human peripheral blood mononuclear cells (PBMCs) and specialized collections are often required. Specialized collections that pose minimal additional risk to study participants should be prioritized (e.g., urine, stool, peripheral blood cells, saliva), while more invasive tissue biopsies (e.g., adipose, muscle, skin) should only be considered if benefit of knowledge to be gained is warranted and efforts to minimize risks are acceptable. Biospecimen repositories provide a unique for emerging basic research and biomarker studies. For example, the endogenous peptide apelin has recently been identified as a biomarker associated with risk of age-related functional decline and sarcopenia, and a potential drug target to prevent or restore physical function, at least in mice (Vinel et al. 2018). Apelin and other markers of interest emerging from basic research could be examined using repository samples and underscore the utility of a well-cultivated repository and importance of continued surveillance for emerging biomarkers.
Conclusion
With recent growth in the field of geroscience, consensus definitions and conceptual frameworks for biomarkers of aging to be used as part of a supporting outcome in clinical trials are imperative. The Biomarkers Workgroup report presents a conceptual framework for inclusion of biomarkers in gerosicence-guided clinical trials and uses TAME as the test case. Conservative application of selection criteria using clinical and epidemiological literature returns a concise list of blood-based biomarkers. The Biomarkers Workgroup also identified several key considerations and areas in need of development. First, selection criteria are designed for inclusion of individual biomarkers, yet analytic approaches based on multi-assay composites or deficit accumulation/frailty indices are likely to outperform any one individual marker as trial biomarker outcomes. Second, currently, there is a striking paucity of markers that could adequately reflect the biologic hallmarks of aging in the context of human research in general and large clinical trials in particular. There is a critical need for biomarker and resource development to discover novel biomarkers and therapeutic targets, and to validate existing biomarkers, including composites of several markers for use in clinical trials on aging. Finally, data-intensive and multi-omic approaches are likely to drive biomarker discovery and uncover new potential therapeutic targets for next-generation geroscience trials.
References
(IOM) (2010) Evaluation of biomarkers and surrogate endpoints in chronic disease. 2101 CONSTITUTION AVE, WASHINGTON, DC 20418 USA
Abualsuod A, Rutland JJ, Watts TE, Pandat S, Delongchamp R, Mehta JL (2015) The effect of metformin use on left ventricular ejection fraction and mortality post-myocardial infarction. Cardiovasc Drugs Ther 29:265–275. https://doi.org/10.1007/s10557-015-6601-x
Administration FaD (2018) Early Alzheimer’s disease: developing drugs for treatment guidance for industry. Rockville, MD
Allard JS, Perez EJ, Fukui K, Carpenter P, Ingram DK, Cabo R (2015) Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behav Brain Res 301:1–9. https://doi.org/10.1016/j.bbr.2015.12.012
Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LO (2009) IGF1 as predictor of all-cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol 160:25–31. https://doi.org/10.1530/EJE-08-0452
Baker GT III, Sprott RL (1988) Biomarkers of aging. Exp Gerontol 23:223–239
Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ (2014) Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab 16:1165–1173. https://doi.org/10.1111/dom.12354
Barron E, Lara J, White M, Mathers JC (2015) Blood-borne biomarkers of mortality risk: systematic review of cohort studies. PLoS One 10:e0127550. https://doi.org/10.1371/journal.pone.0127550
Bartke A, Brown-Borg H, Mattison J, Kinney B, Hauck S, Wright C (2001) Prolonged longevity of hypopituitary dwarf mice. Exp Gerontol 36:21–28
Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L (1998) Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest 101:1353–1361. https://doi.org/10.1172/JCI485
Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA (2016) Metformin as a tool to target aging. Cell Metab 23:1060–1065. https://doi.org/10.1016/j.cmet.2016.05.011
Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM (2006) The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr 38:33–42. https://doi.org/10.1007/s10863-006-9003-8
Bauskin AR et al (2005) The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res 65:2330–2336. https://doi.org/10.1158/0008-5472.CAN-04-3827
Belsky DW et al (2015) Quantification of biological aging in young adults. Proc Natl Acad Sci USA 112:E4104–E4110. https://doi.org/10.1073/pnas.1506264112
Belsky DW, Huffman KM, Pieper CF, Shalev I, Kraus WE (2017a) Change in the rate of biological aging in response to caloric restriction: CALERIE biobank analysis. J Gerontol A Biol Sci Med Sci 73:4–10. https://doi.org/10.1093/gerona/glx096
Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, Schaefer J, Sugden K, Williams B, Poulton R, Caspi A (2017b) Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. https://doi.org/10.1093/aje/kwx346
Biomarkers Definitions Working G (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95. https://doi.org/10.1067/mcp.2001.113989
Blackburn EH (2000) Telomere states and cell fates. Nature 408:53–56. https://doi.org/10.1038/35040500
Blasco MA (2007) Telomere length, stem cells and aging. Nat Chem Biol 3:640–649. https://doi.org/10.1038/nchembio.2007.38
Braunwald E (2008) Biomarkers in heart failure. N Engl J Med 358:2148–2159. https://doi.org/10.1056/NEJMra0800239
Breese CR, Ingram RL, Sonntag WE (1991) Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol 46:B180–B187
Bridges HR, Jones AJ, Pollak MN, Hirst J (2014) Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J 462:475–487. https://doi.org/10.1042/BJ20140620
Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, Ridker PM (2002) Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet 359:2159–2163. https://doi.org/10.1016/S0140-6736(02)09093-1
Brown DA et al (2006) Measurement of serum levels of macrophage inhibitory cytokine 1 combined with prostate-specific antigen improves prostate cancer diagnosis. Clin Cancer Res 12:89–96. https://doi.org/10.1158/1078-0432.CCR-05-1331
Brown-Borg HM, Bartke A (2012) GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci 67:652–660. https://doi.org/10.1093/gerona/gls086
Bruunsgaard H, Andersen-Ranberg K, Hjelmborg J, Pedersen BK, Jeune B (2003) Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med 115:278–283
Burch JB et al (2014) Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S1–S3. https://doi.org/10.1093/gerona/glu041
Burgers AM et al (2011) Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab 96:2912–2920. https://doi.org/10.1210/jc.2011-1377
Burkle A et al (2015) MARK-AGE biomarkers of ageing. Mech Ageing Dev 151:2–12. https://doi.org/10.1016/j.mad.2015.03.006
Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, McNeilly AD, Balfour DJK, Savinko T, Wong AKF, Viollet B, Sakamoto K, Fagerholm SC, Foretz M, Lang CC, Rena G (2016) Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res 119:652–665. https://doi.org/10.1161/CIRCRESAHA.116.308445
Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP (2003) Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab 88:2019–2025. https://doi.org/10.1210/jc.2002-021694
Cesari M et al (2003) Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation 108:2317–2322. https://doi.org/10.1161/01.CIR.0000097109.90783.FC
Chen BH et al (2016) DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY) 8:1844–1865. https://doi.org/10.18632/aging.101020
Cho K, Chung JY, Cho SK, Shin HW, Jang IJ, Park JW, Yu KS, Cho JY (2015) Antihyperglycemic mechanism of metformin occurs via the AMPK/LXRalpha/POMC pathway. Sci Rep 5:8145. https://doi.org/10.1038/srep08145
Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL Jr, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR, American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research (2017) Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation 135:e1054–e1091. https://doi.org/10.1161/CIR.0000000000000490
Chung HK, Ryu D, Kim KS, Chang JY, Kim YK, Yi HS, Kang SG, Choi MJ, Lee SE, Jung SB, Ryu MJ, Kim SJ, Kweon GR, Kim H, Hwang JH, Lee CH, Lee SJ, Wall CE, Downes M, Evans RM, Auwerx J, Shong M (2017) Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol 216:149–165. https://doi.org/10.1083/jcb.201607110
Cohen AA, Legault V, Fuellen G, Fulop T, Fried LP, Ferrucci L (2017) The risks of biomarker-based epidemiology: associations of circulating calcium levels with age, mortality, and frailty vary substantially across populations. Exp Gerontol. https://doi.org/10.1016/j.exger.2017.07.011
Doi T, Shimada H, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Suzuki T (2016) Insulin-like growth factor-1 related to disability among older adults. J Gerontol A Biol Sci Med Sci 71:797–802. https://doi.org/10.1093/gerona/glv167
Dubowitz N et al (2014) Aging is associated with increased HbA1c levels, independently of glucose levels and insulin resistance, and also with decreased HbA1c diagnostic specificity. Diabet Med 31:927–935. https://doi.org/10.1111/dme.12459
Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK (2015) Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med 21:506–511. https://doi.org/10.1038/nm.3787
Engelfriet PM, Jansen EH, Picavet HS, Dolle ME (2013) Biochemical markers of aging for longitudinal studies in humans. Epidemiol Rev 35:132–151. https://doi.org/10.1093/epirev/mxs011
Espeland MA, Crimmins EM, Grossardt BR, Crandall JP, Gelfond JAL, Harris TB, Kritchevsky SB, Manson JAE, Robinson JG, Rocca WA, Temprosa M, Thomas F, Wallace R, Barzilai N, for the Multimorbidity Clinical Trials Consortium (2017) Clinical trials targeting aging and age-related multimorbidity. J Gerontol A Biol Sci Med Sci 72:355–361. https://doi.org/10.1093/gerona/glw220
Evans SJ, Sayers M, Mitnitski A, Rockwood K (2014) The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing 43:127–132. https://doi.org/10.1093/ageing/aft156
Fontana L, Klein S, Holloszy JO (2006) Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am J Clin Nutr 84:1456–1462
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span--from yeast to humans. Science 328:321–326. https://doi.org/10.1126/science.1172539
Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B (2014) Metformin: from mechanisms of action to therapies. Cell Metab 20:953–966. https://doi.org/10.1016/j.cmet.2014.09.018
Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120:2355–2369. https://doi.org/10.1172/JCI40671
Foster MC et al (2013) Novel filtration markers as predictors of all-cause and cardiovascular mortality in US adults. Am J Kidney Dis 62:42–51. https://doi.org/10.1053/j.ajkd.2013.01.016
Fried LP et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
Friedrich N et al (2009) Mortality and serum insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J Clin Endocrinol Metab 94:1732–1739. https://doi.org/10.1210/jc.2008-2138
Gerstein HC et al (2017) Growth differentiation factor 15 as a novel biomarker for metformin. Diabetes Care 40:280–283. https://doi.org/10.2337/dc16-1682
Giovannini S, onder G, Liperoti R, Russo A, Carter C, Capoluongo E, Pahor M, Bernabei R, Landi F (2011) Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc 59:1679–1685. https://doi.org/10.1111/j.1532-5415.2011.03570.x
Guevara-Aguirre J et al (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3:70ra13. https://doi.org/10.1126/scitranslmed.3001845
Hart A, Blackwell TL, Paudel ML, Taylor BC, Orwoll ES, Cawthon PM, Ensrud KE, for the Osteoporotic Fractures in Men (MrOS) Study Group (2017) Cystatin C and the risk of frailty and mortality in older men. J Gerontol A Biol Sci Med Sci 72:965–970. https://doi.org/10.1093/gerona/glw223
Hart A, Paudel ML, Taylor BC, Ishani A, Orwoll ES, Cawthon PM, Ensrud KE, Osteoporotic Fractures in Men Study Group (2013) Cystatin C and frailty in older men. J Am Geriatr Soc 61:1530–1536. https://doi.org/10.1111/jgs.12413
Harvie MN et al (2011) The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 35:714–727. https://doi.org/10.1038/ijo.2010.171
High KP, Kritchevsky SB (2015) Translational research in the fastest growing population: older adults. In: Wehling M (ed) Principles of translational science in medicine, 2nd edn. Academic Press; Elsevier Inc., Cambridge, pp 299–311. https://doi.org/10.1016/B978-0-12-800687-0.00031-1
Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14:R115. https://doi.org/10.1186/gb-2013-14-10-r115
Horvath S et al (2016) An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol 17:171. https://doi.org/10.1186/s13059-016-1030-0
Howlett SE, Rockwood MR, Mitnitski A, Rockwood K (2014) Standard laboratory tests to identify older adults at increased risk of death. BMC Med 12:171. https://doi.org/10.1186/s12916-014-0171-9
Hu D, Pawlikowska L, Kanaya A, Hsueh WC, Colbert L, Newman AB, Satterfield S, Rosen C, Cummings SR, Harris TB, Ziv E, for the Health, Aging, and Body Composition Study (2009) Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: the health, aging, and body composition. Study J Am Geriatr Soc 57:1213–1218. https://doi.org/10.1111/j.1532-5415.2009.02318.x
Jiang J, Wen W, Sachdev PS (2016) Macrophage inhibitory cytokine-1/growth differentiation factor 15 as a marker of cognitive ageing and dementia. Curr Opin Psychiatry 29:181–186. https://doi.org/10.1097/YCO.0000000000000225
Jickling GC, Sharp FR (2015) Biomarker panels in ischemic stroke. Stroke 46:915–920. https://doi.org/10.1161/STROKEAHA.114.005604
Johnson JA, Simpson SH, Toth EL, Majumdar SR (2005) Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with type 2 diabetes. Diabet Med 22:497–502. https://doi.org/10.1111/j.1464-5491.2005.01448.x
Justice J, Miller JD, Newman JC, Hashmi SK, Halter J, Austad SN, Barzilai N, Kirkland JL (2016) Frameworks for proof-of-concept clinical trials of interventions that target fundamental aging processes. J Gerontol A Biol Sci Med Sci 71:1415–1423. https://doi.org/10.1093/gerona/glw126
Jylhava J, Pedersen NL, Hagg S (2017) Biological age predictors. EBioMedicine 21:29–36. https://doi.org/10.1016/j.ebiom.2017.03.046
Kaplan RC, McGinn AP, Pollak MN, Kuller LH, Strickler HD, Rohan TE, Cappola AR, Xue XN, Psaty BM (2007) Association of total insulin-like growth factor-I, insulin-like growth factor binding protein-1 (IGFBP-1), and IGFBP-3 levels with incident coronary events and ischemic stroke. J Clin Endocrinol Metab 92:1319–1325. https://doi.org/10.1210/jc.2006-1631
Karasik D, Cheung CL, Zhou Y, Cupples LA, Kiel DP, Demissie S (2012) Genome-wide association of an integrated osteoporosis-related phenotype: is there evidence for pleiotropic genes? J Bone Miner Res 27:319–330. https://doi.org/10.1002/jbmr.563
Kempf T et al (2007) Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J 28:2858–2865. https://doi.org/10.1093/eurheartj/ehm465
Kennedy BK et al (2014) Geroscience: linking aging to chronic disease. Cell 159:709–713. https://doi.org/10.1016/j.cell.2014.10.039
Kenyon C (2001) A conserved regulatory system for aging. Cell 105:165–168
Khan SS, Singer BD, Vaughan DE (2017) Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 16:624–633. https://doi.org/10.1111/acel.12601
Kickstein E et al (2010) Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci USA 107:21830–21835. https://doi.org/10.1073/pnas.0912793107
Kim KM et al (2018) SCAMP4 enhances the senescent cell secretome. Genes Dev 32:909–914. https://doi.org/10.1101/gad.313270.118
Kim S, Myers L, Wyckoff J, Cherry KE, Jazwinski SM (2017) The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience 39:83–92. https://doi.org/10.1007/s11357-017-9960-3
Kooy A, de Jager J, Lehert P, Bets D, Wulffele MG, Donker AJ, Stehouwer CD (2009) Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 169:616–625. https://doi.org/10.1001/archinternmed.2009.20
Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ (2010) Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 33:322–326. https://doi.org/10.2337/dc09-1380
Lankeit M et al (2008) Growth differentiation factor-15 for prognostic assessment of patients with acute pulmonary embolism. Am J Respir Crit Care Med 177:1018–1025. https://doi.org/10.1164/rccm.200712-1786OC
Lara J, Cooper R, Nissan J, Ginty AT, Khaw KT, Deary IJ, Lord JM, Kuh D, Mathers JC (2015) A proposed panel of biomarkers of healthy ageing. BMC Med 13:222. https://doi.org/10.1186/s12916-015-0470-9
Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D (2004) The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo study. J Clin Endocrinol Metab 89:114–120. https://doi.org/10.1210/jc.2003-030967
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC (2011) Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 11:20. https://doi.org/10.1186/1471-2407-11-20
Lee RY, Hench J, Ruvkun G (2001) Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the DAF-2 insulin-like signaling pathway. Curr Biol 11:1950–1957
Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP (2009) White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci 64:499–502. https://doi.org/10.1093/gerona/gln047
Lettieri-Barbato D, Giovannetti E, Aquilano K (2016) Effects of dietary restriction on adipose mass and biomarkers of healthy aging in human. Aging (Albany NY) 8:3341–3355. https://doi.org/10.18632/aging.101122
Levine ME, Hosgood HD, Chen B, Absher D, Assimes T, Horvath S (2015) DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging (Albany NY) 7:690–700. https://doi.org/10.18632/aging.100809
Levine ME et al (2018) An epigenetic biomarker of aging for lifespan and healthspan. Aging-Us 10:573–591. https://doi.org/10.18632/aging.101414
Li Q, Wang S, Milot E, Bergeron P, Ferrucci L, Fried LP, Cohen AA (2015) Homeostatic dysregulation proceeds in parallel in multiple physiological systems. Aging Cell 14:1103–1112. https://doi.org/10.1111/acel.12402
Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM (2009) New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32:1620–1625. https://doi.org/10.2337/dc08-2175
Lio D et al (2003) Inflammation, genetics, and longevity: further studies on the protective effects in men of IL-10-1082 promoter SNP and its interaction with TNF-alpha -308 promoter SNP. J Med Genet 40:296–299
Liu B, Fan Z, Edgerton SM, Yang X, Lind SE, Thor AD (2011) Potent anti-proliferative effects of metformin on trastuzumab-resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactions. Cell Cycle 10:2959–2966
Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L (2015) Interventions to slow aging in humans: are we ready? Aging Cell 14:497–510. https://doi.org/10.1111/acel.12338
Longo VD, Finch CE (2003) Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 299:1342–1346. https://doi.org/10.1126/science.1077991
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Lu J et al (2015) Activation of AMPK by metformin inhibits TGF-beta-induced collagen production in mouse renal fibroblasts. Life Sci 127:59–65. https://doi.org/10.1016/j.lfs.2015.01.042
Luchsinger JA, Perez T, Chang H, Mehta P, Steffener J, Pradabhan G, Ichise M, Manly J, Devanand DP, Bagiella E (2016) Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis 51:501–514. https://doi.org/10.3233/JAD-150493
Maggio M et al (2007) Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the chianti area (InCHIANTI) study. Arch Intern Med 167:2249–2254. https://doi.org/10.1001/archinte.167.20.2249
Mao K et al (2018) Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat Commun 9:2394. https://doi.org/10.1038/s41467-018-04805-5
Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Gibson J, Henders AK, Redmond P, Cox SR, Pattie A, Corley J, Murphy L, Martin NG, Montgomery GW, Feinberg AP, Fallin M, Multhaup ML, Jaffe AE, Joehanes R, Schwartz J, Just AC, Lunetta KL, Murabito JM, Starr JM, Horvath S, Baccarelli AA, Levy D, Visscher PM, Wray NR, Deary IJ (2015a) DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol 16:25. https://doi.org/10.1186/s13059-015-0584-6
Marioni RE et al (2015b) The epigenetic clock is correlated with physical and cognitive fitness in the Lothian birth cohort 1936. Int J Epidemiol 44:1388–1396. https://doi.org/10.1093/ije/dyu277
Marti CN et al (2014) Soluble tumor necrosis factor receptors and heart failure risk in older adults: health, aging, and body composition (Health ABC) study. Circ Heart Fail 7:5–11. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000344
Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R (2013) Metformin improves healthspan and lifespan in mice. Nat Commun 4:2192. https://doi.org/10.1038/ncomms3192
Martin-Ruiz C et al (2011) Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ study. Mech Ageing Dev 132:496–502. https://doi.org/10.1016/j.mad.2011.08.001
Masson S et al (2006) Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the valsartan heart failure (Val-HeFT) data. Clin Chem 52:1528–1538. https://doi.org/10.1373/clinchem.2006.069575
Masternak MM, Bartke A (2012) Growth hormone, inflammation and aging. Pathobiol Aging Age Relat Dis 2:2. https://doi.org/10.3402/pba.v2i0.17293
McCabe EL, Larson MG, Lunetta KL, Newman AB, Cheng S, Murabito JM (2016) Association of an index of healthy aging with incident cardiovascular disease and mortality in a community-based sample of older adults. J Gerontol A Biol Sci Med Sci 71:1695–1701. https://doi.org/10.1093/gerona/glw077
McKay HS et al (2017) Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine 90:185–192. https://doi.org/10.1016/j.cyto.2016.09.018
Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F (2013) Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc 14:877–882. https://doi.org/10.1016/j.jamda.2013.05.009
Mitnitski A, Collerton J, Martin-Ruiz C, Jagger C, von Zglinicki T, Rockwood K, Kirkwood TB (2015) Age-related frailty and its association with biological markers of ageing. BMC Med 13:161. https://doi.org/10.1186/s12916-015-0400-x
Mitnitski A, Rockwood K (2015) Aging as a process of deficit accumulation: its utility and origin. Interdiscip Top Gerontol 40:85–98. https://doi.org/10.1159/000364933
Mitnitski A, Song X, Rockwood K (2013) Assessing biological aging: the origin of deficit accumulation. Biogerontology 14:709–717. https://doi.org/10.1007/s10522-013-9446-3
Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K (2002) The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev 123:1457–1460
Moiseeva O et al (2013) Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell 12:489–498. https://doi.org/10.1111/acel.12075
Nair V, Sreevalsan S, Basha R, Abdelrahim M, Abudayyeh A, Rodrigues Hoffman A, Safe S (2014) Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. J Biol Chem 289:27692–27701. https://doi.org/10.1074/jbc.M114.592576
Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB (2008) A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci 63:603–609
Newman AB, Sanders JL, Kizer JR, Boudreau RM, Odden MC, Zeki Al Hazzouri A, Arnold AM (2016a) Trajectories of function and biomarkers with age: the CHS all stars study. Int J Epidemiol 45:1135–1145. https://doi.org/10.1093/ije/dyw092
Newman JC, Milman S, Hashmi SK, Austad SN, Kirkland JL, Halter JB, Barzilai N (2016b) Strategies and challenges in clinical trials targeting human aging. J Gerontol A Biol Sci Med Sci 71:1424–1434. https://doi.org/10.1093/gerona/glw149
Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B (2014) Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41:61–68. https://doi.org/10.3233/JAD-131901
O'Connell MDL et al (2018) Mortality in relation to changes in a healthy aging index: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/gly114
Odden MC et al (2010) Age and cystatin C in healthy adults: a collaborative study. Nephrol Dial Transplant 25:463–469. https://doi.org/10.1093/ndt/gfp474
Omland T et al (2007) Prognostic value of B-type natriuretic peptides in patients with stable coronary artery disease: the PEACE trial. J Am Coll Cardiol 50:205–214. https://doi.org/10.1016/j.jacc.2007.03.038
Palta P, Huang ES, Kalyani RR, Golden SH, Yeh HC (2017) Hemoglobin A1c and mortality in older adults with and without diabetes: results from the national health and nutrition examination surveys (1988-2011). Diabetes Care 40:453–460. https://doi.org/10.2337/dci16-0042
Pani LN et al (2008) Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care 31:1991–1996. https://doi.org/10.2337/dc08-0577
Penninx BW et al (2004) Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc 52:1105–1113. https://doi.org/10.1111/j.1532-5415.2004.52308.x
Pijl H, Langendonk JG, Burggraaf J, Frolich M, Cohen AF, Veldhuis JD, Meinders AE (2001) Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab 86:5509–5515. https://doi.org/10.1210/jcem.86.11.8061
Quach A et al (2017) Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY) 9:419–446. https://doi.org/10.18632/aging.101168
Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE (2002) Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc 50:638–644
Rincon M, Rudin E, Barzilai N (2005) The insulin/IGF-1 signaling in mammals and its relevance to human longevity. Exp Gerontol 40:873–877. https://doi.org/10.1016/j.exger.2005.06.014
Rochon J et al (2011) Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci 66:97–108. https://doi.org/10.1093/gerona/glq168
Rodgers BD (2016) The immateriality of circulating GDF11. Circ Res 118:1472–1474. https://doi.org/10.1161/Circresaha.116.308478
Rodgers BD, Eldridge JA (2015) Reduced circulating GDF11 is unlikely responsible for age-dependent changes in mouse heart, muscle, and brain. Endocrinology 156:3885–3888. https://doi.org/10.1210/en.2015-1628
Roubenoff R et al (2003) Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham heart study. Am J Med 115:429–435
Roussel R et al (2010) Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 170:1892–1899. https://doi.org/10.1001/archinternmed.2010.409
Saisho Y (2015) Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets 15:196–205
Sanders JL et al (2018) Association of biomarker and physiologic indices with mortality in older adults: cardiovascular health study. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/gly075
Sanders JL, Boudreau RM, Newman AB (2012a) Understanding the aging process using epidemiologic approaches. In: The epidemiology of aging. Springer, Dordrecht, pp 187–214
Sanders JL, Boudreau RM, Penninx BW, Simonsick EM, Kritchevsky SB, Satterfield S, Harris TB, Bauer DC, Newman AB, for the Health ABC Study (2012b) Association of a modified physiologic index with mortality and incident disability: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci 67:1439–1446. https://doi.org/10.1093/gerona/gls123
Sanders JL et al (2014) Heritability of and mortality prediction with a longevity phenotype: the healthy aging index. J Gerontol A Biol Sci Med Sci 69:479–485. https://doi.org/10.1093/gerona/glt117
Sanders JL, Newman AB (2013) Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev 35:112–131. https://doi.org/10.1093/epirev/mxs008
Sarnak MJ et al (2008) Cystatin C and aging success. Arch Intern Med 168:147–153. https://doi.org/10.1001/archinternmed.2007.40
Saydah S, Graubard B, Ballard-Barbash R, Berrigan D (2007) Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol 166:518–526. https://doi.org/10.1093/aje/kwm124
Schafer MJ et al (2016) Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metabolism 23:1207–1215. https://doi.org/10.1016/j.cmet.2016.05.023
Schramm TK et al (2011) Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J 32:1900–1908. https://doi.org/10.1093/eurheartj/ehr077
Sebastiani P et al (2016) Age and sex distributions of age-related biomarker values in healthy older adults from the long life family study. J Am Geriatr Soc 64:e189–e194. https://doi.org/10.1111/jgs.14522
Sebastiani P, Thyagarajan B, Sun F, Schupf N, Newman AB, Montano M, Perls TT (2017) Biomarker signatures of aging. Aging Cell 16:329–338. https://doi.org/10.1111/acel.12557
Shaver LN, Beavers DP, Kiel J, Kritchevsky SB, Beavers KM (2018) Effect of intentional weight loss on mortality biomarkers in older adults with obesity. J Gerontol A Med Sci. https://doi.org/10.1093/gerona/gly192
Shlipak MG, Wassel Fyr CL, Chertow GM, Harris TB, Kritchevsky SB, Tylavsky FA, Satterfield S, Cummings SR, Newman AB, Fried LF (2006) Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 17:254–261. https://doi.org/10.1681/ASN.2005050545
Sierra F (2016a) The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb Perspect Med 6:a025163. https://doi.org/10.1101/cshperspect.a025163
Sierra F (2016b) Moving geroscience into uncharted waters. J Gerontol A Biol Sci Med Sci 71:1385–1387. https://doi.org/10.1093/gerona/glw087
Smith DL Jr, Elam CF Jr, Mattison JA, Lane MA, Roth GS, Ingram DK, Allison DB (2010) Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci 65:468–474. https://doi.org/10.1093/gerona/glq033
Sprott RL (1988) Biomarkers of aging. Exp Gerontol 23:1–3
Sprott RL (2010) Biomarkers of aging and disease: introduction and definitions. Exp Gerontol 45:2–4. https://doi.org/10.1016/j.exger.2009.07.008
Steuerman R, Shevah O, Laron Z (2011) Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol 164:485–489. https://doi.org/10.1530/EJE-10-0859
Stork S et al (2006) Prediction of mortality risk in the elderly. Am J Med 119:519–525. https://doi.org/10.1016/j.amjmed.2005.10.062
Strong R et al (2016) Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15:872–884. https://doi.org/10.1111/acel.12496
Svensson-Farbom P et al (2014) Cystatin C identifies cardiovascular risk better than creatinine-based estimates of glomerular filtration in middle-aged individuals without a history of cardiovascular disease. J Intern Med 275:506–521. https://doi.org/10.1111/joim.12169
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Task Force for the Universal Definition of Myocardial I (2012) Third universal definition of myocardial infarction. Nat Rev Cardiol 9:620–633. https://doi.org/10.1038/nrcardio.2012.122
van der Spoel E et al (2015) Association analysis of insulin-like growth factor-1 axis parameters with survival and functional status in nonagenarians of the Leiden longevity study. Aging (Albany NY) 7:956–963. https://doi.org/10.18632/aging.100841
Varadhan R et al (2014) Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol a-Biol 69:165–173. https://doi.org/10.1093/gerona/glt023
Vinel C et al (2018) The exerkine apelin reverses age-associated sarcopenia. Nat Med. https://doi.org/10.1038/s41591-018-0131-6
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Wagner KH, Cameron-Smith D, Wessner B, Franzke B (2016) Biomarkers of aging: from function to molecular biology. Nutrients 8:8. https://doi.org/10.3390/nu8060338
Wang T et al (2017) Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol 18:57. https://doi.org/10.1186/s13059-017-1186-2
Welsh JB et al (2003) Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A 100:3410–3415. https://doi.org/10.1073/pnas.0530278100
Wiklund FE et al (2010) Macrophage inhibitory cytokine-1 (MIC-1/GDF15): a new marker of all-cause mortality. Aging Cell 9:1057–1064. https://doi.org/10.1111/j.1474-9726.2010.00629.x
Wu C, Smit E, Sanders JL, Newman AB, Odden MC (2017) A modified healthy aging index and its association with mortality: the National Health and Nutrition Examination Survey, 1999-2002. J Gerontol A Biol Sci Med Sci 72:1437–1444. https://doi.org/10.1093/gerona/glw334
Wu CK, Chang MH, Lin JW, Caffrey JL, Lin YS (2011) Renal-related biomarkers and long-term mortality in the US subjects with different coronary risks. Atherosclerosis 216:226–236. https://doi.org/10.1016/j.atherosclerosis.2011.01.046
Xia X, Chen W, McDermott J, Han JJ (2017) Molecular and phenotypic biomarkers of aging. F1000Res 6:860. https://doi.org/10.12688/f1000research.10692.1
Zheng Z et al (2012) Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes 61:217–228. https://doi.org/10.2337/db11-0416
Funding
This study received financial support from the American Federation for Aging Research (AFAR); the Glenn Center for the Biology of Human Aging (Paul Glenn Foundation for Medical Research); National Institutes of Health: K01 AG059837-01 (JNJ), P30 AG021332 (SKB, JNJ); R01 AG048023, R01 AG052608, R35 GM124922 (GAK); P30 AG038072 (NB); R01 AG023629 (ABN), and supported in part by the Intramural Research Program of the National Institute on Aging, National Institute of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
JNJ, NB, JB, JD, MAE, LF, SBK, SM, ABN, MP, and GAK report no conflicts of interest to this work. VRA was the MedStar Health Research Institute’s principal clinical trial investigator for studies involving Elcelyx (delayed release metformin).
Electronic supplementary material
ESM 1
(DOCX 652 kb)
About this article
Cite this article
Justice, J.N., Ferrucci, L., Newman, A.B. et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. GeroScience 40, 419–436 (2018). https://doi.org/10.1007/s11357-018-0042-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-018-0042-y