Abstract
In this work, the corn straw (CS) with concentrations of 3%, 6%, and 9% (w/v) were pretreated by rumen fluid (RF) and then used for batched mesophilic biogas production. The results showed that after a 6-day pretreatment, volatile fatty acid (VFAs) production of 3.78, 8.27, and 10.4 g/L could be found in 3%, 6%, and 9%, respectively. When concerning with biogas production, the highest accumulative methane production of 149.1 mL CH4/g volatile solid was achieved by 6% pretreated CS, which was 22% and 45% higher than 3% and 9%, respectively. Also, it was 3.6 times higher than the same concentration of unpretreated CS. The results of the microbial community structure analysis revealed that the 6% CS pretreatment not only maintained a microbial community with the highest richness and diversity, but also exhibited the highest relative abundance of Firmicutes (45%) and Euryarchaeota (3.9%). This high abundance was conducive to its elevated production of VFAs and methane. These findings provide scientific reference for the utilization of CS and support the development of agricultural waste resource utilization and environmental protection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increase in global energy demand and growing environmental problems, the effective utilization of biomass energy has become a research hotspot (Zhu et al. 2022). The corn straw (CS) is an abundant and renewable agricultural waste, with an annual production of about 340 million tons in China in 2021, making it a biomass energy resource with great potential (Hu et al. 2021). However, in most developing countries, unutilized CS is either abandoned on farmland or openly burnt, resulting in the waste of biomass resources and causing serious environmental pollution (Abraham et al. 2020). The technology of biogas production from crop residues can effectively solve the problem of waste disposal and generate sustainable bioenergy, making a positive contribution to sustainable development (Li et al. 2019). Anaerobic fermentation can convert cellulose, hemicellulose, and other organic matter in CS into biogas and organic fertilizer. These products have significant applications in the fields of energy and agriculture, contributing to sustainable development (Li et al. 2020a).
However, the cellulose, hemicellulose, and lignin in CS are entangled with each other, forming a recalcitrant structure. This leads to a long time for biogas production and low methane yield (Zhu et al. 2022; Woiciechowski et al. 2020). Previous studies have shown that the digestibility of lignocellulosic biomass can be improved by adding a pretreatment step before the biogas production process can improve the digestibility of lignocellulosic biomass, especially for biomass rich in lignocellulose. The aim is to enhance the methane yield of the biogas production process by removing lignin, reducing crystallinity, and improving porosity (Koyama 2017; Dollhofer 2018). There are four main methods of pretreatment, physical, chemical, physicochemical, and biological pretreatment (Dong et al. 2018; Takizawa et al. 2018). However, the first three methods involve high energy consumption, high reagent costs, and low pretreatment rates (Takizawa et al. 2018). The biological pretreatment is performed by microorganisms capable of decomposing and utilizing lignocellulose, making it environmentally friendly with low energy input (Hu et al. 2021). The rumen of ruminants is considered a natural cellulose-degrading system because rumen fluid (RF) contains a variety of efficient microorganisms and associated enzymes for decomposing herbaceous plants. Therefore, the pretreatment of RF is considered a promising biological method for enhancing the degradation of lignocellulosic biomass (Jin et al. 2018).
Livestock production has historically been a significant source of greenhouse gases. Consequently, researchers have focused on the rumen as a specific organ and its associated microorganisms (Huuki et al. 2022). The unique dietary structure of ruminants has prompted extensive research on the utilization of RF in lignocellulosic biomass applications. In this emerging field, limited literature has demonstrated varying enhancements in lignocellulose degradation rates and methane production following RF treatment in different types of lignocellulosic biomass, which includes wheat straw (Li et al. 2017a), waste paper sludge (Takizawa et al. 2018), chopped grass (Wang et al. 2018), rice straw (Liang et al. 2021b), barley straw (Meyer et al. 2022), CS (Xu et al. 2024) and other crop residues (Kara et al. 2021). Most notably, RF treatment excels in enhancing the production of volatile fatty acids (VFAs). A comparison between RF and anaerobic sludge revealed that rumen microorganisms yield higher levels of VFAs from lignocellulosic biomass (Nguyen et al. 2019). Liang et al. (2021b) found that a 10% (w/v) rice straw yielded 10.8 g/L of VFAs when inoculated with RF (Liang et al. 2021b). Also, ruminant stomach membrane reactors for VFAs extraction from crop residues have yielded promising results (Nguyen et al. 2020). However, most of the current literature on the in vitro application of RF focuses on using it as an inoculum rather than a pretreatment stage or emphasizes VFAs production. While RF shows significant potential in enhancing both VFAs and methane production from lignocellulosic biomass, the existing research and data on its use as a pretreatment for methane production are limited (Xu et al. 2024). Therefore, further detailed investigation is necessary to optimize the process for specific feedstocks, such as CS, in order to quantify and determine the rational parameters and application scope of RF pretreatment for promoting VFAs and methane generation. The optimal concentration of CS during RF pretreatment is one of the most crucial foundational parameters in practical applications.
With the rapid advancement of microbiological analysis technology, the study of microbial physiological and ecological mechanisms underlying the high degradability of lignocellulosic biomass by RF has emerged as a prominent research focus. It is now understood that Prevotella, Butyrivibrio, Fibrobacter, Bacteroides, Alistipes, Clostridium, and Ruminococcus are the major rumen bacteria that hydrolyze lignocellulosic biomass (Li et al. 2022; Takizawa et al. 2023). These rumen microorganisms secrete high levels of extracellular multi-enzyme complexes and carbohydrate-active enzymes (CAZymes) (Xing et al. 2020; Bhujbal et al. 2022), converting cellulose, hemicellulose, and lignin into oligosaccharides and monosaccharides (Liang et al. 2021a). The symbiotic relationship between fiber solubilizing microbes and methanogens in the rumen microflora helps to maintain a balance between the production and consumption of the metabolites, facilitating the efficient conversion into the final biofuels such as methane, hydrogen, and VFAs (Bhujbal et al. 2022). Generally, the predominant rumen methanogens are Methanobrevibacter, Methanosphaera, and Methanomicrobium (Bhujbal et al. 2022). When employing microbiological analysis technology in the artificial rumen fermentation system, Jo et al. (2021) discovered that the use of RF as a bio-fungicide during prolonged reactor operation enhanced microbial diversity and bolstered the community’s resilience to disturbances (Jo et al. 2021). Notably, the in vitro rumen microbial pretreatment is significantly different from the in situ rumen environment (Xu et al. 2024). Therefore, to investigate the microbial principles underlying the optimal conditions for RF pretreatment of CS, microbial community structure and functional analysis are essential.
In this study, the CS underwent pretreatment with RF at concentrations of 3%, 6%, and 9% (w/v). Subsequently, anaerobic sludge was added to the pretreated system for methane production. The pH, VFAs, and cumulative methane production were analyzed to assess the effects and optimal CS concentration of RF pretreatment on subsequent anaerobic digestion. Additionally, the diversity, abundance, and community structure of rumen microbes in the pretreatment fluid were analyzed using 16S rRNA gene sequences to elucidate the microbial ecological basis underlying the optimal pretreatment conditions. This study will highlight the potential and the ecological mechanisms of RF pretreatment at optimal concentrations for enhancing the anaerobic digestion of CS. It will also contribute to a better understanding of the impact of in vitro factors on rumen microflora cultivation.
Materials and methods
Materials
The harvested dried CS was collected from an agricultural field in Lianyungang, Jiangsu Province, China. It was mechanically crushed until the particles were sieved through a 40-mesh sieve, then sealed and stored in self-sealing bags before use. The RF was collected from a slaughterhouse in Jiaxing, Zhejiang Province, China. The entire rumen was removed from the abdomen of freshly slaughtered cattle. The rumen digestate was kept in an insulated container and taken to the laboratory as soon as possible. The RF was extracted before being pre-insulated at 39 °C for 1 h. The inoculum for biogas production was in the digestate from a biogas plant with a UASB reactor in Jiaxing, Zhejiang Province, China. The characteristics of feedstock and inoculum are shown in Table 1.
Corn straw pretreatment with rumen fluid
The experiment involved adding a mixture of RF and artificial saliva (400 g) to a 500 mL fermentation flask. Subsequently, 12 g, 24 g, and 36 g CS were added to the flask to achieve concentrations of 3%, 6%, and 9% (w/w), respectively. The artificial saliva was prepared according to the formula of Menke and Steingass (Menke 1988), and the mass to volume ratio of RF to artificial saliva was 1:1 (w/w). A control group was also included, with a CS concentration of 0% (w/w). Three parallel runs were established for each experimental group. R0, R3, R6, and R9 denoted the 0% CS group, 3% CS group, 6% CS group, and 9% CS group, respectively. The flasks were flushed with N2 for 2 min before being sealed with silicone plugs. Pretreatment was performed in a constant temperature water bath shaker at 39.0 ± 0.5 °C and 100 rpm for 6 days. Liquid samples were taken on days 1, 2, 3, 4, and 6 for determination of pH and VFAs. Gas production was analyzed volumetrically and compositionally on a daily basis. Samples were collected on the sixth day and stored in an −80 °C fridge for 16S rRNA gene sequencing analysis.
Methane production
In each group for biochemical methane production from pretreated CS slurry (PRCS), the addition of inoculum and substrate were 160 g and 240 g, respectively. Therefore, the inoculum to substrate (I/S) ratios were 2.4, 1.4, and 1.0 (volatile solid (VS) basis) for the 3% PRCS, 6% PRCS, and 9% PRCS groups, respectively. The I/S ratios align with the recommended values in the published literatures (Raposo et al. 2011; Labatut 2012). Additionally, to set up the corresponding control groups, 3%, 6%, and 9% CS without pretreatment were prepared with distilled water instead of RF. After N2 was introduced into each flask for 2 min to maintain the anaerobic condition, the flasks were immediately sealed with silicone stoppers. Two holes were reserved on the silicone stopper, one of which was used to connect 1 L airbags for biogas collection and the other for sampling. Biogas production was performed in a water bath shaker at a constant temperature of 36.0 ± 0.5 °C at 100 rpm. A syringe was used to extract 10 mL of gas from the airbags daily for gas composition analysis. The gas volume in the airbags was measured by the water displacement method. Four milliliters of digestate was sampled periodically from the port on the silicone stopper for VFAs and pH determination.
Analysis methods
The total solid (TS) and VS were measured in a 105 °C oven and a 550 °C muffle furnace, respectively (APHA 2012). The pH was determined using a pH meter (PHS-25 Shanghai Yidian Scientific Instrument Co., Ltd., China). The gas composition and VFAs were determined by a gas chromatograph (7820A, Agilent Technologies Ltd., USA) equipped with a thermal conductivity detector and flame ionization detector, and acetic, propionic, butyric, isobutyric, isovaleric, and valeric acids were used as standard VFAs, which were analyzed according to Zhang et al. (2016). The biogas volume was counted using the drainage method, and cellulose, hemicellulose, and lignin were determined using the Van Soest method for determining cellulose content (Van Soest et al. 1991).
Microbial sequencing analysis
The control plus pretreated CS samples after 6 days were collected for characterizing the diversity of microbial communities via high throughput sequencing technology. The DNA was extracted with the FastDNA® Spin Kit for Soil (MP Biomedicals, USA) according to the manufacturer’s instructions. A polymerase chain reaction (PCR) targeting 16S rRNA genes was performed using the forward primer 515FmodF (5′-GTGYCAGCMGCCGCGGTAA-3′) and the reverse primer 806RmodR (5′-GGACTACNVGGGTWTCTAAT-3′) for the 16S rRNA gene. PCR amplification was performed as described by Liang et al. (2022) using TransGen AP221-02: TransStart Fastqpfu DNA Polymerase, 20 μL reaction system, and 3 replicates per sample. After being purified and quantified, the PCR products of the V4 region of the 16S rRNA gene were pyrosequenced using the Illumina MiSeq PE300 sequencer. The UPARSE software (version 7.0) was used to cluster operational taxonomic units with a similarity cutoff of 97% and to identify and remove chimeric sequences (Edgar 2013). QIIME (version 1.9.1) was used to generate the classification table for each classification level, and USEARCH software (version 11) was used to count OUTs.
Modeling
The methane production data and digestion time were analyzed using a modified Gompertz model (Eq. 1).
where Y refers to the cumulative biogas production at moment x (mL CH4/g VS), P is the maximum methane production potential (mL CH4/g VS), R is the maximum gas production rate (mL CH4/ (g VS·d)), and a is the lag time (d).
Statistics
The experiments were conducted out in triplicate, and the data was expressed as the mean ± standard deviation. Data processing was performed using Excel 2016, while data fitting and plotting were done using Origin 2021. The collected data was analyzed using a one-way analysis of variance. If the results show a significant difference, further analysis will be conducted using Duncan’s new multiple-range test (Booth et al. 1981).
Results and discussion
Acid production analysis
Figure 1 illustrates the yield of VFAs and pH status during both the pretreatment and biogas production stages. Specifically, Fig. 1(A) displays the variation of total VFAs and pH during the pretreatment stage. The production of VFAs stage increased with the increase of pretreatment time, indicating that rumen microorganisms were able to convert lignocellulose into VFAs. On day 6 of 3%, 6%, and 9% PRCS pretreatment, the VFAs produced were 5.5, 2.2, and 1.9 times higher than on day 1, respectively. Nguyen et al. (2019) investigated the production of biogas, VFAs, and other soluble organic matter from four different lignocellulosic biomasses using RF and anaerobic sludge. They found that rumen microorganisms were more effective than sludge in producing VFAs specifically acetic, propionic, and butyric acid from lignocellulosic biomass (Nguyen et al. 2019). The concentration of CS was found to be positively correlated with the production of VFAs. The VFAs produced by 3% PRCS, 6% PRCS, and 9% PRCS after 6 days of pretreatment were 3.78, 8.27, and 10.4 g/L, respectively. The pH of the experimental groups of 3% PRCS, 6% PRCS, and 9% PRCS on day 6 of the pretreatment decreased from 7.48, 6.75, and 6.42 to 6.80, 6.18, and 5.72, respectively. This decrease in pH was due to the production of VFAs, as reported by Zhang et al. (2016). Li and Wang (2017, 2018) pretreated CS and grass clippings with RF for 3 days (Li et al. 2017b; Wang et al. 2018). The total concentration of VFAs increased with substrate concentration, while pH decreased gradually, which is consistent with the findings of this study.
The changes of VFAs and pH during pretreatment and anaerobic fermentation ((A) and (B) the VFAs and pH changes of PRCS during pretreatment and anaerobic fermentation, respectively; (C) the VFAs and pH changes of CS during anaerobic fermentation; (a–c): the changes of VFAs in the pretreatment stage for 3%, 6%, and 9% PRCS, respectively; (a'–c'): the changes of VFAs in the anaerobic fermentation stage for 3%, 6%, and 9% PRCS, respectively)
The production of biogas involves several metabolic intermediates, including acetic acid, propionic acid, and butyric acid, which are collectively known as VFAs. The efficiency of fermentation is directly influenced by the concentration of VFAs (Li et al. 2022). Among these VFAs, acetic acid is the most favorable for conversion to methane, while propionic acid and butyric acid cannot be directly utilized by methanogenic archaea, making them susceptible to accumulation during high-load anaerobic fermentation (Stams and Plugge 2009). At a concentration of 900 mg/L, propionic acid was found to inhibit the methanogens, as reported by Wang et al. (2009).
Njokweni et al. (2021) reported that the main VFAs produced from woody fibrous biomass using RF were acetic acid, propionic acid, and butyric acid (Njokweni et al. 2021). The percentages of acetic, propionic, and butyric acid content in the VFAs produced by RF pretreatment of CS are shown in Fig. 1(a, b, and c). Acetic acid accounted for the highest percentage (89–92%), which is approximately 50–60% higher than the acetic acid content reported by Liang et al. (2022) using rumen microorganisms for long-term in vitro fermentation of CS (2.5%, w/v) (Liang et al. 2022). Butyric acid accounted for 4–5% of the content, while propionic acid accounted for only about 2%. The concentrations of propionic acid in 3–9% PRCS were highest at 124, 190, and 212 mg/L, respectively. None of these concentrations exceeded the reported toxic threshold of 900 mg/L. The high percentage of acetic acid in the pretreated solution, along with the low percentages of propionic acid and butyric acid, indicate that the composition of VFAs in the pretreatment solution will not reduce pH and affect the production of methane.
Figure 1(B) illustrates the changes in total VFAs and pH during biogas production. The initial VFAs content in the fermentation system was high and increased with higher CS concentration, resulting in lower pH. Over the first 3 days of the fermentation process, the VFA concentration in the 3% PRCS, 6% PRCS, and 9% PRCS groups gradually decreased while the pH increased. From the 7th day of fermentation onwards, the pH of all experimental groups was maintained between 7.5 and 8. Figure 1(a')–(c') shows the concentration of acetic acid, propionic acid, and butyric acid in 3% PRCS, 6% PRCS, and 9% PRCS during anaerobic fermentation. On the 1st day of fermentation, the concentration of acetic acid was 3.58 g/L, 6.12 g/L, and 6.88 g/L, respectively. The concentration of propionic acid was 0.10 g/L, 0.13 g/L, and 0.15 g/L, respectively. The concentration of butyric acid was 0.17 g/L, 0.33 g/L, and 0.41 g/L, respectively. After the 10th day of fermentation, the concentration of acetic acid remained constant at 0.37 g/L, 0.09 g/L, and 0.08 g/L in the 3% PRCS, 6% PRCS, and 9% PRCS groups, respectively. The degradation of propionic acid and butyric acid varied among the groups. Propionic acid was not detected on the 9th, 14th, and 16th days of fermentation, while butyric acid was not detected on the 6th, 8th, and 10th days. The yields of VFAs were similar for both 6% PRCS and 9% PRCS during RF pretreatment. However, VFAs degraded faster in 6% PRCS than in 9% PRCS during biogas production.
Figure 1(C) illustrates the changes in VFAs and pH during anaerobic fermentation of unpretreated CS (control groups). The VFA concentrations generated by the three different CS concentrations during the fermentation process were comparable. Specifically, on the first day of anaerobic fermentation, the VFAs produced by 3%, 6%, and 9% CS were 0.34 g/L, 0.37 g/L, and 0.36 g/L, respectively. By the 9th day of fermentation, the VFAs concentrations for all three concentrations remained steady at 0.03 g/L. Throughout the anaerobic fermentation, the pH level was maintained around 7.5. A comparison between the VFAs concentration in pretreated CS and unpretreated CS revealed 10–17 times increase, primarily attributed to the RF pretreatment effect.
During the biogas production phase, the rate of VFAs consumption was faster at 6% PRCS. By the 10th day of fermentation, the VFAs concentration of 0.2 g/L was the lowest among the three groups. Although the higher the concentrations of CS resulted in more VFAs production through RF pretreatment, the pH trend was opposite to that of VFAs. The concentration of VFAs should be considered during the pretreatment of woody fibrous biomass using RF. High levels of VFAs are not conducive to subsequent biogas production.
Gas production analysis
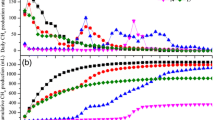
The biogas composition and cumulative methane production during RF pretreatment are shown in Fig. 2a and b. The biogas production was dominated by CO2 during the pretreatment stage, with the CO2 content increasing rapidly in the first 2 days. After the first 3 days of pretreatment, the CO2 content in the biogas production stabilized at 65–70%. Methane production increased more rapidly in the first 3 days of pretreatment, with the final methane percentage ranging from 20 to 25%. Pretreatment of rice straw with RF resulted in a biogas composition similar to that of the control group. CO2 accounted for 79.5% to 90.6% of the biogas, while methane accounted for only 6.2% to 19.6% (Zhang et al. 2016). This suggests that rumen microorganisms primarily produce CO2 during the process, and excessive CO2 production could lead to carbon loss in the system, which is unfavorable for subsequent methane fermentation.
Biogas production diagram of pretreatment stage and biogas production stage. (a, b Gas composition and cumulative methane production in the pretreatment stage, respectively. c, d Daily and cumulative methane production during anaerobic fermentation of PRCS and CS, respectively. e Gompertz kinetic models of CS and PRCS in the anaerobic fermentation stage. f Total methane yield diagram)
Figure 2b shows that after 6 days of pretreatment, the cumulative methane production was 11.40 ± 0.79, 12.76 ± 0.14, and 9.16 ± 0.46 mL CH4/g VS for 3% PRCS, 6% PRCS, and 9% PRCS, respectively (P < 0.05). Although the methane production during the pretreatment stage was not high, it increased rapidly in the first 3 days and then slowed down. Previous reports suggested that methanogens were the primary microflora in ruminants, despite their low abundance (Mizrahi et al. 2021). However, some researchers have used RF as inoculum for biogas production from lignocellulosic biomass, although the methane production was suboptimal (Li et al. 2017a). It is important to note that the optimal working pH range for methanogenic archaea is between 6.5 and 7.5. However, with an increase in pretreatment time, the VFAs in the system increased while the pH decreased. The pH was lower than 6.6 on the second day of pretreatment in both the 6% PRCS and 9% PRCS groups.
Figure 2c displays the daily methane production of each group during anaerobic fermentation. The analysis revealed that during the first 5 days of fermentation, the daily methane production of the pretreatment group was lower than that of the control group. This might be attributed to the high content of VFAs in the pretreatment solution affecting the microbial activities. On the 6th day of fermentation, the 3% PRCS, 6% PRCS, and 9% PRCS groups produced 6.6, 3.2, and 1.9 times more methane than the control group, respectively. From then on, the daily methane production of each pretreatment group was higher than that of its equivalent control group.
Figure 2d shows a graph of cumulative methane production of pretreated and unpretreated CS during biogas production. During the first 14 days of fermentation, the order of cumulative methane production was 3% PRCS > 6% PRCS > 9% PRCS. Although the 3% PRCS showed slow biogas production in the first 2 days, it increased from day 2 onwards. In contrast, the 6% PRCS and 9% PRCS groups began to increase biogas production from the fourth day of fermentation. This increase may be due to the high concentration of VFAs, which is the substrate for methane production. However, this high concentration also affected the microbial community structure and delayed methane production. From the 14th day of fermentation, the cumulative methane production curves for each experimental group began to level off. The cumulative methane production of 3%, 6%, and 9% CS was 19.40 ± 0.27, 41.4 ± 0.90, and 87.57 ± 1.73 (P < 0.05), respectively. The cumulative methane production for the 3% PRCS, 6% PRCS, and 9% PRCS groups was 110.86 ± 3.15, 133.59 ± 2.78, and 93.57 ± 0.35 mL CH4/g VS (P < 0.05), respectively. This was 6.3, 3.6, and 1.2 times higher than the unpretreated group. The results indicate that RF pretreatment can significantly improve the methane production efficiency of biogas production from CS. According to the daily and cumulative methane production, the methane production from CS only lasted for approximately 10 days. This phenomenon may be attributed to the use of distilled water instead of RF rich in microorganisms in CS. Additionally, the CS might enter a slow hydrolysis stage of lignocellulose, and the anaerobic fermentation time of 16 days was relatively short. This likely resulted in the feedstock not being fully fermented by the conclusion of the study period.
The Gompertz model is a population growth model that follows an S-shaped curve. Among various modifications, the modified Gompertz model has proven to be a valuable tool in the study of biohydrogen, biomethane, and related fermentation processes (Yin and Wang 2021; Zhang et al. 2022). The addition of reflecting the stagnation period in this model allows predicting not only the methane potential and the maximum rate of methane production but also aligning it with the actual anaerobic digestion reaction (Wang and Guo 2024). In this study, the modified Gompertz model was employed to predict and compare the theoretical maximum methane productions, gas production rates, and lag times of anaerobic fermentation between PRCS and CS, evaluating the impact of RF pretreatment on the methane production process from a kinetic perspective. The results are presented in Table 2 and Fig. 2e. The Gompertz fitted curves demonstrated excellent agreement with the experimental results, with R2 values ranging from 0.990 to 0.994. The maximum cumulative methane production obtained was 122 ± 4.38 mL CH4/g VS, 161 ± 13.8 mL CH4/g VS, and 98.7 ± 5.29 mL CH4/g VS for 3% PRCS, 6% PRCS, and 9% PRCS, respectively. Compared to the control groups, pretreatment significantly increased the maximum gas production rate, with an increase ranging from 3.1 to 11.3 mL CH4/(g VS·d). Among these, the 6% PRCS group exhibited the highest gas production rate at 14.7 mL CH4/(g VS·d). On the other hand, pretreatment also increased the lag time for methane production by 2.1–4.2 days, which did not shorten the initiation time of anaerobic fermentation, as observed in typical pretreatment studies. This is attributed to the inhibitory effect of the high concentration of VFAs (3.8–10.4 g/L) produced during pretreatment on methanogens.
The calculation of the total methane yield involves adding the cumulative methane yield from both the pretreatment and fermentation processes. As shown in Fig. 2f, the total methane yields for the 3% CS, 6% CS, 9% CS, 3% PRCS, 6% PRCS, and 9% PRCS groups were 19.4 ± 0.27 mL CH4/g VS, 41.39 ± 0.90 mL CH4/g VS, 87.57 ± 1.73 mL CH4/g VS, 121.90 mL CH4/g VS, 146.35 mL CH4/g VS, and 102.73 mL CH4/g VS (P < 0.05), respectively. The methane production of the 6% PRCS was higher than that of 3% and 9% by 22% and 45%, respectively. Furthermore, it was 3.6 times higher than the same concentration of CS. The CS pretreated with a 6% concentration showed 1.2 and 1.4 times higher cumulative total methane yields than the 3% and 9% concentrations, respectively. Li et al. (2020a) obtained a cumulative methane yield of 143.4 mL CH4/g VS from a 30-day batch fermentation of corn silage with an I/S ratio of 2:1 and a TS concentration of 5% at 38 ± 1 °C (Li et al. 2020b). The cumulative methane production of 6% CS was 4.3% higher than that of corn silage. The results indicated that 6% CS achieved the highest and fastest methane production rate when applied with RF pretreatment previously. Out of the three pretreatment concentrations tested (3%, 6%, and 9% w/v), 6% was found to be the most effective concentration for pretreating CS RF. It is important to note that this evaluation is based solely on objective data and not subjective opinions.
Analysis of microbial diversity during pretreatment
Rumen microorganisms are crucial in the production of methane and VFAs during RF pretreatment of CS. To investigate how the rumen promotes the pretreatment process, microbial diversity was analyzed on day 6 of pretreatment. Table 3 displays the diversity and abundance of rumen microorganisms during pretreatment (OTUs sequence similarity: 97%). The Sobs and Chao indices reflect community richness, while the Shannon and Simpson indices reflect community diversity. The Shannon index indicates higher community diversity, while the Simpson index indicates lower community diversity. The coverage index reflects the community coverage, and all groups have a coverage index value of 1, indicating that the sequencing results accurately represent the microorganisms in the samples. Table 3 date indicates that the richness of the 3%, 6%, and 9% PRCS group was higher than that of the control group. The 6% PRCS group exhibited the highest Shannon index value (5.57) and the lowest Simpson index value (0.01) among the four groups, suggesting the greatest microbial diversity in this group.
Figure 3 shows the structural composition of rumen microbial populations. The Venn diagram in Fig. 3a shows that there were 863 common species in the RF, 3% PRCS, 6% PRCS, and 9% PRCS groups, while 56, 22, 31, and 23 OTUs species were individually unique to each group, respectively. Notably, the 6% PRCS group had the largest number of species, indicating a significant difference from the 3% PRCS and 9% PRCS groups. The analysis of rumen microbes was conducted at the phylum level, and the top 10 phylum in Fig. 3b, while the remaining species were grouped as “others.” The phylum with a relative abundance higher than 1% were Firmicutes, Bacteroidota, Verrucomicrobiota, Spirochaetota, Euryarchaeota, Proteobacteria, Actinobacterioa, Synergistota, Planctomycetota, and Chloroflexi. The three main phyla, Firmicutes, Bacteroidota, and Verrucomicrobiota, dominated with a combined relative abundance of over 70%. Studies have shown that the major phylum listed in rumen microflora studies are Fibrobacteres, Bacteroidetes, and Firmicutes (Moraïs and Mizrahi 2019; Won et al. 2020). The abundance of Fibrobacteres was reported to be affected by the fiber content of the ruminant diet, with an increase in their number when the diet of cattle is high in fiber (Ozbayram et al. 2018). However, the relative abundance of Fibrobacteres in this study exceeded 1%. The reason for this result may be the low number of Fibrobacteres in the original RF.
Rumen microbial composition map (a Venn map of rumen microbial species composition. b Rumen microbial community composition map of the first 10 rumen microbial communities in the top 10 of phylum level abundance. c Rumen microbial community composition map of the top 30 rumen microorganisms of genus abundance)
Firmicutes represented over 35% of the microbial relative abundance in samples of 3%, 6%, and 9% PRCS, and reached 45% in 6% PRCS. Firmicutes are significant contributors in VFA production (Ma et al. 2017) and also produce cellulases, proteases, and other extracellular enzymes during organic matter degradation (Chen et al. 2017; Yue et al. 2013). The increase in VFAs in the pretreatment fluid after RF pretreatment could be attributed to this. The analysis showed that the highest relative abundance of Bacteroidetes was found in the 9% PRCS sample, at 43%. The VFAs were also highest in the 9% PRCS sample after 6 days of pretreatment. According to Accetto and Avguštin (2021), Bacteroidetes are capable of converting various sugars, including polysaccharides, xylan, fructose, and galactose, into short-chain fatty acids and other metabolites for CH4 production. Additionally, Fig. 3b shows that the relative abundance of Euryarchaeota decreased as the CS concentration increased, with a relative abundance of only 1.8% in 9% PRCS. The reduction in Euryarchaeota colonies supports the argument that high levels of VFAs can hinder methanogens. This suggests that the concentration of CS used in pretreatment with RF can impact biogas production for methane. Selecting an appropriate pretreatment concentration can optimize CS utilization.
To gain a better understanding of the composition of the rumen microbial community, we conducted an analysis of the top 30 genera in abundance at the genus level. The results are presented in Fig. 3c. Following Mizrahi’s report (Mizrahi et al. 2021), we have listed the genus that belong to the core rumen microorganisms among the top 30 in abundance at the genus level in Table 4. Rikenellaceae_RC9_gut_group, Prevotella, Succiniciasticum, and Ruminococcus are key bacteria for degrading lignocellulose and producing VFAs (Huws et al. 2021; Henderson et al. 2016). Rikenellaceae_RC9_gut_group had the highest relative abundance share (11.8%, 11.3%, 14.7%, and 13.2% in the four groups, respectively), while Succiniciasticum had a relative abundance share of 2.0%, 3.9%, 4.4%, and 3.2%, respectively. According to Ahmad et al. (2020), Rikenellaceae_RC9_gut_group and Succiniciasticum are highly prevalent among the production of acetic and propionic acids. Additionally, the relative abundance of both Prevotella and Prevotellaceae_UCG-003 increased with increasing CS concentration with Prevotella increasing by 4.0% and Prevotellaceae_UCG-003 increasing by 2.4%. Prevotella is a significant genus in the rumen ecosystem due to its high efficiency in degrading xylan, xyloglucan, and pectin and converting resulting sugars into acetic, succinic, and propionic acids (Accetto and Avguštin 2021). The increase in the relative abundance of Prevotella and Prevotellaceae_UCG-003 indicates that RF pretreatment of CS was feasible, contributing to the increase in VFAs production with increasing CS concentration. The abundance of Ruminococcus decreased from 1.2 to 0.5% with the increase in pretreated CS concentration. Ruminococcus secretes enzymes that degrade cellulose and plays a crucial role in lignocellulose hydrolysis and acidification (Ozbayram et al. 2020). Papillibacter has been shown to produce acetic acid and butyric acid (Liu et al. 2021). The relative abundance of Papillibacter increased in with the concentration of CS, reaching its highest level in the 6% PRCS group, which was 4.9% higher than that in RF.
In Table 4, in addition to the core genera of rumen microorganisms, a relatively high proportion is accounted for by a large number of unclassified genera, such as norank_f__F082. This situation has also been observed in other studies on rumen microbe distribution (Liang et al. 2022). Further investigation is required to explore the diversity and function of rumen microorganisms.
To improve the analysis of the effect of RF pretreatment on methane production, the microorganisms in the phylum Methanobacteriaceae were analyzed at the genus level, as shown in Fig. 4. The samples contained four genera: Methanobrevibacter, Methanosphaera, unclassified_f__Methanobacteriaceae, and Methanothermobaceter. The RF showed a high relative abundance percentage of Methanobrevibacter, accounting for 91.82%. This percentage increased with the concentration of CS in 3%, 6%, and 9% PRCS, reaching 71.82%, 87.19%, and 91.23%, respectively. Research has demonstrated that Methanobrevibacter is the primary genus of rumen archaea, comprising 70% of the total population (Friedman et al. 2017; Mizrahi et al. 2021). Methanosphaera and unclassified_f__ Methanobacteriaceae exhibited a similar distribution pattern in the pretreated samples, with both decreasing as CS concentration increased. The groups showed a relative abundance of 11.53% and 14.38% for Methanosphaera and unclassified__f__Methanobacteriaceae, respectively, in 3% PRCS and 6% PRCS. In 6% PRCS, the relative abundance was 6.90% and 3.45%, while in 9% PRCS, it was 4.83% and 2.86%. However, all values were lower than that of RF (3.94% and 1.97%). Both Methanobrevibacter and Methanosphaera are hydrogenotrophic rumen methanogens capable of producing methane from H2 and CO2 (Ozbayram et al. 2018; Bayané and Guiot 2011). Methanothermobacter, a thermophilic methanogenic bacterium, is found in only 6% of PRCS with a relative abundance of 0.2% (Chen and Chang 2020). It metabolizes acetate to methane (Sun et al. 2020). Methane production relies on various types of flora working in concert with each other. The abundance values of Archaea in Fig. 4b demonstrate that the number of Archaea in the 6% PRCS group is significantly larger and more diverse than in the other groups. On day 6 of pretreatment, the 6% PRCS group exhibited the highest daily methane production, and subsequent anaerobic fermentation with 6% PRCS resulted in the highest cumulative methane production. Therefore, 6% PRCS is deemed more suitable for methane production than 3% and 9% PRCS. This conclusion is based on the data presented in the study.
The hierarchical cluster analysis of Beta diversity phylum level samples of microbial data (Fig. 5) revealed a significant gap between the 6% PRCS group and the control group at the phylum level. The species responsible for the gap were mainly Firmicutes, Bacteroidetes, Verrucomicrobiota, Spirochaetota, and Euryarchaeota. The relative abundance of Firmicutes and Bacteroidetes in the RF did not differ significantly, at approximately 40 ± 0.7%. However, in the 6% PRCS, the relative abundance of Firmicutes was 13.43% higher than that of Bacteroidetes. Additionally, Verrucomicrobiota was 4.23% less abundant than in the control group in the 6% PRCS. The relative abundance of Euryarchaeota was 2.5% higher in the RF. The study found that the relative abundance of Spirochaetota increased with the concentration of CS, while the relative abundance of Euryarchaeota was 0.2% and 0.6% higher in 3% PRCS and 6% PRCS, respectively, and 1.5% lower in 9% PRCS compared to the control. This suggests that high CS concentration in RF pretreatment may have an impact on the survival of Euryarchaeota, which are the typical rumen microbial methanogens. It is noteworthy that in the context of community structure similarity, R9 exhibited closer resemblance to R3 rather than R6. The underlying reasons for this phenomenon are multifaceted, as community composition is shaped by a multitude of environmental factors working together, with the CS concentration during pretreatment being just one of these factors. One potential explanation for the unique community structure of R6 is its notably high concentration of isobutyric acid (199 mg/L), surpassing that in all other groups. Previous reports indicated a negative correlation between isobutyric acid concentration and the abundance of Firmicutes (Liu et al. 2019). In brief, the analysis results showed that the concentration of CS had a significant impact on the structure of the rumen microbial community. The acid and biogas production during pretreatment and biogas production of combined 3% PRCS, 6% PRCS, and 9% PRCS indicated that 6% was the optimal pretreatment concentration among the three concentrations. The population of Euryarchaeota increased with the optimal pretreatment concentration, which was beneficial for the full conversion of VFAs produced during pretreatment into methane.
In conclusion, the microbial diversity analysis revealed that the pretreatment and anaerobic fermentation stages with 6% CS concentration exhibited the highest methane production and maximum gas production rate. This phenomenon was underpinned by the ability of the 6% pretreatment group to sustain the most abundant and diverse microbial community. Notably, within this community, Firmicutes exhibited higher abundance compared to other experimental groups, while Euryarchaeota showed the most significant increase. Further genus-level analysis of Euryarchaeota highlighted that the 6% CS concentration pretreatment leads to the highest abundance of methanogens, particularly Methanobrevibacter. This emphasizes the crucial role of Methanobrevibacter in enhancing methane production during RF pretreatment.
The effects of acetic acid, propionic acid, and butyric acid on microorganisms were consistent, while the correlation of the first three genera was opposite to that of pH (Fig. 6). The pH showed significant negative correlation to Prevotella, Prevotellaceae_UCG 003, Sphaerochaeta, and Treponema, and for Lachnospiraceae_NK3A20_group, Ruminococcus, norank_f__Eubacterium_coprostanoligenes_group, and UCG 005 were significantly positively correlated.
The pH level is a crucial environmental factor for the growth of rumen microorganisms. Variations in pH can lead to changes in the growth and metabolic activity of these microorganisms, which can ultimately affect the degradation of cellulose and the production of VFAs (Eryildiz et al. 2020). In this study, the pH values of 3%, 6%, and 9% PRCS were reduced to 6.80, 6.18, and 5.72, respectively, after 6 days of pretreatment. Sundberg et al. (2013) reported that Firmicutes were susceptible to pH-induced changes in the environment (Sundberg et al. 2013). The pH had an inhibitory effect on their growth, which also explained the lower relative abundance of Firmicutes in the 9% PRCS compared to the RF. Liang et al. (2021c) found a strong positive correlation between pH and Ruminococcus. The results of the pretreatment process indicate that pH decreased with increased pretreatment time and CS concentration (Liang et al. 2021c). The significant decrease in the relative abundance of Ruminococcus in 9% PRCS may have been influenced by the pH. Previous studies have shown that lower pH levels make it more difficult for microorganisms to attach to the fiber matrix, resulting in a lower rate of fiber degradation (Farenzena et al. 2014; Hu et al. 2004). Therefore, pH has a strong influence on acid production during pretreatment.
According to Liang et al. (2021a), the main cause of pH reduction and limited microbial fermentation was the accumulation of VFAs (Liang et al. 2021a). Acetic, propionic, and butyric acids were found to have a significant positive correlation with Prevotella, Prevotellaceae_UCG-003, Sphaerochaeta, and Treponema, and a significant negative correlation with Lachnospiraceae_NK3A20_group, Ruminococcus, and norank_f__ Eubacterium_coprostanoligenes_group. Ahmad et al. (2020) analyzed the microbial diversity of the rumen in yaks and found a positive correlation between Prevoteaceae UCG-003, Prevoella, and Christensenellaceae_R7 with acetic acid, propionic acid, and butyric acid (Ahmad et al. 2020). Liang et al. (2021b) conducted a study on biogas production from rice straw using RF (Liang et al. 2021b). The study found a positive correlation between VFAs and Prevotella, Prevotellaceae_UCG-003, and Fibrobacter, and a negative correlation with Ruminococcus, Christensenellaceae_R7, and Treponema. The effects of VFAs on individual microorganism abundance and diversity varied in different studies.
Conclusion
The production of VFAs and methane varied with different CS concentrations during RF pretreatment and anaerobic fermentation. Regarding VFAs production, the optimum of CS concentration of RF pretreatment was found to be 9% (10.40 g/L). For biogas production, the optimum CS concentration of RF pretreatment was determined to be 6% (146.35 mL CH4/g VS), which was 22% and 45% higher than the concentrations of 3% and 9%, respectively. A higher CS concentration increased the relative abundance of Prevotella and decreased the relative abundance of Methanobrevibacter in the rumen microorganisms. Prevotella contributed to VFA production, while Methanobrevibacter contributed to methane production. Based on apparent acids and methane production index and microbial population analysis, the economic benefits of an over-low CS concentration when applying RF pretreatment were unsatisfactory. On the contrary, the over-high proportion of CS led to the sharp increase of VFAs, which showed a negative impact on biogas production. Optimizing the CS proportion during RF pretreatment is essential for follow-up research and industrial applications.
Data availability
Data will be made available on request.
References
Abraham A, Mathew AK, Park H, Choi O, Sindhu R, Parameswaran B, Pandey A, Park JH, Sang B-I (2020) Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour Technol 301:122725. https://doi.org/10.1016/j.biortech.2019.122725
Accetto T, Avguštin G (2021) Non-oral Prevotella stepping into the spotlight. Anaerobe, Special Issue: Anaerobes in the Microbiome 68:102321. https://doi.org/10.1016/j.anaerobe.2021.102321
Ahmad AA, Yang C, Zhang J, Kalwar Q, Liang Z, Li C, Du M, Yan P, Long R, Han J, Ding X (2020) Effects of dietary energy levels on rumen fermentation, microbial diversity, and feed efficiency of yaks (Bos grunniens). Front Microbiol 11:625. https://doi.org/10.3389/fmicb.2020.00625
APHA, 2012. Standard methods for the examination of water and wastewater.
Bayané A, Guiot SR (2011) Animal digestive strategies versus anaerobic digestion bioprocesses for biogas production from lignocellulosic biomass. Rev Environ Sci Biotechnol 10:43–62. https://doi.org/10.1007/s11157-010-9209-4
Bhujbal SK, Ghosh P, Vijay VK, Rathour R, Kumar M, Singh L, Kapley A (2022) Biotechnological potential of rumen microbiota for sustainable bioconversion of lignocellulosic waste to biofuels and value-added products. Sci Total Environ 814:152773. https://doi.org/10.1016/j.scitotenv.2021.152773
Booth GD, Steel RGD, Torrie JH (1981) Principles and procedures of statistics: a biometrical approach. J Am Stat Assoc 76:753. https://doi.org/10.2307/2287561
Chen H, Chang S (2020) Dissecting methanogenesis for temperature-phased anaerobic digestion: impact of temperature on community structure, correlation, and fate of methanogens. Bioresour Technol 306:123104. https://doi.org/10.1016/j.biortech.2020.123104
Chen Y, Jiang X, Xiao K, Shen N, Zeng RJ, Zhou Y (2017) Enhanced volatile fatty acids (VFAs) production in a thermophilic fermenter with stepwise pH increase – investigation on dissolved organic matter transformation and microbial community shift. Water Res 112:261–268. https://doi.org/10.1016/j.watres.2017.01.067
Dollhofer V (2018) Accelerated biogas production from lignocellulosic biomass after pre-treatment with Neocallimastix frontalis. Bioresour Technol 264:219–227
Dong C, Chen J, Guan R, Li X, Xin Y (2018) Dual-frequency ultrasound combined with alkali pretreatment of corn stalk for enhanced biogas production. Renew Energy 127:444–451. https://doi.org/10.1016/j.renene.2018.03.088
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Eryildiz B, Lukitawesa, Taherzadeh MJ (2020) Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour Technol 302:122800. https://doi.org/10.1016/j.biortech.2020.122800
Farenzena R, Kozloski GV, Mezzomo MP, Fluck AC (2014) Forage degradability, rumen bacterial adherence and fibrolytic enzyme activity in vitro: effect of pH or glucose concentration. J Agric Sci 152:325–332. https://doi.org/10.1017/S0021859613000427
Friedman N, Shriker E, Gold B, Durman T, Zarecki R, Ruppin E, Mizrahi I (2017) Diet-induced changes of redox potential underlie compositional shifts in the rumen archaeal community. Environ Microbiol 19:174–184. https://doi.org/10.1111/1462-2920.13551
Henderson G, Cox F, Ganesh S, Jonker A, Young W, Global Rumen Census Collaborators, Janssen PH (2016) Erratum: Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep 6:19175. https://doi.org/10.1038/srep19175
Hu P, Li H, Xiao W, Xie X, Yang Y, Duan L, Zhou S, Hu Y, Qiao Q, Ran Q, Jiang Z (2021) Effect of Rhodococcus sp. pretreatment on cellulose hydrolysis of corn stalk. Prep Biochem Biotechnol 51:137–143. https://doi.org/10.1080/10826068.2020.1799391
Hu Z-H, Wang G, Yu H-Q (2004) Anaerobic degradation of cellulose by rumen microorganisms at various pH values. Biochem Eng J 21:59–62. https://doi.org/10.1016/j.bej.2004.05.004
Huuki H, Tapio M, Mäntysaari P, Negussie E, Ahvenjärvi S, Vilkki J, Vanhatalo A, Tapio I (2022) Long-term effects of early-life rumen microbiota modulation on dairy cow production performance and methane emissions. Front Microbiol 13:983823. https://doi.org/10.3389/fmicb.2022.983823
Huws SA, Edwards JE, Lin W, Rubino F, Alston M, Swarbreck D, Caim S, Stevens PR, Pachebat J, Won M-Y, Oyama LB, Creevey CJ, Kingston-Smith AH (2021) Microbiomes attached to fresh perennial ryegrass are temporally resilient and adapt to changing ecological niches. Microbiome 9:143. https://doi.org/10.1186/s40168-021-01087-w
Jin W, Xu X, Yang F (2018) Application of rumen microorganisms for enhancing biogas production of corn straw and livestock manure in a pilot-scale anaerobic digestion system: performance and microbial community analysis. Energies 11:920. https://doi.org/10.3390/en11040920
Jo Y, Rhee C, Choi H, Shin J, Shin SG, Lee C (2021) Long-term effectiveness of bioaugmentation with rumen culture in continuous anaerobic digestion of food and vegetable wastes under feed composition fluctuations. Bioresour Technol 338:125500. https://doi.org/10.1016/j.biortech.2021.125500
Kara GK, Doluk R, Civelek Yoruklu H, Demir A, Ozkaya B (2021) Biomethane production kinetics of rumen pretreated lignocellulosic wastes. Clean Techn Environ Policy 23:2941–2954. https://doi.org/10.1007/s10098-021-02214-9
Koyama M (2017) Effect of alkaline pretreatment on mesophilic and thermophilic anaerobic digestion of a submerged macrophyte: inhibition and recovery against dissolved lignin during semi-continuous operation. Bioresour Technol 238:666–674
Labatut R (2012) Anaerobic Biodegradability of complex substrates: performance and stability at mesophilic and thermophilic conditions. Cornell University Library
Li F, Zhang P, Zhang G, Tang X, Wang S, Jin S (2017b) Enhancement of corn stover hydrolysis with rumen fluid pretreatment at different solid contents: Effect, structural changes and enzymes participation. Int Biodeterior Biodegrad 119:405–412. https://doi.org/10.1016/j.ibiod.2016.10.038
Li J, Wachemo AC, Yuan H, Zuo X, Li X (2019) Natural freezing-thawing pretreatment of corn stalk for enhancing anaerobic digestion performance. Bioresour Technol 288:121518. https://doi.org/10.1016/j.biortech.2019.121518
Li K, Zhu H, Zhang Y, Zhang H (2017a) Characterization of the microbial communities in rumen fluid inoculated reactors for the biogas digestion of wheat straw. Sustainability 9:243. https://doi.org/10.3390/su9020243
Li MM, Ghimire S, Wenner BA, Kohn RA, Firkins JL, Gill B, Hanigan MD (2022) Effects of acetate, propionate, and pH on volatile fatty acid thermodynamics in continuous cultures of ruminal contents. J Dairy Sci 105:8879–8897. https://doi.org/10.3168/jds.2022-22084
Li P, He C, Li G, Ding P, Lan M, Gao Z, Jiao Y (2020a) Biological pretreatment of corn straw for enhancing degradation efficiency and biogas production. Bioengineered 11:251–260. https://doi.org/10.1080/21655979.2020.1733733
Li Y, Hua D, Haipeng X, Jin F, Hui M, Zhao Y, Fang X (2020b) Acidogenic and methanogenic properties of corn straw silage: regulation and microbial analysis of two-phase anaerobic digestion. Bioresour Technol 307:123180. https://doi.org/10.1016/j.biortech.2020.123180
Liang J, Fang W, Chang J, Zhang G, Ma W, Nabi M, Zubair M, Zhang R, Chen L, Huang J, Zhang P (2022) Long-term rumen microorganism fermentation of corn stover in vitro for volatile fatty acid production. Bioresour Technol 358:127447. https://doi.org/10.1016/j.biortech.2022.127447
Liang J, Fang W, Wang Q, Zubair M, Zhang G, Ma W, Cai Y, Zhang P (2021a) Metagenomic analysis of community, enzymes and metabolic pathways during corn straw fermentation with rumen microorganisms for volatile fatty acid production. Bioresour Technol 342:126004. https://doi.org/10.1016/j.biortech.2021.126004
Liang J, Zhang H, Zhang P, Zhang G, Cai Y, Wang Q, Zhou Z, Ding Y, Zubair M (2021b) Effect of substrate load on anaerobic fermentation of rice straw with rumen liquid as inoculum: hydrolysis and acidogenesis efficiency, enzymatic activities and rumen bacterial community structure. Waste Manag 124:235–243. https://doi.org/10.1016/j.wasman.2021.02.017
Liang J, Zheng W, Zhang H, Zhang P, Cai Y, Wang Q, Zhou Z, Ding Y (2021c) Transformation of bacterial community structure in rumen liquid anaerobic digestion of rice straw. Environ Pollut 269:116130. https://doi.org/10.1016/j.envpol.2020.116130
Liu C, Wu H, Liu S, Chai S, Meng Q, Zhou Z (2019) Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front Microbiol 10:1116. https://doi.org/10.3389/fmicb.2019.01116
Liu YS, Li S, Wang XF, Xing T, Li JL, Zhu XD, Zhang L, Gao F (2021) Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult Sci 100:273–282. https://doi.org/10.1016/j.psj.2020.09.089
Ma H, Liu H, Zhang L, Yang M, Fu B, Liu H (2017) Novel insight into the relationship between organic substrate composition and volatile fatty acids distribution in acidogenic co-fermentation. Biotechnol Biofuels 10:137. https://doi.org/10.1186/s13068-017-0821-1
Menke KH (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Animal Res Dev 28:7–55
Meyer G, Okudoh V, van Rensburg E (2022) A rumen based anaerobic digestion approach for lignocellulosic biomass using barley straw as feedstock. South African J Chem Eng 41:98–104. https://doi.org/10.1016/j.sajce.2022.05.005
Mizrahi I, Wallace RJ, Moraïs S (2021) The rumen microbiome: balancing food security and environmental impacts. Nat Rev Microbiol 19:553–566. https://doi.org/10.1038/s41579-021-00543-6
Moraïs S, Mizrahi I (2019) The road not taken: the rumen microbiome, functional groups, and community states. Trends Microbiol 27:538–549. https://doi.org/10.1016/j.tim.2018.12.011
Nguyen AQ, Nguyen LN, Johir MAH, Ngo H-H, Chaves AV, Nghiem LD (2020) Derivation of volatile fatty acid from crop residues digestion using a rumen membrane bioreactor: a feasibility study. Bioresour Technol 312:123571. https://doi.org/10.1016/j.biortech.2020.123571
Nguyen LN, Nguyen AQ, Johir MAH, Guo W, Ngo HH, Chaves AV, Nghiem LD (2019) Application of rumen and anaerobic sludge microbes for bio harvesting from lignocellulosic biomass. Chemosphere 228:702–708. https://doi.org/10.1016/j.chemosphere.2019.04.159
Njokweni SG, Weimer PJ, Botes M, Van Zyl WH (2021) Effects of preservation of rumen inoculum on volatile fatty acids production and the community dynamics during batch fermentation of fruit pomace. Bioresour Technol 321:124518. https://doi.org/10.1016/j.biortech.2020.124518
Ozbayram E, Ince O, Ince B, Harms H, Kleinsteuber S (2018) Comparison of rumen and manure microbiomes and implications for the inoculation of anaerobic digesters. Microorganisms 6:15. https://doi.org/10.3390/microorganisms6010015
Ozbayram EG, Kleinsteuber S, Nikolausz M (2020) Biotechnological utilization of animal gut microbiota for valorization of lignocellulosic biomass. Appl Microbiol Biotechnol 104:489–508. https://doi.org/10.1007/s00253-019-10239-w
Raposo F, Fernández-Cegrí V, De la Rubia MA, Borja R, Béline F, Cavinato C, Demirer G, Fernández B, Fernández-Polanco M, Frigon JC, Ganesh R, Kaparaju P, Koubova J, Méndez R, Menin G, Peene A, Scherer P, Torrijos M, Uellendahl H et al (2011) Biochemical methane potential (BMP) of solid organic substrates: evaluation of anaerobic biodegradability using data from an international interlaboratory study. J Chem Technol Biotechnol 86:1088–1098. https://doi.org/10.1002/jctb.2622
Stams AJM, Plugge CM (2009) Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat Rev Microbiol 7:568–577. https://doi.org/10.1038/nrmicro2166
Sun X, Atiyeh HK, Li M, Chen Y (2020) Biochar facilitated bioprocessing and biorefinery for productions of biofuel and chemicals: a review. Bioresour Technol 295:122252. https://doi.org/10.1016/j.biortech.2019.122252
Sundberg C, Yu D, Franke-Whittle I, Kauppi S, Smårs S, Insam H, Romantschuk M, Jönsson H (2013) Effects of pH and microbial composition on odour in food waste composting. Waste Manag 33:204–211. https://doi.org/10.1016/j.wasman.2012.09.017
Takizawa S, Asano R, Abe K, Fukuda Y, Baba Y, Sakurai R, Tada C, Nakai Y (2023) Relationship between rumen microbial composition and fibrolytic isozyme activity during the biodegradation of rice straw powder using rumen fluid. Microbes Environ 38:ME23041. https://doi.org/10.1264/jsme2.ME23041
Takizawa S, Baba Y, Tada C, Fukuda Y, Nakai Y (2018) Pretreatment with rumen fluid improves methane production in the anaerobic digestion of paper sludge. Waste Manag 78:379–384. https://doi.org/10.1016/j.wasman.2018.05.046
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Wang J, Guo X (2024) The Gompertz model and its applications in microbial growth and bioproduction kinetics: past, present and future. Biotechnol Adv 72:108335. https://doi.org/10.1016/j.biotechadv.2024.108335
Wang S, Zhang G, Zhang P, Ma X, Li F, Zhang H, Tao X, Ye J, Nabi M (2018) Rumen fluid fermentation for enhancement of hydrolysis and acidification of grass clipping. J Environ Manag 220:142–148. https://doi.org/10.1016/j.jenvman.2018.05.027
Wang Y, Zhang Y, Wang J, Meng L (2009) Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 33:848–853. https://doi.org/10.1016/j.biombioe.2009.01.007
Woiciechowski AL, Dalmas Neto CJ, de Souza P, Vandenberghe L, de Carvalho Neto DP, Novak Sydney AC, Letti LAJ, Karp SG, Zevallos Torres LA, Soccol CR (2020) Lignocellulosic biomass: acid and alkaline pretreatments and their effects on biomass recalcitrance – conventional processing and recent advances. Bioresour Technol 304:122848. https://doi.org/10.1016/j.biortech.2020.122848
Won M-Y, Oyama LB, Courtney SJ, Creevey CJ, Huws SA (2020) Can rumen bacteria communicate to each other? Microbiome 8:23. https://doi.org/10.1186/s40168-020-00796-y
Xing B-S, Cao S, Han Y, Wang XC, Wen J, Zhang K (2020) A comparative study of artificial cow and sheep rumen fermentation of corn straw and food waste: batch and continuous operation. Sci Total Environ 745:140731. https://doi.org/10.1016/j.scitotenv.2020.140731
Xu F, Mu L, Wang Y, Peng H, Tao J, Chen G (2024) Pretreatment with rumen fluid improves methane production in the anaerobic digestion of corn straw. Fuel 363:130831. https://doi.org/10.1016/j.fuel.2023.130831
Yin Y, Wang J (2021) Mechanisms of enhanced hydrogen production from sewage sludge by ferrous ion: insights into functional genes and metabolic pathways. Bioresour Technol 321:124435. https://doi.org/10.1016/j.biortech.2020.124435
Yue Z, Chen R, Yang F, MacLellan J, Marsh T, Liu Y, Liao W (2013) Effects of dairy manure and corn stover co-digestion on anaerobic microbes and corresponding digestion performance. Bioresour Technol 128:65–71. https://doi.org/10.1016/j.biortech.2012.10.115
Zhang H, Zhang P, Ye J, Wu Y, Fang W, Gou X, Zeng G (2016) Improvement of methane production from rice straw with rumen fluid pretreatment: a feasibility study. Int Biodeterior Biodegrad 113:9–16. https://doi.org/10.1016/j.ibiod.2016.03.022
Zhang Q, Liu H, Shui X, Li Y, Zhang Z (2022) Research progress of additives in photobiological hydrogen production system to enhance biohydrogen. Bioresour Technol 362:127787. https://doi.org/10.1016/j.biortech.2022.127787
Zhu R, Zhang Y, Zou H, Zheng Y, Guo R-B, Fu S-F (2022) Understanding the mechanisms behind enhanced anaerobic digestion of corn straw by humic acids. Bioresour Technol 359:127454. https://doi.org/10.1016/j.biortech.2022.127454
Funding
This work was supported by the National Key R&D Program of China (2021YFE0104600) and the Natural Science Foundation of Zhejiang Province (LY22E060002).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Qing Yu has performed the experiments and prepared the first draft of the manuscript. The experimental guidance and manuscript modification were performed by Weixing Cao and Chen Sun. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent for publication
All authors consent to publish this paper.
Consent to participate
All authors consented to participate in this work.
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Q., Sun, C., Cao, W. et al. Rumen fluid pretreatment promotes anaerobic methane production: revealing microbial dynamics driving increased acid yield from different concentrations of corn straw. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-33615-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-024-33615-0