Abstract
Detergents are highly produced pollutants with environmental problems like foam generation and toxic effects in biota. Nonylphenol ethoxylates (NPEs) are efficient, economical, and versatile surfactants, used in detergents for more than 40 years due to their detergency capacity. In the environment, NPE biodegrades into the metabolite nonylphenol (NP), classified as an endocrine disruptor. The identification and quantification of 4-NP in a designed detergent and 30 commercially available detergents were performed to prove the degradation of NPE into 4-NP during storage time. This investigation introduces the first evidence of NPE degradation during storage in commercially available detergents, demonstrating a novel exposure pathway in humans that has not been explored before, representing potential human health risks. Therefore, simple, easy, low-cost, and available approaches to remove and substitute NP is paramount. Alkyl polyglucoside (APG) was assessed as a substitute, and the feasibility of this substitution was proven according to physical and chemical properties, cleaning performance, and antimicrobial properties. NPE substitution in detergents is demonstrated as a viable strategy to minimize exposure risks in humans and the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alkylphenol ethoxylates (APEs) are non-ionic surfactants widely used in detergents, plastic additives, emulsifiers, and pesticide manufacturing (Najim and Radeef 2023). Therefore, most of APEs are removed from wastewater with conventional treatment plants (Grześkowiak et al. 2021); however, APEs are one of the most reported surfactants in wastewaters, effluents, rivers, drinking water, sediments, and soil (Belmont et al. 2006; Chen et al. 2013; Dong et al. 2015; Jie et al. 2017; Van Zijl et al. 2017). Nonylphenol ethoxylate (NPE) is an efficient, low-cost, and versatile surfactant. It has been used in detergents for more than 40 years due to its ability to form micelles in solution and detergency capacity (Araujo et al. 2018; Yu et al. 2008). In some cases, domestic and industrial wastes are discharged without previous treatment, affecting significantly water quality (Bilal et al. 2019). Therefore, NP is one of the most frequently identified organic pollutants in wastewater, rivers, drinking water, sediments, and soil (Belmont et al. 2006; Dong et al. 2015; Van Zijl et al. 2017). After its disposal, NPE is degraded by microorganisms or ultraviolet light in environmental conditions, transforming into nonylphenol (NP) (Cheng et al. 2017). NP and intermediate biodegradation products are known for their estrogenic properties (Bhandari et al. 2021). NP mimics the female hormone 17β-estradiol, causing adverse health effects on organisms and humans; therefore, it is classified as an endocrine disruptor (Hong et al. 2020). Previous studies have shown adverse effects on humans, like decreased sperm count, reproductive malformations, immune deficiency, and an increment in prostate, breast, ovarian, and testicular cancer. Moreover, effects on children, fetuses, and newborns include neurological effects and poor intellect development (Kim et al. 2020; Lussier et al. 2000; Prasad et al. 2023; Scaia et al. 2019; Shirdel et al. 2020; Tabassum et al. 2017), and concentrations have been found in rivers (Lalonde and Garron 2021, Pakzad Toochaei and Kazemi 2022, Zhao et al. 2024), lakes (Liu et al. 2020), sediments (Crane 2021, Komaki and Riyahi Bakhtiari 2020), and sewage (Ryu et al. 2024; Sá et al. 2022). Consequently, regulations to restrict the use of NPE have been implemented. The European Union (EU), through Directive 2003/53/EC, establishes that NPE “…may not be placed on the market or used as a substance or constituent of preparations in concentrations equal or higher than 0,1% by mass…” (Directive 2003), and Directive 775/2004(02/2076) prohibits the use of NPE in pesticides formulations (Commission Regulation 2004). Also, the Environmental Protection Agency (EPA) from the United States added NPE to the Toxics Release Inventory (TRI) list. Also, the NPE meets the toxicity listing criteria of the EPA’s Emergency Planning and Community Right-to-know Act (EPCRA) Section. 313(d)(2)(C), indicating that the NPE is highly toxic to aquatic organisms (EPA 2005). Likewise, Canada enlists NP as a toxic substance in the Canadian Environmental Protection Act (Act and Pentafluoroethane 1999), and the Rotterdam Convention classifies it as a severely restricted compound. However, there are still countries without regulations, like Latin American countries (Vargas-Berrones et al. 2020a). Due to the occurrence and potential exposure risks, the substitution of NPE in detergents as a development of sustainable technology is proposed. Usually, NPE is substituted with alcohol ethoxylates, less effective surfactants with side effects like eye irritation, skin irritation and sensitization, genetic toxicity, oral and dermal acute toxicity, and oral repeat-dose toxicity (Li et al. 2018a, Li et al. 2021, Maurer and Kung 2020, Yu et al. 2008). Therefore, natural surfactants from renewable sources like fatty acid esters, amino acid amides, lecithin, and various sugar types have been introduced. Sugar-based surfactants are of primary interest because of their cleaning performance, consumers’ health, and environmental compatibility (Deleu and Paquot 2004, Uzwatania et al. 2017, Holmberg 2001). Alkyl polyglucosides (APG) are sugar-based surfactants with synergic effects, low irritation potential, full biodegradation, and non-toxic (Wasilewski et al. 2016; Yu et al. 2008). More evidence of sustainable alternatives is needed, mainly in developing countries, where higher concentrations in water bodies and health effects of exposure have been found (Vargas-Berrones et al. 2020a). There is a major concern about the future and transport of NP through the environment and humans because of its continuous detection and identification in water sources. In addition, due to the lack of regulations in developing countries, most commercially available detergents have NPE in their formula. This study suggests NPE degradation during the shelf time of detergents, introducing a new exposure pathway with associated potential health risks. Thus, the aim of this study is to investigate the NPEs’ degradation of detergents during shelf time and develop a detergent using a natural surfactant (APG) to assess its possible substitution as a strategy to minimize exposure risks in humans and the environment (Fig. 1).
Materials and methods
Preparation of detergents
Two liquid detergents using NPE and APG, respectively (13%), were designed following the recommendations of ISO 6330:2012. Ten percent of fatty alcohol was added as a complementary surfactant. A builder (5%) was used for water softening and as a preservative to improve detergent performance (Lim et al. 2019). Enzymes (0.1%) were included to reduce the use of large quantities of chemicals and to improve cleaning capacity (Hasan et al. 2010). Finally, sodium hydroxide (0.9%) was used as an alkali to maintain pH and favor the cleaning processes (Chiplunkar et al. 2017). Solutions were stirred for 30 min at room temperature. On the other hand, thirty commercially available detergents were purchased in Mexico for further analysis.
Degradability in detergents
The biological oxygen demand (BOD) represents the oxygen consumed by the bacteria and microorganisms while the organic matter is decomposed under aerobic conditions. Therefore, NPE degradability in the designed detergents is determined by measuring the consumed oxygen following the International Standard of the Organization for Economic Cooperation and Development (OECD) 301D Closed Bottled Test. BOD measures were taken on days 0, 7, 14, 21, and 28 using a dissolved oxygen meter (MeterTo, model JPB-607 A). The flasks were previously prepared with 4% of the designed detergents and 1% of inoculum provided by the Electrolytic Zinc Refinery, Grupo México. Duplicate control blanks were prepared to eliminate any potential bias or matrix effect. The chemical oxygen demand (COD) was calculated following the closed reflux method. The experiment was performed in triplicate to assure reproducibility; averages are reported. NPE degradation was confirmed by identifying and quantifying 4-NP with gas chromatography–mass spectrometry (GC–MS) following the method described previously by our research team. The linear range (r2 = 0.99) of the calibration curve was from 0.5 to 50 µg L−1 and was determined from the average of five curves obtained for 3 days. Repeatability and reproducibility of the method were determined by evaluating different concentrations in triplicate on the same day and in duplicate on five different days. Blanks and calibration check samples were analyzed as quality and assurance control (QA/QC). The obtained values for detection (LOD) and quantification (LOQ) were 0.01 µg L−1 and 0.15 µg L−1, respectively. Complete data can be consulted in the paper by our research group (Vargas-Berrones et al. 2020b). Also, directed monitoring of 30 samples of commercially available detergents (manufactured and sold in Mexico) was performed to detect NP after shelf time.
Detergent evaluation
The evaluation of the designed detergents was divided into three: physicochemical properties, detergency tests, and antibacterial activity assessment. Every determination was performed in triplicate to assure reproducibility; averages are reported.
Physicochemical properties
The following physicochemical properties were determined: surface tension, critical micellar concentration (CMC), and viscosity. Surface tension was determined following the Spanish Standard UNE 55–501-90 (Española 1990) using a test temperature of 20 °C and a tensiometer with a 4 cm diameter platinum foil (MGW-LAUDA Hucoa-Erlôss). Accordingly, CMC was calculated by measuring the surface tension of a concentration series (5%, 20%, 50%, and 100%); the surface tension changes strongly just before reaching the CMC. Viscosity was determined following the ASTM D-445–06 (Standard ASTM 2006) using a calibrated viscosimeter, a bath at a controlled temperature (± 0.02 °C), a glass thermometer accurately calibrated (± 0.02 °C), and a chronometer that allows lectures with 0.1 s of discrimination.
Detergency tests
Regarding the detergency tests, the foaming power was determined following the Spanish Standard UNE-EN 14371 (UNE Normalización Española 2005). A graduated test tube and a gear pump with an outlet flow of 0 L h−1 up to 280 L h−1, which allows flow adjustment of 200 L h−1, were used. The height of the foam produced in a 1 g L−1 solution at 50 ± 2 °C was determined at 30 s, 3 min, and 5 min. The wetting power by immersion was performed according to ISO 8022:1990 using a cotton woven disc (DIN 53901). The cotton discs were placed in solutions of a concentration series (2, 3, 4, and 5 g L−1) to establish the wetting time (time that it takes to immerse). The cleaning performance (decontamination rate) was calculated following the ISO 6330:2012 using a front-loading horizontal rotating drum-type machine (Type A) and polyester fabric squares of 20 ± 4 cm by 20 ± 4 cm with a mass per area of 310 ± 20 g cm−3, and individual weight of 50 ± 5 g (purchased from the Center for Test Materials B.V.). The decontamination rate was determined by the reflectance lecture with a photocolorimeter (GretagMacbeth-Color i5) before and after washing the test cloth materials with the test detergents (8 g L−1).
Antibacterial activity assessment
The minimum inhibitory concentration (MIC) was determined to establish the antibacterial activity. MIC was measured following the turbidimetry methodology (ISO 20776–1) using sterile phosphate-buffered saline (PBS) as a liquid culture medium. Four different strains were evaluated to have a wide range of test organisms: (i) Staphylococcus epidermis, (ii) Escherichia coli, (iii) Candida sp., and (iv) Pseudomonas aeruginosa grown in agar coliform at 37 °C for 24 h. Results were obtained with a microplate reader Infinite M200, Tecan Software Magellan®.
Results and discussion
NPE degradability in-storage detergents
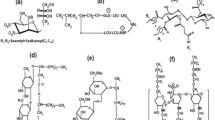
Table 1 shows the dissolved oxygen values and the chemical oxygen demand from the designed detergents stored at normal shelf conditions and demonstrates the decomposition of organic matter. Previous studies have proved that APE metabolites degrade more easily under aerobic conditions than under anaerobic conditions (Arora et al. 2023, Haq and Raj 2019, Khalil and Liu 2021). NPE initial biodegradability occurs rapidly by losing surfactant properties (Quiroga et al. 2019). Chemical and environmental factors like agitation, temperature, and the addition of yeast extracts affect and influence NPE biodegradation. Also, previous studies have demonstrated that aluminum sulfate, hydrogen peroxide, Pb, Cd, Cu, Zn, phthalic esters, and NaCl inhibit NP aerobic degradation (Mao et al. 2012). NP half-live in sewage, sludge, and sediments under aerobic conditions has been established from 1.1 to 99 days (Chang et al. 2005, 2008; Yuan et al. 2004), while under anaerobic conditions is from 23.9 to 69.3 (Chang et al. 2005). Aerobic degradation starts with the shortening of the EO chain and is completed with the diethoxylation (AP formation). The EO chain oxidation forms alkyl phenoxy ethoxy acetic acid and alkyl phenoxy acetic acid (Fig. 2). The three more reported intermediaries are APs (NP and OP), short-chain APEs with EO units (1 to 4), mainly APE 2, and a series of ether carboxylates including alkyl phenoxy acetic acid and alkylphenoxy ethoxy acetic acid (Bhandari et al. 2021; Ying et al. 2002). Previous studies have shown that even if 4-n-NP is persistent in the environment, it is readily biodegradable during activated sludge processes (Stasinakis et al. 2008). For example, 4-n-NP sorption on suspended solids was achieved with higher values of sorption coefficient at a sludge retention time (SRT) of 3 days, and the biodegradation was enhanced at SRT of 20 days (Stasinakis et al. 2010). Moreover, different methods like adsorption and degradation with fungi, bacteria, microalgae, and yeasts have been tested to remove NP. These organisms can use NP as a carbon and energy source for their growth and development (Bhandari et al. 2021). For sustainable bioremediation, a microbial approach is established as the most efficient, low-cost, and sustainable because physicochemical techniques present several drawbacks like high cost, toxic by-products, and the use of large quantities of solvents (Bilal et al. 2019; Różalska et al. 2015). However, conventional water treatment plants are ineffective for NP degradation (Lara-Moreno et al. 2022).
Estimated aerobic NPE degradation route (Ying et al. 2002). Ying G-G, Williams B, Kookana R (2002): Environmental fate of alkylphenols and alkylphenol ethoxylates—a review. Environment International 28, 215–226
The degradation of NPE in the designed detergents under controlled conditions is proven by identifying and quantifying 4-NP using GC–MS. Concentrations of 4-NP in the test detergents increased throughout time, reaching 5 µg L−1 after 28 days (peak at 11.2 min) (Fig. 3); the other peaks observed may correspond to NP isomers (Lu and Gan 2014). Also, Table 2 shows that 36% of the commercially available detergents presented significant concentrations of 4-NP (from 0.87 to 134.80 µg L−1), suggesting high levels of this pollutant when released into the environment. Therefore, concentrations of 4-NP above the Water Framework Directive of the European Union (2 µg L−1) (European Union 2013) and the EPA recommendation (6.6 µg L−1 for surface water and 1.7 µg L−1 for saltwater) (EPA 2005) are expected. It is critical to acknowledge that concentrations of 4-NP were found in the commercially available detergents that include non-ionic surfactants in their ingredients list, while 4-NP was not found in detergents with anionic and amphoteric surfactants as ingredients. Even when the labeling does not openly include NPE as an ingredient, NPE has been recognized as the predominant APE and the most used for over five decades due to their efficiency and cost-effectiveness in detergent formulation (Jawaid et al. 2024). Currently, detergents are being designed using mostly anionic surfactants, although there is no regulatory framework on the use of NPE in detergents in developing countries (i.e., Mexico). This behavior could be due to the increasing trend of environmental and social responsibility to minimize endocrine disruptor occurrence. Therefore, cost-effective, high-performance, and environmentally friendly alternative development are paramount for the prompt implementation by industries.
The following limitations have been considered when interpreting the results of this study: (i) the concentration of 4-NP increases over time until day 28 as the standard establishes; thus, it is crucial to analyze if there is still an increment in the 4-NP concentration throughout time considering that shelf life of a detergent is of 12 to 24 months (Kübelbeck et al. 2018; Ramnani et al. 2005; Riedler et al. 2003); (ii) 4-NP was the only isomer considered in this study because it is the most toxic and reported (Calafat et al. 2005; Lara-Moreno et al. 2022); however, NP is a mixture of approximately 20 isomers with intermediaries structures so, the identification of other isomers is needed. Also, each isomer presents a different degradation range, and only a few persist after 45 days (Li et al. 2018b; Metcalfe et al. 2022; Zhang et al. 2023); (iii) NPE biodegradability in detergents during shelf time was proved applying the OECD protocol; however, NPE degradation was not calculated. Future research is suggested to establish biodegradability percentages according to common commercial detergent formulations associated to environmental and health implications.
NP has been widely reported in water samples (Babay et al. 2008; Belmont et al. 2006; Bina et al. 2018; Félix–Cañedo et al. 2013; Jardim et al. 2012; Martín et al. 2014; Martinez and Peñuela 2013; Montagner 2011; Vargas-Berrones et al. 2020a); nonetheless, no information about NP in detergents was found when the literature review was carried out; only an investigation by Cheng and Ding (2002) reported NPEOs in detergents with concentrations from 0.2 to 21% in 41% of 90 household detergents analyzed. The presence of NP in detergents may represent potential risk effects for human health, mainly through dermal contact. Previous studies have demonstrated NP dermal absorption in humans and animals with effects like skin irritation (Talmage 2020); however, exposure through detergents has not been studied before because, to date, exposure through food and water intake has been the main and only route analyzed (Chang et al. 2019; Osimitz et al. 2015; Raecker et al. 2011).
Detergent performance
Some of the following results have been previously shared in a preprint (Bernal-Jácome et al. 2023). The detergent with APG (DAPG) showed better results regarding surface tension and CMC. The DAPG presented lower surface tension (25% lower than the detergent with NPE); therefore, the CMC of DAPG was achieved using only 20% of the surfactant in the solution, while the detergent with NPE (DNPE) presented this point at 50%. Previous studies have demonstrated that lower surface tension values benefit soil removal (Mousavi and Khodadoost 2019). Also, lower CMC implies that less detergent is needed to achieve the maximum micelle formation, improving cleaning processes and minimizing economic and environmental costs (Singh et al. 2018). These results are similar to previous studies where APG significantly reduces surface tension and CMC in detergents, allowing better penetration of the detergent in textiles and achieving better soil removal (Karthick et al. 2018; Pukale et al. 2017). On the other hand, the viscosity of the detergent with NPE (DNPE) and with APG is 445.74 and 20.38 mpoise, respectively, meaning that DAPG presents a viscosity 22 times lower than DNPE. Detergents with high viscosity hinder production processes and kinetically limit water solubility (Calero et al. 2017); hence, DAPG offers a cleaner and more efficient production.
DAPG presents higher foam formation (550 ml of height in 5 min) than DNPE (153 ml of height in 5 min). This behavior is expected because compounds with lower surface tension usually favor foam generation due to the energy decrease of the molecular interface (Chiplunkar et al. 2017). However, previous studies have shown that APG foam height decreases as the alkyl chain length increases, and increases depending on the concentration in the solution. Therefore, APG could be selected according to the desired foam stability (Chiplunkar et al. 2017; El-Sukkary et al. 2008). In addition, natural antifoaming agents may be used in the production processes to control foam generation (Schilling and Zessner 2011). Unstable and poor foam formation is desirable because foam blocks the oxygen-mass transformation from air to water, affecting organisms (Effendi et al. 2017).
The wetting time of both detergents decreased as the concentration increased. The wetting power of DNPE at 5 g L−1 was 37 s, and the DAPG was 163 s. These values represent 4.4 times higher wetting power for the detergent with NPE related to soil removal efficiency (Moulay et al. 2005), assessed through the decontamination rate test; results are shown in Table 3. Several stains, like grass, rice starch, pigment, oil, wine, cocoa, and others, present similar decontamination rates with both detergents, though each detergent works better in different stains; however, DAPG presents a better decontamination rate in 64% of the samples tested. APG’s capability as a surfactant was confirmed; nonetheless, proportions in formulations need to be considered and assessed accordingly to their use. These results are similar to previous studies where APG in detergent formulations shows a better decontamination rate in different fabric types (Karthick et al. 2018; Pukale et al. 2017). Other natural surfactant assessment is recommended; for example, methyl ester sulfonate (MES) has proven stable in hard water and meets cleaning standards (Tai et al. 2018).
Table 4 presents MIC results for each detergent considering the four strains evaluated. Values show that microbial growth has been inhibited since time cero in the four strains studied. However, it is crucial to consider lower concentrations (< 10%) to establish the minimum concentrations in which the detergent presents antimicrobial properties. These results are similar to others previously reported in which APG demonstrates appropriate antimicrobial properties without toxicity problems (Boonprakobsak and Rachtanapun 2022, Smułek et al. 2021).
To our knowledge, there are no studies regarding 4-NP identification and quantification in detergents and the formulations of APG detergent as a direct substitute for NPE. This study introduces the first evidence that NPE degrades under normal storage conditions in commercial detergents. Also, the first comparison between NPE and APG as a surfactant in detergents is presented. The feasibility of APG as a sustainable substitute for an emerging pollutant and prohibited compound in several countries (NPE) was demonstrated. The quantity of non-ionic surfactants used in detergent industries is not explicit; however, previous studies have found concentrations of NPE in detergents from 0.2 to 21% (Huang et al. 2014). The NPE market is estimated to be worth $ 928.89 million by 2027, registering a growth rate of 7.3% from 2020 to 2027 (Research DBM 2020), and the growing demand for NPE in the detergent, fertilizer, and paint industries will continue to increase. Global cleaning product market growth will achieve a rate of $ 59.5 billion by 2025 with an annual growth rate of 4.8% (Markets and Markets 2023). Thus, the environmental risks associated are hard to predict; the countries without regulations (Latin American countries) are the most likely to be affected. The chemical industry represents one of the most important economic sectors worldwide, and the introduction of new technologies is constantly increasing (Pearce and Tombs 2019). Accelerated industrialization and economic development have caused exponential population and urbanization growth (Chen 2018; Zafra et al. 2015). This situation has led to higher consumption of natural resources and environmental pollution (Li &Yu 2011; Nations 2015; Zhang et al. 2015), for example, detergent consumption. In 2017, more than sixty billion dollars were used for detergent production (Giagnorio et al. 2017), and the surfactant market represented thirty-three million dollars in 2016 (Karray et al. 2016). Even though these potential risks and adverse effects in the industry are well known, it is still a challenge to persuade the industries to replace conventional surfactants due to the costs that this may represent (up to 50 times higher than synthetic surfactants) (Farias et al. 2021).
Conclusions
Water quality and availability have deteriorated due to anthropogenic activities, population growth, industrialization, and urbanization. Among the pollutants, detergents represent a critical source of pollution with associated health and environmental risks. Therefore, efforts in investigation and strategies to obtain a responsible approach to the use and handling of NP and its ethoxylate in detergents are required to promote consciousness of the impact of NP as a pollutant. This study proved the degradation of NPE and formation of 4-NP in detergents during shelf time, presenting an exposure pathway that has not been explored. Concentrations of 4-NP up to 5 µg L−1 were found after 28 days in the designed detergents, and 36% of the commercially available detergents tested presented concentrations above the European Union and the EPA regulatory framework. Thus, a detergent was developed using APG to substitute NPE. The detergent with APG presented lower surface tension and CMC, showing a better penetration of the detergent in the fabrics tested. Also, better detergent capacity in 64% of the stains tested and effective inhibition of bacterial growth were demonstrated. Overall, results show that APG is an adequate substitute for NPE. This substitution allows cleaner and more efficient production, resulting in lower economic and environmental costs. Countries without regulation in this regard are reluctant due to the monetary costs that the substitution of NPE represents; however, the environmental cost associated has not been balanced with the ecological and human risks. The green technology developed proved the multiple benefits that natural surfactants offer in terms of availability, performance, and environmental health. It is paramount to promote awareness of the negative impacts of endocrine disrupters. Also, environmental regulations and continuous monitoring are recommended to address the contamination in ecological receptors that could develop into significant effects on human health. The results of this study provide new and valuable information to establish a basis for further studies and for industries to improve and implement greener detergent production.
Data availability
Data available on request from the authors.
References
Act CEP, Pentafluoroethane CAS (1999) Canadian Environmental Protection Act. Erişim adresi: https://laws-lois.justice.gc.ca/eng/acts/c-15.31/FullText.html
Araujo FG, Bauerfeldt GF, Cid YP (2018) Nonylphenol: properties, legislation, toxicity and determination. Anais Da Academia Brasileira De Ciencias 90:1903–1918
Arora U, Khuntia H, Chanakya H, Kapley A (2023) Surfactants: combating the fate, impact, and aftermath of their release in the environment. Int J Environ Sci Technol 20:11551–11574
Babay PA, Romero Ale EE, Itria RF, Becquart ET, Thiele B, Batistoni DA (2008) Simplified determination of lipophilic metabolites of nonylphenol ethoxylates: method development and application in aqueous samples from Buenos Aires, Argentina. J Environ Monit 10:443–452
Belmont MA, Ikonomou M, Metcalfe CD (2006) Presence of nonylphenol ethoxylate surfactants in a watershed in central Mexico and removal from domestic sewage in a treatment wetland. Environ Toxicol Chem 25:29–35
Bernal-Jácome LA, Izar-Landeta JM, Flores-Ramirez R, i Farreras JM, Vargas-Berrones KX (2023) Sustainable development and a performance assessment of Alkyl Polyglucoside as a substitute for Nonylphenol Ethoxylates in detergents
Bhandari G, Bagheri AR, Bhatt P, Bilal M (2021) Occurrence, potential ecological risks, and degradation of endocrine disrupter, nonylphenol, from the aqueous environment. Chemosphere 275:130013
Bilal M, Adeel M, Rasheed T, Zhao Y, Iqbal HMN (2019) Emerging contaminants of high concern and their enzyme-assisted biodegradation – a review. Environ Int 124:336–353
Bina B, Mohammadi F, Amin MM, Pourzamani HR, Yavari Z (2018) Determination of 4-nonylphenol and 4-tert-octylphenol compounds in various types of wastewater and their removal rates in different treatment processes in nine wastewater treatment plants of Iran. Chin J Chem Eng 26:183–190
Boonprakobsak P, Rachtanapun C (2022) Antimicrobial activity of alternative surfactants on foodborne pathogenic bacteria. J Food Sci Agric Technol (JFAT) 6:99–103
Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL (2005) Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113:391–395
Calero N, Santos J, Muñoz J (2017) Methodology to estimate the yield stress applied to ultraconcentrated detergents as model systems. Chem Eng Sci 166:115–121
Chang BV, Chiang F, Yuan SY (2005) Biodegradation of nonylphenol in sewage sludge. Chemosphere 60:1652–1659
Chang BV, Liu CL, Yuan SY, Cheng CY, Ding WH (2008) Biodegradation of nonylphenol in mangrove sediment. Int Biodeterior Biodegradation 61:325–330
Chang W-H, Liu S-C, Chen H-L, Lee C-C (2019) Dietary intake of 4-nonylphenol and bisphenol A in Taiwanese population: integrated risk assessment based on probabilistic and sensitive approach. Environ Pollut 244:143–152
Chen Y-C (2018) Evaluating greenhouse gas emissions and energy recovery from municipal and industrial solid waste using waste-to-energy technology. J Clean Prod 192:262–269
Chen HW, Liang CH, Wu ZM, Chang EE, Lin TF, Chiang PC, Wang GS (2013) Occurrence and assessment of treatment efficiency of nonylphenol, octylphenol and bisphenol-A in drinking water in Taiwan. Sci Total Environ 449:20–28
Cheng C-Y, Ding W-H (2002) Determination of nonylphenol polyethoxylates in household detergents by high-performance liquid chromatography. J Chromatogr A 968:143–150
Cheng Y, Shan Z, Zhou J, Bu Y, Li P, Lu S (2017) Effects of 4-nonylphenol in drinking water on the reproductive capacity of Japanese quails (Coturnix japonica). Chemosphere 175:219–227
Chiplunkar PP, Shinde VV, Pratap AP (2017) Synthesis and application of palm fatty acid distillate based alkyd resin in liquid detergent. J Surfactants Deterg 20:137–149
Commission Regulation (EC) (2004) No 775/2004 of 26 April 2004 amending Annex I to regulation (EC) No 304/2003 of the European parliament and of the council concerning the export and import of dangerous chemicals (Text with EEA relevance). Official J L 123:0027–0030
Crane JL (2021) Distribution and toxic potential of alkylphenols, nonylphenol ethoxylates, and pyrethroids in Minnesota, USA lake sediments. Sci Total Environ 776:145974
Deleu M, Paquot M (2004) From renewable vegetables resources to microorganisms: new trends in surfactants. C R Chim 7:641–646
Directive EC (2003) Directive 2003/53/EC of the European Parliament and of the Council of 18 June 2003 amending for the 26th time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (nonylphenol, nonylphenol ethoxylate and cement). Official J Eur Commun L 178:24–27
Dong CD, Chen CW, Chen CF (2015) Seasonal and spatial distribution of 4-nonylphenol and 4-tert-octylphenol in the sediment of Kaohsiung Harbor Taiwan. Chemosphere 134:588–597
Effendi I, Nedi S, Pakpahan R (2017) Detergent disposal into our environment and its impact on marine microbes. In IOP conference series: Earth and environmental science (Vol. 97, No. 1, p. 012030). IOP Publishing
El-Sukkary MMA, Syed NA, Aiad I, El-Azab WIM (2008) Synthesis and characterization of some alkyl polyglycosides surfactants. J Surfactants Deterg 11:129–137
EPA U (2005) Aquatic life ambient water quality criteria—nonylphenol, vol. 5. EPA-822-R-05. Addition of NPEs Category to TRI List Final Rule | US EPA. Federal Register/Vol. 83, No. 113/Tuesday, June 12, 2018/Rules and Regulations 27291
Española UN (1990) Agentes de superficie. Determinación de la tensión superficial por estirado de una película líquida.
EU, (2013) Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off JEur Communities L226(1):1–17
Farias CBB, Almeida FC, Silva IA, Souza TC, Meira HM, Rita de Cássia F, Luna JM, Santos VA, Converti A, Banat IM (2021) Production of green surfactants: market prospects. Electron J Biotechnol 51:28–39
Félix-Cañedo TE, Durán-Álvarez JC, Jiménez-Cisneros B (2013) The occurrence and distribution of a group of organic micropollutants in Mexico City’s water sources. Sci Total Environ 454–455:109–118
Giagnorio M, Amelio A, Grüttner H, Tiraferri A (2017) Environmental impacts of detergents and benefits of their recovery in the laundering industry. J Clean Prod 154:593–601
Grześkowiak T, Szymański A, Zgoła-Grześkoviak A, Beata C-G, Frankowskil R (2021) Alkylphenols and alkylphenol ethcpwlates their impact on living organisms, biodegradation, and environmental pollution, Biodegradation. Pollutants and Bioremediation Principles. CRC Press, pp 1–32
Hasan F, Shah AA, Javed S, Hameed A (2010) Enzymes used in detergents: lipases. African J Biotechnol 9(31):4836–4844
Haq I, Raj A (2019) Endocrine-disrupting pollutants in industrial wastewater and their degradation and detoxification approaches. Emerging and eco friendly approaches for waste management, Singapore, pp 121–142
Holmberg K (2001) Natural surfactants. Curr Opin Colloid Interface Sci 6:148–159
Hong Y, Feng C, Yan Z, Wang Y, Liu D, Liao W, Bai Y (2020) Nonylphenol occurrence, distribution, toxicity and analytical methods in freshwater. Environ Chem Lett 18:2095–2106
Huang Y-F, Wang P-W, Huang L-W, Yang W, Yu C-J, Yang S-H, Chiu H-H, Chen M-L (2014) Nonylphenol in pregnant women and their matching fetuses: placental transfer and potential risks of infants. Environ Res 134:143–148
Jardim WF, Montagner CC, Pescara IC, Umbuzeiro GA, Di Dea Bergamasco AM, Eldridge ML, Sodré FF (2012) An integrated approach to evaluate emerging contaminants in drinking water. Sep Purif Technol 84:3–8
Jawaid J, Hussain A, Khan AM (2024) Exploring the effects of interactions between nonylphenol ethoxylate and ionic surfactants on interfacial and foaming properties. J Mol Liq 394:123673
Jie Y, Jie Z, Ya L, Xuesong Y, Jing Y, Yu Y, Jiaqi Y, Jie X (2017) Pollution by nonylphenol in river, tap water, and aquatic in an acid rain-plagued city in southwest China. Int J Environ Health Res 27:179–190
Karray F, Mezghani M, Mhiri N, Djelassi B, Sayadi S (2016) Scale-down studies of membrane bioreactor degrading anionic surfactants wastewater: isolation of new anionic-surfactant degrading bacteria. Int Biodeterior Biodegradation 114:14–23
Karthick RA, Jangir K, Chattopadhyay P (2018) Foaming and cleaning performance comparison of liquid detergent formulations using mixtures of anionic and nonionic surfactants. Tenside, Surfactants, Deterg 55:162–168
Khalil M, Liu Y (2021) Greywater biodegradability and biological treatment technologies: a critical review. Int Biodeterior Biodegradation 161:105211
Kim YB, Cheon YP, Choi D, Lee SH (2020) Histological analysis of reproductive system in low-dose nonylphenol-treated F1 female mice. Dev Reprod 24:159–165
Komaki NA, Riyahi Bakhtiari A (2020) Evaluation of the concentration of 4-nonylphenol and octylphenol estrogen-like compounds in surface sediments of the South and Southeast Rivers of the Caspian Sea in Mazandaran Province. J Water and Wastewater Ab va Fazilab (in persian) 31:76–87
Kübelbeck S, Mikhael J, Keller H, Konradi R, Andrieu-Brunsen A, Baier G (2018) Enzyme–polymer conjugates to enhance enzyme shelf life in a liquid detergent formulation. Macromol Biosci 18:1800095
Lalonde B, Garron C (2021) Nonylphenol, octylphenol, and nonylphenol ethoxylates dissemination in the Canadian freshwater environment. Arch Environ Contam Toxicol 80:319–330
Lara-Moreno A, Aguilar-Romero I, Rubio-Bellido M, Madrid F, Villaverde J, Santos JL, Alonso E, Morillo E (2022) Novel nonylphenol-degrading bacterial strains isolated from sewage sludge: application in bioremediation of sludge. Sci Total Environ 847:157647
Li W-W, Yu H-Q (2011) From wastewater to bioenergy and biochemicals via two-stage bioconversion processes: a future paradigm. Biotechnol Adv 29:972–982
Li B-x, Pang X-y, Zhang P, Lin J, Li X-x, Liu Y, Li H, Liu F, Mu W (2018a) Alcohol ethoxylates significantly synergize pesticides than alkylphenol ethoxylates considering bioactivity against three pests and joint toxicity to Daphnia magna. Sci Total Environ 644:1452–1459
Li C, Jin F, Snyder SA (2018b) Recent advancements and future trends in analysis of nonylphenol ethoxylates and their degradation product nonylphenol in food and environment. TrAC Trends Anal Chem 107:78–90
Li J, Wu Y, Bai H, Wen X, Zhou Q, Yuan Y, Liu Y, Chen C, Guo L (2021) Highly efficient adsorption and mechanism of alkylphenols on magnetic reduced graphene oxide. Chemosphere 283:131232
Lim YS, Baharudin NB, Ung YW (2019) Methyl ester sulfonate: a high-performance surfactant capable of reducing builders dosage in detergents. J Surfactants Deterg 22:549–558
Liu R, Luo X, Shu S, Ding J, Zhang G, Wang Z, Zou H, Zhang Y (2020) Impact of rainfall on the occurrence, spatiotemporal distribution, and partition trend of micropollutants in Taihu Lake, China: bisphenol A and 4-nonylphenol as examples. Ecotoxicol Environ Saf 204:111064
Lu Z, Gan J (2014) Analysis, toxicity, occurrence and biodegradation of nonylphenol isomers: a review. Environ Int 73:334–345
Lussier SM, Champlin D, LiVolsi J, Poucher S, Pruell RJ (2000) Acute toxicity of para-nonylphenol to saltwater animals. Environ Toxicol Chem 19:617–621
Mao Z, Zheng X-F, Zhang Y-Q, Tao X-X, Li Y, Wang W (2012) Occurrence and biodegradation of nonylphenol in the environment. Int J Mol Sci 13:491–505
Markets and Markets (2023) Industrial cleaning market by ingredient type (surfactant, solvent, chelating agent), product type (general and metal cleaners), application (manufacturing & commercial offices, healthcare, retail & foodservice), Region - global forecast to 2028. https://www.marketsandmarkets.com/Market-Reports/industrial-institutional-cleaning-chemicals-market-52902227.html
Martín J, Camacho-Muñoz D, Santos JL, Aparicio I, Alonso E (2014) Determination of emerging and priority industrial pollutants in surface water and wastewater by liquid chromatography–negative electrospray ionization tandem mass spectrometry. Anal Bioanal Chem 406:3709–3716
Martinez M, Peñuela GA (2013) Analysis of triclosan and 4n-nonylphenol in Colombian reservoir water by gas chromatography-mass spectrometry. Water Environ J 27:387–395
Maurer LL, Kung MH (2020) Mammalian toxicity testing of semilinear and branched alcohol ethoxylates. J Surfactants Deterg 23:921–935
Metcalfe C, Bayen S, Desrosiers M, Muñoz G, Sauvé S, Yargeau V (2022) An introduction to the sources, fate, occurrence and effects of endocrine disrupting chemicals released into the environment. Environ Res 207:112658
Montagner CC (2011) Contaminantes emergentes em água tratada e seus mananciais= sazonalidade, remoção e atividade estrogênica (Doctoral dissertation, [sn])
Moulay S, Zenimi A, Dib M (2005) Rosin/acid oil-based liquid soap. J Surfactants Deterg 8:169–174
Mousavi SA, Khodadoost F (2019) Effects of detergents on natural ecosystems and wastewater treatment processes: a review. Environ Sci Pollut Res 26:26439–26448
Najim AA, Radeef AY (2023) Surfactants: hygiene’s first line of defence against pollution. J Environ Eng Sci 40:1–20
Nations U (2015) Department of Economic and Social Affairs. Population Division. Current Economic Developments
Osimitz TG, Droege W, Driver JH (2015) Human risk assessment for nonylphenol. Hum Ecol Risk Assess Int J 21:1903–1919
Pakzad Toochaei S, Kazemi A (2022) Evaluating the concentration of bisphenol a and nonylphenol and water quality in caspian sea rivers estuarine in Mazandaran Province by using WQI Index. J Water and Wastewater Ab va Fazilab (in persian) 33:12–26
Pearce F, Tombs S (2019) Toxic capitalism: corporate crime and the chemical industry. Routledge
Prasad GS, Rout SK, Malik MM, Karmakar S, Amin A, Ahmad I (2023) Occurrence of xenoestrogen alkylphenols (octylphenols and nonylphenol) and its impact on the aquatic ecosystem. Xenobiotics in Aquat Anim Reprod Dev Impacts 275–284
Pukale DD, Bansode AS, Pinjari DV, Kulkarni RR, Sayed U (2017) Application of silicone surfactant along with hydrocarbon surfactants to textile washing for the removal of different complex stains. J Surfactants Deterg 20:287–295
Quiroga J, Garrido M, Romero L, Márquez DS, Perales J (2019) Assessment of the biodegradability and ecotoxicty of a nonylphenol ethoxylate surfactant in littoral waters. Int J Environ Sci Dev 10:130–136
Raecker T, Thiele B, Boehme R, Guenther K (2011) Endocrine disrupting nonyl- and octylphenol in infant food in Germany: considerable daily intake of nonylphenol for babies. Chemosphere 82:1533–1540
Ramnani P, Kumar SS, Gupta R (2005) Concomitant production and downstream processing of alkaline protease and biosurfactant from Bacillus licheniformis RG1: Bioformulation as detergent additive. Process Biochem 40:3352–3359
Research DBM (2020) Global nonylphenol ethoxylates market – industry trends and forecast to 2027. Nonylphenol ethoxylates market players, size, share, report, value, & forecast trends by 2029 (databridgemarketresearch.com). https://www.databridgemarketresearch.com/reports/global-nonylphenol-ethoxylates-market
Riedler GF, Haycox AR, Duggan AK, Dakin HA (2003) Cost-effectiveness of solvent/detergent-treated fresh-frozen plasma. Vox Sang 85:88–95
Różalska S, Soboń A, Pawłowska J, Wrzosek M, Długoński J (2015) Biodegradation of nonylphenol by a novel entomopathogenic Metarhizium robertsii strain. Biores Technol 191:166–172
Ryu H-D, Han H, Park T-J, Park J-H, Kim YS (2024) New findings on the occurrence, removal, and risk assessment of nonylphenol and octylphenol in industrial wastewater treatment plants in Korea. J Hazard Mater 461:132615
Sá MF, Castro V, Gomes AI, Morais DF, Braga RVS, Saraiva I, Souza-Chaves BM, Park M, Fernández-Fernández V, Rodil R (2022) Tracking pollutants in a municipal sewage network impairing the operation of a wastewater treatment plant. Sci Total Environ 817:152518
Scaia MF, de Gregorio LS, Franco-Belussi L, Succi-Domingues M, de Oliveira C (2019) Gonadal, body color, and genotoxic alterations in Lithobates catesbeianus tadpoles exposed to nonylphenol. Environ Sci Pollut Res 26:22209–22219
Schilling K, Zessner M (2011) Foam in the aquatic environment. Water Res 45:4355–4366
Shirdel I, Kalbassi MR, Esmaeilbeigi M, Tinoush B (2020) Disruptive effects of nonylphenol on reproductive hormones, antioxidant enzymes, and histology of liver, kidney and gonads in Caspian trout smolts. Comp Biochem Physiol C Toxicol Pharmacol 232:108756
Singh A, Sharma A, Bansal S, Sharma P (2018) Comparative interaction study of amylase and surfactants for potential detergent formulation. J Mol Liq 261:397–401
Smułek W, Burlaga N, Hricovíni M, Medveďová A, Kaczorek E, Hricovíniová Z (2021) Evaluation of surface active and antimicrobial properties of alkyl D-lyxosides and alkyl L-rhamnosides as green surfactants. Chemosphere 271:129818
Standard ASTM (2006) D445: standard test method for kinematic viscosity of transparent and opaque liquids (and calculation of dynamic viscosity). American Society for Testing and Materials, West Conshohocken, PA (USA)
Stasinakis AS, Petalas AV, Mamais D, Thomaidis NS (2008) Application of the OECD 301F respirometric test for the biodegradability assessment of various potential endocrine disrupting chemicals. Biores Technol 99:3458–3467
Stasinakis AS, Kordoutis CI, Tsiouma VC, Gatidou G, Thomaidis NS (2010) Removal of selected endocrine disrupters in activated sludge systems: effect of sludge retention time on their sorption and biodegradation. Biores Technol 101:2090–2095
Tabassum H, Ashafaq M, Parvez S, Raisuddin S (2017) Role of melatonin in mitigating nonylphenol-induced toxicity in frontal cortex and hippocampus of rat brain. Neurochem Int 104:11–26
Tai X-m, Song J-y, Du Z-p, Liu X, Wang T, Wang G (2018) The performance test of fatty acid methyl ester sulfonates and application in the dishwashing liquid detergent. J Dispersion Sci Technol 39:1422–1426
Talmage SS (2020) Environmental and human safety of major surfactants: alcohol ethoxylates and alkylphenol ethoxylates. CRC Press
UNE Normalización Española (2005) UNE-EN 14371:2005. Surface active agents - Determination of foamability and degree of foamability - Circulation test method. https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0032927
Uzwatania F, Hambali E, Suryani A (2017) Sintesis surfaktan alkil poliglikosida (APG) berbasis dodekanol dan heksadekanol dengan reaktan glukosa cair 75%. Jurnal Teknologi Industri Pertanian 27(1)
Van Zijl MC, Aneck-Hahn NH, Swart P, Hayward S, Genthe B, De Jager C (2017) Estrogenic activity, chemical levels and health risk assessment of municipal distribution point water from Pretoria and Cape Town, South Africa. Chemosphere 186:305–313
Vargas-Berrones K, Bernal-Jácome L, Díaz de León-Martínez L, Flores-Ramírez R (2020a) Emerging pollutants (EPs) in Latin América: a critical review of under-studied EPs, case of study -Nonylphenol. Sci Total Environ 726:138493
Vargas-Berrones K, Díaz de León-Martínez L, Bernal-Jácome L, Rodriguez-Aguilar M, Ávila-Galarza A, Flores-Ramírez R (2020b) Rapid analysis of 4-nonylphenol by solid phase microextraction in water samples. Talanta 209:120546
Wasilewski T, Seweryn A, Krajewski M (2016) Improvement in the safety of use of hand dishwashing liquids through the addition of hydrophobic plant extracts. J Surfactants Deterg 19:1315–1326
Ying GG, Williams B, Kookana R (2002) Environmental fate of alkylphenols and alkylphenol ethoxylates–a review. Environ Int 28:215–226
Yu Y, Zhao J, Bayly AE (2008) Development of surfactants and builders in detergent formulations. Chin J Chem Eng 16:517–527
Yuan SY, Yu CH, Chang BV (2004) Biodegradation of nonylphenol in river sediment. Environ Pollut 127:425–430
Zafra G, Moreno-Montaño A, Absalón ÁE, Cortés-Espinosa DV (2015) Degradation of polycyclic aromatic hydrocarbons in soil by a tolerant strain of Trichoderma asperellum. Environ Sci Pollut Res Int 22:1034–1042
Zhang X, He W, Ren L, Stager J, Evans PJ, Logan BE (2015) COD removal characteristics in air-cathode microbial fuel cells. Biores Technol 176:23–31
Zhang J, Liu L, Ning X, Lin M, Lai X (2023): Isomer-specific analysis of nonylphenol and their transformation products in environment: a review. Sci Total Environ 165982
Zhao Y, Ji J, Wu Y, Chen S, Xu M, Cao X, Liu H, Wang Z, Bi H, Guan G (2024) Nonylphenol and its derivatives: environmental distribution, treatment strategy, management and future perspectives. Chemosphere 141377
Funding
The authors received grants and fellowships from the National Council on Science and Technology Initial Academic Postdoctoral Research CVU 815662 “Ocurrencia de sales cuaternarias de amonio y sus subproductos de purificación en muestras de agua. Un desarrollo tecnológico sostenible para su sustitución en desinfectantes” and Sectoral Research Fund for Education Basic-Science # A1-S-28176.
Author information
Authors and Affiliations
Contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Sampling, analytical methods, and manuscript writing by Luis Armando Bernal-Jácome; analytical methods by Juan Manuel Izar-Landeta and Luis Fernando González-Salazar; conceptualization and funding by Rogelio Flores-Ramírez; conceptualization, analytical methods, writing—reviewing, and manuscript editing by Karla Ximena Vargas-Berrones.
Corresponding author
Ethics declarations
Ethics approval
The paper reflects the authors’ own research and analysis in a truthful and complete manner.
Consent to participate and for publication
All persons who meet authorship criteria are listed as authors, and all authors consent to participate and publish in the work to take public responsibility for the content.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bernal-Jácome, L.A., Izar-Landeta, J.M., Flores-Ramírez, R. et al. Nonylphenol ethoxylate degradation in detergents during shelf time, a new exposure pathway, and a perspective on their substitution. Environ Sci Pollut Res 31, 30497–30508 (2024). https://doi.org/10.1007/s11356-024-33260-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33260-7