Abstract
Trichoderma asperellum H15, a previously isolated strain characterized by its high tolerance to low (LMW) and high molecular weight (HMW) PAHs, was tested for its ability to degrade 3–5 ring PAHs (phenanthrene, pyrene, and benzo[a]pyrene) in soil microcosms along with a biostimulation treatment with sugarcane bagasse. T. asperellum H15 rapidly adapted to PAH-contaminated soils, producing more CO2 than uncontaminated microcosms and achieving up to 78 % of phenanthrene degradation in soils contaminated with 1,000 mg Kg−1 after 14 days. In soils contaminated with 1,000 mg Kg−1 of a three-PAH mixture, strain H15 was shown to degrade 74 % phenanthrene, 63 % pyrene, and 81 % of benzo[a]pyrene. Fungal catechol 1,2 dioxygenase, laccase, and peroxidase enzyme activities were found to be involved in the degradation of PAHs by T. asperellum. The results demonstrated the potential of T. asperellum H15 to be used in a bioremediation process. This is the first report describing the involvement of T. asperellum in LMW and HMW-PAH degradation in soils. These findings, along with the ability to remove large amounts of PAHs in soil found in the present work provide enough evidence to consider T. asperellum as a promising and efficient PAH-degrading microorganism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Degradation of polycyclic aromatic hydrocarbons (PAHs) in soils has become an environmental priority, mainly because of their elevated persistence and potential harmful effects on human and animal health. PAHs are recalcitrant organic compounds with potential cytotoxic, carcinogenic, genotoxic, and mutagenic effects, characterized by a high hydrophobicity and low aqueous solubility (US-EPA 2008). Low molecular weight (LMW), high molecular weight (HMW) PAHs as well as their toxic intermediary products can be absorbed and accumulated in diverse organisms. Microbial degradation is thought to be the main natural method of degradation of PAHs in soils and biochemical degradation pathways are well documented; several fungal, bacterial, and algal species have been reported as PAH-degrading organisms (Cerniglia and Sutherland 2010; Seo et al. 2009; Todd et al. 2002), making bioremediation an effective and promising technology to remove pollutants from soils.
Fungi belonging to Trichoderma genus are worldwide ubiquitous organisms commonly found in soils, known to possess a versatile and powerful enzymatic machinery (e.g. cellulases, hemicellulases, chitinases, proteases, glucanases) useful for the degradation of a wide range of substrates in soils, but specially, cellulosic material (Jaklitsch 2009). Trichoderma is one of the biological control agents more commonly used against plant pathogens mainly due to its production of hydrolytic enzymes and secondary metabolites, besides interacting through antibiosis, competing for space and resources, and improving growth and resistance to biotic and abiotic stress (Chernin and Chet 2002). Within the Trichoderma genus, T. asperellum stands out as a species with a wide range of substrate utilization, high production of antimicrobial compounds and an ability for environmental opportunism through saprotrophic, biotrophic, and mycoparasitic interactions (Chutrakul et al. 2008; Ding et al. 2012; Druzhinina et al. 2011). Trichoderma asperellum is used as a biological control agent against a wide range of plant pathogens including Colletotrichum gloeosporioides, Phytophthora megakarya, other pathogenic fungi, and nematodes (de los Santos-Villalobos S et al. 2013; Sharon et al. 2007; Slusarski and Pietr 2009; Tondje et al. 2007).
Although Trichoderma species are commonly used for the commercial production of lytic enzymes and as biological control agents, their use in pollutant bioremediation is limited. Several studies have shown the ability of Trichoderma to biotransform heavy metals (Atagana 2009; Su et al. 2011) and hydrocarbons (Matsubara et al. 2006). In fact, it is known that several species of the genus Trichoderma possess the ability to degrade and metabolize PAHs such as naphthalene, phenanthrene, pyrene, and benzo[a]pyrene, even in the presence of heavy metals (Atagana 2009; Verdin et al. 2004). The species reported as metabolizers include T. hamatum, T. harzianum, T. koningii, T. viride and T. virens (Argumedo-Delira et al. 2012; Cerniglia and Sutherland 2010). However, there are no reports involving T. asperellum as a hydrocarbon or PAH-degrading organism. The use of T. asperellum as bioremediation agent on PAH-polluted soils may present additional advantages over the use of other soil microorganisms, such as its high growth rate, wide range of substrates, growth-promoting effects on plants, and the production of oxidizing hydrolytic enzymes including laccases, peroxidises, and dioxygenases (Cazares-Garcia et al. 2013; Hadibarata et al. 2007). Thus, the aim of this work was to evaluate the degradation capability of a strain of T. asperellum tolerant to LMW and HMW-PAHs in solid culture, for the bioremediation of PAH-polluted soils.
Material and methods
Fungal strain and inoculum preparation
T. asperellum H15 is a strain previously isolated from a heavy crude oil-contaminated soil, showing increased tolerance levels to 3, 4, and 5-ring PAHs and the ability to use them as sole carbon source (Zafra et al. 2014). This strain has been deposited in the Agricultural Research Service (ARS) patent culture collection with registration number NRRL50869. T. asperellum H15 was maintained on potato dextrose agar (PDA) plates at 30 °C. Production of spores was carried out in 250-mL flasks containing 30 mL of PDA, inoculated with strain H15 and incubated at 30 °C. Spores were collected on day 4 with the addition of 20 mL of 0.1 % Tween 80 solution, sterile glass beads and gently shaking the flasks for 2 min. The spore suspension concentration was quantified in a Neubauer haematocytometer chamber using a optical microscope.
Degradation of PAHs by T. asperellum H15 in solid culture
Degradation ability of analytical grade phenanthrene (Phe) and a mixture of Phe, pyrene (Pyr), and benzo[a]pyrene (BaP) by T. asperellum H15 was evaluated in microcosm solid culture systems using sugarcane bagasse (34.34 % carbon, 0.18 % nitrogen, 0.00343 % phosphorus) as fungal growth support, texturizing agent and alternative carbon source. Sterile sugarcane bagasse (0.35 g dry weight) was placed in 50-mL glass flasks with Czapeck medium (g L−1: sucrose, 30; sodium nitrate, 3; dipotassium phosphate, 1; magnesium sulfate, 0.5; potassium chloride, 0.5; ferrous sulphate, 0.01; pH 7.3) to reach 30 % moisture content, inoculated with a concentration of 2 × 107 spores of strain H15 per gram of contaminated soil and incubated for 5 days at 30 °C. The inoculated sugarcane bagasse was then mixed with 6.65 g of sterile soil (sandy loam with 2.4 % organic matter, 1.4 % total organic carbon, 0.063 % nitrogen, 0.0023 % phosphorus and pH of 8.41) spiked with 1,000 mg Kg−1 of Phe or 1,000 mg Kg−1 of a mixture of Phe, Pyr, and BaP (1:1:1 ratio). Soil/sugarcane bagasse mixture was incubated at 30 °C for 14 (Phe-contaminated soil) or 18 days (PAH mixture-contaminated soil). Control samples were prepared by inoculating a non-contaminated soil under the same culture conditions. Abiotic controls, consisting of sterile non-inoculated microcosms treated under the same conditions as those of Trichoderma-inoculated systems, were included to confirm that the disappearance of PAHs was caused by biodegradation and not by abiotic factors such as absorption or volatilization. Assays were carried out in triplicate.
Heterotrophic activity measurements
Headspace in each of the microcosms flasks was flushed every 48 h for 10 min with sterile and moistened air, to preserve aerobic conditions and avoid carbon dioxide accumulation. CO2 evolution in the microcosms was measured every 48 h using an Agilent 6890 Series Gas Chromatograph equipped with a thermal conductivity detector and a GS-CarbonPLOT column. Instantaneous and accumulated CO2 was reported as milligrams of CO2 per gram of initial dry matter (IDM).
Enzymatic assays
Activity of T. asperellum H15 extracellular laccase and peroxidase enzymes was screened in agar plates by means of the oxidation of chromogenic dyes ABTS (Saparrat et al. 2000), o-anisidine (OA) (Conesa et al. 2000) and azure B (AB) (Archibald 1992), respectively, in the presence and absence of 1,000 mg L−1 of a mixture of Phe, Pyr, and BaP. Laccase screening was performed in minimal medium plates (g L−1: glucose, 2; (NH4)2 C4H4O6, 1; KH2PO4, 0.26; NaHPO4, 0.26; MgSO4 · 7H2O, 0.5; CuSO4 · 5H2O, 0.5; CaCl2 · 5H2O, 0.01; FeSO4, 0.005; ZnSO4 7H2O, 0.005; NaMoO4 7H2O, 0.0002; MnCl2 · H2O, 0.00009; H3BO3, 0.0007; malt extract, 2; ABTS, 0.2; agar, 16. pH 5.5); OA-oxidizing peroxidase activity was evaluated in plates with modified Kirk medium (g L−1: glucose, 10; KH2PO4, 2; MgSO4 · 7H2O, 0.5; CaCl2, 0.1; 2,2-dimethylsuccinate, 2,2; (NH4)2 C4H4O6, 0.5; yeast extract, 0.2; o-anisidine, 0.3; agar, 16. pH 5.0), and AB-oxidizing peroxidase activity was evaluated in plates with 20 ml of Czapeck medium supplemented with azure B (0.0066 g L−1). Plates were inoculated with PDA discs (5 mm diameter), containing 3-day-old active mycelia. Plates were incubated at 30 °C for 10 days. Determination of specific enzymatic activities was carried out in liquid culture. Glass flasks with 50 mL of minimal medium contaminated with a mixture of Phe (25 mg L−1) and Pyr (25 mg L−1) were inoculated with 1 × 106 spores mL−1 of strain H15 and incubated at 30 °C for 10 days. Enzymatic activities were assessed every 48 h from culture supernatants. Laccase extracellular activity was determined spectrophotometrically by the oxidation of ABTS (Nagai et al. 2002). Cationic radical formation was detected by measuring the increase in absorbance at 420 nm (e420 = 36,000 M−1 cm−1). Catechol 1,2 dioxygenase extracellular activity was determined spectrophotometrically by the formation of cis, cis-muconic acid at 260 nm (e260 = 16,800 M−1 cm−1) (Wojcieszynska et al. 2011). Catechol 2,3 dioxygenase extracellular activity was determined by the formation of 2-hydroxymuconic semialdehyde at 375 nm (e375 = 36,000 M−1 cm−1) (Wojcieszynska et al. 2011). Phenol red (PSP)-oxidizing peroxidase activity was determined spectrophotometrically at 37 °C by the oxidation of phenol red at 610 nm (Kuwahara et al. 1984). Veratryl alcohol (VA)-oxidizing peroxidase activity was determined spectrophotometrically at 37 °C by the oxidation of veratryl alcohol to verytraldehyde at 310 nm (Tien and Kirk 1988). One unit of enzyme activity (U/l) was defined as the amount of enzyme required to generate 1 μmol of each reaction product in 1 min. Protein concentrations of the culture supernatants were determined by the bicinchoninic acid method (BCA) using bovine serum albumin as standard (Smith et al. 1985).
PAH measurements
Residual PAHs were extracted from 1 g of initial dry matter (for solid culture) with the addition of 25 mL of a dichloromethane-acetone solution (7:3 ratio) using a Multiwave 3000 SOLV apparatus (Anton Paar) for 20 min, according to EPA method 3546. The resulting extracts were evaporated, suspended in 2 mL of acetonitrile and analyzed in an HP Agilent 1100 HPLC system equipped with a C18 reverse-phase column, with an UV absorbance detector set at 245–360 nm under an isocratic ambient in acetonitrile:water (90:10) and a flow rate of 1 ml min−1. For liquid culture, residual PAHs were extracted from mycelium and liquid medium; first the mycelium was filtered from 50 mL medium through cellulose filter paper with medium retention (Whatman grade 1) and resuspended in 10 mL acetone, then, it was sonicated for 10 min, and the organic phase was recovered by filtration with the same type of filter paper. Residual PAHs were extracted from the filtered liquid medium by stirring with 50 mL of ethyl acetate for 30 min, then, this organic phase was mixed with acetone fraction obtained from the mycelium, the mixture were evaporated, resuspended in acetonitrile, and quantified by HPLC as described above.
Statistical analysis
Data were analyzed by analysis of variance (ANOVA) followed by a multiple comparison test (LSD) with SPSS Statistics Software version 19 (IBM), considering statistically significant differences those with a p value <0.05.
Results
Heterotrophic activity of strain H15 in soil microcosms
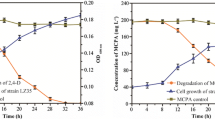
Figures 1 and 2 show the growth of T. asperellum H15 during the PAH biodegradation process in microcosms in the presence of Phe and a mixture of Phe, Pyr and BaP, respectively. CO2 production by strain H15 in uncontaminated microcosms increased gradually throughout the process, reaching the highest value on day 2 (6.914 mg CO2 g−1 IDM) and a marked and constant decrease from day 4 to 6, with a lower but relatively constant production of CO2 from day 8 to the end of the process. The presence of a single hydrocarbon (Phe) in soil initially delayed the growth of strain H15 but after day 4, Phe-contaminated microcosms produced CO2 levels higher than those in uncontaminated microcosms (Fig. 1) with an accumulated CO2 production of 27.615 versus 22.063 mg CO2 g−1 IDM, respectively. On the other hand and as observed with Phe, the presence of a three-PAH mixture in soil also delayed the initial growth of strain H15, although from day 4, it produced accumulated CO2 levels higher than those obtained in uncontaminated microcosms (Fig. 2). CO2 production differences between PAH-contaminated and PAH-uncontaminated microcosms were not significant.
PAH biodegradation in solid culture
The biodegradation efficiency of Phe, Pyr, and BaP by T. asperellum H15 in solid culture after 8, 14, and 18 days of incubation is shown in Fig. 3. A high degradation was obtained when Phe was added individually to microcosms, reaching a degradation efficiency of 78.3 % after 14 days of incubation. When a mixture of thee PAHs was added, the degradation efficiency of Phe by strain H15 was relatively low for day 8 (23.4 %), but for day 18 had increased substantially (74.4 %). The same situation was observed for Pyr and BaP; even though there was a lower degradation of Pyr and BaP during the first week of growth (15.3 and 16.31 %, respectively), there was a marked increase by day 18 (62.63 and 80.94 %, respectively). The abiotic losses of PAHs during the biodegradation experiments were similar and negligible.
Enzymatic assays and PAH removal in liquid culture
Plate-based screening of laccase and peroxidase activity by strain H15 is shown in Fig. 4. Extracellular laccase activity was observed from day 2 of incubation, when there was an evident increase in ABTS oxidation in plates containing 1,000 mg L−1 of the PAH mixture compared with those without PAHs (Fig. 4a). By day 10, plates with and without PAHs showed a complete oxidation of ABTS. Peroxidase activity, assessed by the oxidation of OA and AB, was detected only from day 7. Strain H15 was able to oxidize OA and AB in plates with no substantially appreciable differences regarding the presence or absence of PAHs in medium, although AB showed a lower intensity of the halo coloration in comparison with OA plates (Fig. 4b, c). By day 10 of incubation, the oxidation of both dyes was clear, but the overall oxidation rate was slower, and the effect of the presence of PAHs was less evident than ABTS plates.
Figures 5 and 6 show the specific activities of five PAH-oxidizing enzymes, as well as Phe and Pyr degradation in liquid medium. The presence of PAHs in the liquid cultures led to an increase in the specific activity of laccase from day 4 to 8 in comparison to controls without PAHs, maintaining relatively constant values from day 4 to 8. VA-oxidizing peroxidase activity was also higher by day 6 in comparison to controls without PAHs, reaching its highest value (23.94 U mg−1), although by day 8, enzyme activity decreased (20.4 U mg−1) and controls without PAHs reached their maximum (25.29 U mg−1). In contrast, PSP-oxidizing peroxidase activity was identical in cultures with and without PAHs, showing low or basal activity except at day 4 (Fig. 5). The presence of PAHs in the medium also produced an evident initial increase in the specific activity of catechol 1,2 dioxygenase (from day 2 to 6) and catechol 2,3 dioxygenase (from day 2 to 6) in comparison with controls without PAHs, although the enzyme activity in the latter was higher in medium without PAHs from day 6 (Fig. 6). Maximum degradation values of PAHs were 85.39 % for Phe and 41.06 % for Pyr at day 10. Phe degradation was significantly higher than Pyr degradation from day 2 until the last day of evaluation.
Discussion
In this study, we investigated the ability of T. asperellum H15, a strain previously isolated from a heavy crude oil-contaminated soil, to degrade several LMW and HMW PAHs in soil as well as to produce several PAH-oxidizing enzymes in the presence of PAHs associated with the degradation in liquid culture. Few data on PAH degradation by Trichoderma species are available; however, studies have shown that several Trichoderma species are capable of tolerating and metabolizing hydrocarbons including LMW and HMW PAHs (Argumedo-Delira et al. 2012; Atagana 2009; Hadibarata et al. 2007; Ravelet et al. 2000; Saraswathy and Hallberg 2002; Verdin et al. 2004). Previous work in our group showed remarkably high tolerance levels of T. asperellum H15 (up to 6,000 mg Kg−1) toward the same LMW and HMW PAHs tested in this work, as well as the ability to use them as sole carbon source (Zafra et al. 2014).
Many fungal species possess the ability to degrade PAHs and the potential to remediate polluted soils. However, one limiting factor in the success of these organisms is the inability to adapt and grow properly on extensively contaminated soils (Tabak et al. 2003). Solid-state fermentation in microcosms showed that although the presence of PAHs initially delayed the growth of strain H15 in soil, CO2 production remained higher in contaminated microcosms from day 4 when compared to uncontaminated microcosms. This suggests a rapid and successful adaption of strain H15 in PAH-polluted soils, as well as an ability to use the sugarcane bagasse and PAHs for growth. Although CO2 production does not correlate with PAH degradation levels, strain H15 produced remarkably higher amounts of CO2, at least twice, in comparison with other reported native and transformant PAH-degrading fungi when growing in microcosm at comparable PAH concentrations (Cortes-Espinosa et al. 2006, 2011; Reyes-Cesar et al. 2014). Unlike some reports indicating low tolerance levels to PAHs in several Trichoderma members such as T. harzianum, T. viride or Trichoderma sp. strains (Argumedo-Delira et al. 2012; Matsubara et al. 2006; Verdin et al. 2004), the elevated tolerance levels showed by T. asperellum H15 could facilitate its adaptation to polluted soils and thus improve the rate of removal/degradation of PAHs.
Biodegradation assays showed that soils contaminated with T. asperellum H15 led to a rapid degradation of considerable amounts of Phe, Pyr, and Bap. Although low Phe biodegradation took place by day 8 (29.32 % for Phe-contaminated soil and 23.42 % for PAH mixture-contaminated soils), by the end of the process, it had increased importantly (78.3 and 74.4 %, respectively). The same situation was observed in microcosms contaminated with a mixture of three PAHs, where scarce Pyr and BaP degradation was observed during the first week of growth (15.3 and 16.31 %) but by the end of the second week also increased substantially (62.63 and 80.94 %). This notorious shift in the PAH degradation rate could be associated with the induction of enzymes involved in the degradation of PAHs at different stages. HMW PAHs, particularly BaP, are barely metabolized substrates or used as a sole carbon and energy source and are poor inducers of their own degradative enzymes (Bouchez et al. 1995). The presence of alternative substrates, including LMW-PAHs, favors microbial growth and induces the expression of PAH catabolic enzymes (Juhasz and Naidu 2000). Our results are consistent with previous reports showing an increased cometabolic degradation of HMW PAHs in response to the presence of LMW PAHs and their corresponding degradative pathways (Juhasz and Naidu 2000; Yuan et al. 2003). PAH degradation by strain H15, particularly of HMW PAHs, showed to be higher and faster than with other reported non-ligninolytic and ligninolytic degrading fungi. For example, Irpex lacteus, Coprinus cinereus, and Pleurotus ostreatus degraded 65–80 % Phe and 30–65 % Pyr out of 400 mg Kg−1 after 28 days of incubation (Matsubara et al. 2006), while Anthracophyllum discolor degraded 75 % out of 45 mg Kg−1 BaP in soil after 60 days of incubation (Acevedo et al. 2011). The same was observed with strains of Fusarium sp., Monilinia sp., Aspergillus terreus and Talaromyces spectabilis strains in soil microcosms and solid culture when using similar LMW and HMW-PAH mixtures, even in the presence of concentrations below 100 mg Kg−1 of individual PAHs (Reyes-Cesar et al. 2014; Thion et al. 2013; Wu et al. 2008;) and regarding the degradation of 10 mg Kg−1 of Pyr in liquid culture by Trichoderma harzianum (Ravelet et al. 2000). This depicts the high potential of strain H15 to remediate PAH in contaminated soils.
The metabolism of PAHs by fungi has been extensively studied, especially in basidiomycetes. Most fungi metabolize PAHs with enzymes that include LiP, MnP, laccases, cytochrome P450 monooxygenases, and epoxide hydrolases (Cerniglia and Sutherland 2010). In contrast, aromatic-ring-hydroxylating dioxygenases are more commonly found in bacteria and greatly contribute to the initial ring cleavage of aromatic compounds, including LMW PAHs (Hadibarata and Tachibana 2010). Probable mechanisms for PAH degradation in T. asperellum could include the production of laccases (Cazares-Garcia et al. 2013), peroxidases (Cristica et al. 2010), and dioxygenases (Hadibarata et al. 2007) among others. We observed that the presence of PAHs in liquid cultures led to a significant increase in the activity of catechol 1,2 and 2,3 dioxygenases during the initial 4 days of incubation, reaching a maximum production at day 6 and a subsequent decrease from day 8. This could indicate an involvement of these enzymes in the early stages of degradation, which play a key role in the initial oxidation of aromatic compounds in other microorganisms (Seo et al. 2009). Hadibarata et al. (2007) reported a direct implication of catechol 1,2 and 2,3 dioxygenases in the degradation of Phe by Trichoderma sp. 109, a strain similar to T. asperellum H15 that possesses the ability to grow with PAHs as sole carbon source. 1-Hydroxy-2-naphthoic acid, salicylic acid, and catechol were identified as major intermediaries, indicating a Phe degradation pathway in Trichoderma via dioxygenation at positions 3 and 4 and subsequent meta-cleavage, leading to PAH mineralization. A high production of extracellular catechol 1,2 and 2,3 dioxygenases associated with PAH metabolism have been also observed in the degradation of chrysene by Fusarium sp. (Hidayat et al. 2012), as well naphthalene and BaP by ligninolytic fungi Armillaria sp. and Polyporus sp. (Hadibarata et al. 2012; Hadibarata and Kristanti 2012). In contrast, laccase activity was nearly undetectable during the initial 2 days of degradation but increased notoriously from day 4, with a higher production in response to the presence of PAHs. It is likely that T. asperellum laccases are involved in the oxidation of aromatic rings and play a crucial role in the degradation of PAHs by T. asperellum, as they are strongly linked to aromatic hydrocarbon degradation in other fungi (Baldrian 2006; Haritash and Kaushik 2009). Three different T. asperellum laccase genes have been identified in silico (Cazares-Garcia et al. 2013), two of them being extracellular enzymes with probable PAH-oxidizing capacity. On the other hand, there is no evidence of classic fungal peroxidases such as MnP and LiP in T. asperellum genome, nor in the secretome of this fungus grown in sugarcane bagasse (Marx et al. 2013). However, we found evidence suggesting that strain H15 produced peroxidases with OA/PSP-oxidizing activity (related with MnP activity) and AB/VA-oxidizing activity (related with LiP activity) in solid and liquid culture. Increased activity of VA-oxidizing peroxidase activity was observed in treatments with soil contaminated with PAHs; this result suggests that this enzyme is involved in the degradation of PAHs; in contrast, PSP-oxidizing peroxidase activity, although detectable during the first days, did not appear to be involved in the degradation of PAHs by T. asperellum. Identified T. asperellum peroxidases include cytochrome C peroxidases, catalase peroxidases, glutathione peroxidise, and dye decolorizing (DyP-type) peroxidases (Fawal 2014). Although peroxidase activity in T. asperellum has been described mainly as a response against oxidative stress (Fawal et al. 2013), VA-oxidizing peroxidase activity detected in strain H15 could in fact be related to DyP-type peroxidase (TaspDyPrx01, PeroxiBase ID 12842). DyP peroxidases possess a broad substrate specificity and have been previously described as LiP in Actinobacteria, having a significant role in bacterial lignin degradation (Ahmad et al. 2011) as well as a role in fungal degradation of lignin by basidiomycetes (Liers et al. 2011).
Sugarcane bagasse, in addition to serving as soil texturizer, could have also contributed with carbohydrates that could have been utilized by T. asperellum as carbon source. In fact, T. asperellum produces a potent lignocellulolytic cocktail when grown on sugarcane bagasse (Marx et al. 2013), favoring the use of alternative carbon sources (including PAHs). This is particularly relevant in soil, where a complex mixture of substrates can be found and greatly favors the use of biostimulation in conjunction with T. asperellum for the bioremediation of soils.
The results of this study indicate that T. asperellum H15 possesses the ability to degrade high amounts of LMW and HMW PAHs from contaminated soils and has a great potential for use in soil remediation processes. Based on our findings, we suggest that PAH degradation mechanisms in T. asperellum H15 could be mediated by dioxygenase enzymes, which could contribute to the initial degradation of LMW-PAHs (Phe) and subsequently, laccase, peroxidise, and dioxygenase enzymes continue the degradation process of the remaining LMW and HMW-PAHs.
Conclusions
Our results show that T. asperellum H15 is an efficient PAH degrader in soil and is able to tolerate high amounts of PAHs and that the presence of PAHs induces the production of enzymes involved in the oxidation of PAH aromatic rings at different stages. This, along with its great ability to grow in soil, make T. asperellum a microorganism with great potential for use in the bioremediation of PAH-contaminated soils. To our knowledge, this is the first report on the biodegradation of LMW and HMW PAHs by T. asperellum. Further studies testing the bioremediation of impacted soils at field scale, as well as intermediate production are necessary to better address the degradation mechanisms and bioremediation potential of this microorganism.
References
Acevedo F, Pizzul L, Castillo MD, Cuevas R, Diez MC (2011) Degradation of polycyclic aromatic hydrocarbons by the Chilean white-rot fungus Anthracophyllum discolor. J Hazard Mater 185(1):212–219. doi:10.1016/j.jhazmat.2010.09.020
Ahmad M, Roberts JN, Hardiman EM, Singh R, Eltis LD, Bugg TD (2011) Identification of DypB from Rhodococcus jostii RHA1 as a lignin peroxidase. Biochemistry 50(23):5096–5107. doi:10.1021/bi101892z
Archibald FS (1992) A new assay for lignin-type peroxidases employing the dye azure B. Appl Environ Microbiol 58(9):3110–3116
Argumedo-Delira R, Alarcon A, Ferrera-Cerrato R, Almaraz JJ, Pena-Cabriales JJ (2012) Tolerance and growth of 11 Trichoderma strains to crude oil, naphthalene, phenanthrene and benzo[a]pyrene. J Environ Manage 95(Suppl):S291–S299. doi:10.1016/j.jenvman.2010.08.011
Atagana HI (2009) Biodegradation of PAHs by fungi in contaminated-soil containing cadmium and nickel ions. Afr J Biotechnol 8(21):5780–5789
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30(2):215–242. doi:10.1111/j.1574-4976.2005.00010.x
Bouchez M, Blanchet D, Vandecasteele JP (1995) Substrate availability in phenanthrene biodegradation: transfer mechanism and influence on metabolism. Appl Microbiol Biotechnol 43(5):952–960
Cazares-Garcia SV, Vazquez-Garciduenas MS, Vazquez-Marrufo G (2013) Structural and phylogenetic analysis of laccases from Trichoderma: a bioinformatic approach. PLoS One 8 (1).doi: 10.1371/journal.pone.0055295
Cerniglia CE, Sutherland GR (2010) Degradation of polycyclic aromatic hydrocarbons by fungi. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin, pp 2079–2110. doi:10.1007/978-3-540-77587-4_151
Chernin L, Chet I (2002) Microbial enzymes in the biocontrol of plant pathogens and pests. In: Burns R, Dick R (eds) Enzymes in the environment: activity, ecology, and applications. CRC Press, Columbus, pp 171–226. doi:10.1201/9780203904039.ch7
Chutrakul C, Alcocer M, Bailey K, Peberdy JF (2008) The production and characterisation of trichotoxinpeptaibols, by Trichoderma asperellum. Chem Biodivers 5(9):1694–1706. doi:10.1002/cbdv.200890158
Conesa A, van den Hondel CA, Punt PJ (2000) Studies on the production of fungal peroxidases in Aspergillus niger. Appl Environ Microbiol 66(7):3016–3023
Cortes-Espinosa DV, Fernandez-Perrino FJ, Arana-Cuenca A, Esparza-Garcia F, Loera O, Rodriguez-Vazquez R (2006) Selection and identification of fungi isolated from sugarcane bagasse and their application for phenanthrene removal from soil. J Environ Sci Health A Tox Hazard Subst Environ Eng 41(3):475–486. doi:10.1080/10934520500428351
Cortes-Espinosa DV, Absalon AE, Sanchez N, Loera O, Rodriguez-Vazquez R, Fernandez FJ (2011) Heterologous expression of manganese peroxidase in Aspergillus niger and its effect on phenanthrene removal from soil. J Mol Microbiol Biotechnol 21(3-4):120–129. doi:10.1159/000331563
Cristica M, Manoliu A, Barbaneagra T, Ciornea E (2010) Compared analysis of catalase and peroxidase activity in cellulolytic fungus Trichoderma reesei grown on medium with different concentrations ofgrinded wheat and barley straws. An StiintUniv Al I Cuza Iasi, Sect II a Biol 12(3):89–93
de los Santos-Villalobos S, Guzmán-Ortiz DA, Gómez-Lim MA, Délano-Frier JP, de-Folter S, Sánchez-García P, Peña-Cabriales JJ (2013) Potential use of Trichoderma asperellum (Samuels, Liechfeldt et Nirenberg) T8a as a biological control agent against anthracnose in mango (Mangifera indica L.). Biol Control 64 (1):37-44. doi:http://dx.doi.org/10.1016/j.biocontrol.2012.10.006
Ding G, Chen AJ, Lan J, Zhang H, Chen X, Liu X, Zou Z (2012) Sesquiterpenes and cyclopeptides from the endophytic fungus Trichoderma asperellum SAMUELS LIECKF. & NIRENBERG. Chem Biodivers 9(6):1205–1212. doi:10.1002/cbdv.201100185
Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kenerley CM, Monte E, Mukherjee PK, Zeilinger S, Grigoriev IV, Kubicek CP (2011) Trichoderma: the genomics of opportunistic success. Nat Rev Microbiol 9(10):749–759
Fawal N (2014) The PeroxiBase.TrichodermaasperellumPeroxidases. https://peroxibase.toulouse.inra.fr/tools/search.php?searchString[]=Trichoderma+asperellum&searchType[]=Organism&od=1.
Fawal N, Li Q, Savelli B, Brette M, Passaia G, Fabre M, Mathe C, Dunand C (2013) PeroxiBase: a database for large-scale evolutionary analysis of peroxidases. Nucleic Acids Res 41(Database issue):D441–D444. doi:10.1093/nar/gks1083
Hadibarata T, Kristanti RA (2012) Identification of metabolites from benzo[a]pyrene oxidation by ligninolytic enzymes of Polyporus sp. S133. J Environ Manage 111:115–119. doi:10.1016/j.jenvman.2012.06.044
Hadibarata T, Tachibana S (2010) Characterization of phenanthrene degradation by strain Polyporus sp. S133. J Environ Sci (China) 22(1):142–149
Hadibarata T, Tachibana S, Itoh K (2007) Biodegradation of phenanthrene by fungi screened from nature. Pak J Biol Sci 10(15):2535–2543
Hadibarata T, Yusoff AR, Aris A, Kristanti RA (2012) Identification of naphthalene metabolism by white rot fungus Armillaria sp. F022. J Environ Sci (China) 24(4):728–732
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169(1-3):1–15. doi:10.1016/j.jhazmat.2009.03.137
Hidayat A, Tachibana S, Itoh K (2012) Determination of chrysene degradation under saline conditions by Fusarium sp. F092, a fungus screened from nature. Fungal biol 116(6):706–714. doi:10.1016/j.funbio.2012.04.004
Jaklitsch WM (2009) European species of Hypocrea Part I. The green-spored species. Stud Mycol 63:1–91. doi:10.3114/sim.2009.63.01
Juhasz AL, Naidu R (2000) Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: a review of the microbial degradation of benzo[a]pyrene. Int Biodet Biodegr 45:57–88
Kuwahara M, Glenn JK, Morgan MA, Gold MH (1984) Separation and characterization of two extracelluar H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169 (2):247-250. doi.10.1016/0014-5793(84)80327-0
Liers C, Arnstadt T, Ullrich R, Hofrichter M (2011) Patterns of lignin degradation and oxidative enzyme secretion by different wood- and litter-colonizing basidiomycetes and ascomycetes grown on beech-wood. FEMS Microbiol Ecol 78(1):91–102. doi:10.1111/j.1574-6941.2011.01144.x
Marx IJ, van Wyk N, Smit S, Jacobson D, Viljoen-Bloom M, Volschenk H (2013) Comparative secretome analysis of Trichoderma asperellum S4F8 and Trichoderma reesei Rut C30 during solid-state fermentation on sugarcane bagasse. Biotechnol Biofuels 6(1):172. doi:10.1186/1754-6834-6-172
Matsubara M, Lynch JM, De Leij FAAM (2006) A simple screening procedure for selecting fungi with potential for use in the bioremediation of contaminated land. Enzyme Microb Tech 39(7):1365–1372. doi:10.1016/j.enzmictec.2005.04.025
Nagai M, Sato T, Watanabe H, Saito K, Kawata M, Enei H (2002) Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl Microbiol Biotechnol 60(3):327–335. doi:10.1007/s00253-002-1109-2
Ravelet C, Krivobok S, Sage L, Steiman R (2000) Biodegradation of pyrene by sediment fungi. Chemosphere 40(5):557–563
Reyes-Cesar A, Absalon AE, Fernandez FJ, Gonzalez JM, Cortes-Espinosa DV (2014) Biodegradation of a mixture of PAHs by non-ligninolytic fungal strains isolated from crude oil-contaminated soil. World J Microbiol Biotechnol 30(3):999–1009. doi:10.1007/s11274-013-1518-7
Saparrat MC, Margarita-Bucsinszky AM, Alfio-Tournier H, Cabello MN, Arambarri AM (2000) Extracellular ABTS-oxidizing activity of autochthonous fungal strains from Argentina in solid medium. Rev Iberoam Micol 17(2):64–68
Saraswathy A, Hallberg R (2002) Degradation of pyrene by indigenous fungi from a former gasworks site. FEMS Microbiol Lett 210(2):227–232
Seo JS, Keum YS, Li QX (2009) Bacterial degradation of aromatic compounds. Int J Environ Res Public Health 6(1):278–309. doi:10.3390/ijerph6010278
Sharon E, Chet I, Viterbo A, Bar-Eyal M, Nagan H, Samuels G, Spiegel Y (2007) Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. Eur J Plant Pathol 118(3):247–258. doi:10.1007/s10658-007-9140-x
Slusarski C, Pietr SJ (2009) Combined application of dazomet and Trichoderma asperellum as an efficient alternative to methyl bromide in controlling the soil-borne disease complex of bell pepper. Crop Prot 28(8):668–674. doi:10.1016/j.cropro.2009.03.016
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150 (1):76-85. doi:10.1016/0003-2697(85)90442-7
Su SM, Zeng XB, Bai LY, Li LF, Duan R (2011) Arsenic biotransformation by arsenic-resistant fungi Trichoderma asperellum SM-12F1, Penicillium janthinellum SM-12F4, and Fusarium oxysporum CZ-8F1. Sci Total Environ 409(23):5057–5062. doi:10.1016/j.scitotenv.2011.08.039
Tabak HH, Lazorchak JM, Lei L, Khodadoust AP, Antia JE, Bagchi R, Suidan MT (2003) Studies on bioremediation of polycyclic aromatic hydrocarbon-contaminated sediments: bioavailability, biodegradability, and toxicity issues. Environ Toxicol Chem 22(3):473–482
Thion C, Cebron A, Beguiristain T, Leyval C (2013) Inoculation of PAH-degrading strains of Fusarium solani and Arthrobacter oxydans in rhizospheric sand and soil microcosms: microbial interactions and PAH dissipation. Biodegradation 24(4):569–581. doi:10.1007/s10532-013-9628-3
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. In: Willis A. Wood STK (ed) Methods in Enzymology, vol Volume 161. Academic Press, pp 238-249.doi:http://dx.doi.org/10.1016/0076-6879(88)61025-1
Todd SJ, Cain RB, Schmidt S (2002) Biotransformation of naphthalene and diaryl ethers by green microalgae. Biodegradation 13(4):229–238
Tondje PR, Roberts DP, Bon MC, Widmer T, Samuels GJ, Ismaiel A, Begoude AD, Tchana T, Nyemb-Tshomb E, Ndoumbe-Nkeng M, Bateman R, Fontem D, Hebbar KP (2007) Isolation and identification of mycoparasitic isolates of Trichoderma asperellum with potential for suppression of black pod disease of cacao in Cameroon. Biol Control 43(2):202–212. doi:10.1016/j.biocontro1.2007.08.004
US-EPA (2008) Polycyclic aromatic hydrocarbons (PAHs). Office of Solid Waste, United States Environmental Protection Agency.http://www.epa.gov/wastes/hazard/wastemin/minimize/factshts/pahs.pdf. Accessed March 2012
Verdin A, Sahraoui L, Durand R (2004) Degradation of benzo[a]pyrene by mitosporic fungi and extracellular oxidative enzymes. Int Biodeter Biodegr 53(2):65–70. doi:10.1016/j.ibiod.2003.12.001
Wojcieszynska D, Guzik U, Gren I, Perkosz M, Hupert-Kocurek K (2011) Induction of aromatic ring: cleavage dioxygenases in Stenotrophomonas maltophilia strain KB2 in cometabolic systems. World J Microbiol Biotechnol 27(4):805–811. doi:10.1007/s11274-010-0520-6
Wu Y, Luo Y, Zou D, Ni J, Liu W, Teng Y, Li Z (2008) Bioremediation of polycyclic aromatic hydrocarbons contaminated soil with Monilinia sp.: degradation and microbial community analysis. Biodegradation 19(2):247–257. doi:10.1007/s10532-007-9131-9
Yuan SY, Chang SW, Chang BV (2003) Biodegradation of polycyclic aromatic hydrocarbons in sludge. Bull Environ Contam Toxicol 71(3):625–632
Zafra G, Absalón ÁE, Cuevas MC, Cortés-Espinosa DV (2014) Isolation and selection of a highly tolerant microbial consortium with potential for PAH biodegradation from heavy crude oil-contaminated soils. Water Air Soil Poll 225(2):1826. doi:10.1007/s11270-013-1826-4
Acknowledgments
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACYT) project CB2008-105643, Instituto Politécnico Nacional project SIP20144071 and CONACYT grant 269828.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Zafra, G., Moreno-Montaño, A., Absalón, Á.E. et al. Degradation of polycyclic aromatic hydrocarbons in soil by a tolerant strain of Trichoderma asperellum . Environ Sci Pollut Res 22, 1034–1042 (2015). https://doi.org/10.1007/s11356-014-3357-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3357-y