Abstract
It is widespread of endemic fluorosis in China, and the exposure of excessive fluoride will cause nervous system disease and activate microglia. However, the mechanism of the damage is not clear. It is well-known that NLRP3/Caspase-1/GSDMD pathway, a classic pyroptosis pathway, is widely involved in the occurrence and development of nervous system–related diseases, infectious diseases, and atherosclerotic diseases. This research aimed to explore the molecular mechanism of sodium fluoride on inflammation and pyroptosis in BV2 microglia based on the NLRP3/Caspase-1/GSDMD signaling pathway. BV2 microglia was treated with sodium fluoride at the dose of 0.25, 1, and 2 mmol/L for 24, 48, and 72 h, respectively. Cell viability, cell morphology, lactate dehydrogenase content, and related proteins and genes were examined to investigate if sodium fluoride caused damage to BV2 microglia through the pyroptosis pathway. Dithiolam (5 μmol/L), a pyroptosis inhibitor, was added for further verification. NaF could induced BV2 cells injury in a dose-dependent fashion through disrupting the integrity of cell membranes and increasing IL-1β via upregulating NLRP3, Caspase-1, and its downstream protein GSDMD. Disulfiram could improve these changes caused by NaF. In conclusion, our results suggested that NLRP3/Caspase-1/GSDMD-mediated classical pyroptosis pathway was involved in fluoride-induced BV2 microglia damage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluorine is a halogen element in the periodic table. Fluoride widely exists in daily drinking water, soil, and atmosphere, and most of it exist in the human body in the form of compounds (Li et al. 2022). As per the guideline adopted by the WHO (WHO 2011) and Chinese standard (GB5749-2022), the concentration of fluoride in drinking water should not exceed beyond 1.5 mg/L and 1.0 mg/L, respectively. China locates in high F− zone in the world. The results showed that 15% of the groundwater contains F−. The average fluoride content in water is higher than 2.0 mg/L and even reaches 10.4 mg/L in endemic areas (Feng et al. 2020). Fluoride is an essential trace element for human body, mostly distributing in bones and teeth. Proper intake of fluoride is beneficial to the prevention and treatment of dental caries, as well as the development of the bone. However, fluoride is also one of the major toxicants in the environment, and excessive intake of fluoride will cause a significant risk on human health, which will result in skeletal fluorosis and dental fluorosis which is a typical feature of chronic systemic diseases (Godebo et al. 2020; Rezaee et al. 2020; Zheng et al. 2020). Furthermore, fluoride plays an adverse role in affecting tissues of the kidneys, liver, heart, and testicle (Dharmaratne 2019; Kanagaraj et al. 2015; Xie et al. 2020) with a broad array of symptoms and various pathological changes. In addition, studies have shown that fluoride could penetrate both the placental and the blood–brain barrier (BBB) as well as caused central nervous system functional impairments (Grandjean 2019). Fluoride inside the brain caused remarkable histopathological alterations, extensive cellular damage, and apoptosis. The accumulated data revealed that fluorosis patients often occurred in the central nervous system dysfunction, such as headache, dizziness, and memory loss (Choi et al. 2012). Chronic fluorosis could cause intellectual changes. High fluoride exposure reduced children’s reaction speed and abstracted thinking ability and further affected children’s learning ability. Studies have identified that excessive fluoride intake showed a significant negative correlation with children’s IQ (Chen et al. 2018; Grandjean 2019; Sabour and Ghorbani 2013). Animal studies showed that long-term fluoride exposure would activate oxidative stress reaction in the brain. The increase of ROS concentration led to the disorder of cell metabolism and the increase of the expression of pro-inflammatory genes and caused inflammation, cell apoptosis, and degenerative diseases (Akinrinade et al. 2015; Ma et al. 2017).
Microglia are dormant macrophages in brain tissue, which participate in the immune regulation of the central nervous system and the repair of brain tissue damage and pathogens. Therefore, they are the first line of defense in the central nervous system against the external damage. Activated microglia could protect neurons through phagocytosis. On the other hand, they could induce inflammation in the brain. Activated microglia release neurotoxic cytokines, such as IL-1β, IL-6, tumor necrosis factor α (TNF-α), and prostaglandins, and initiate the expression level of phospholipase A2 and cyclooxygenase, which result in the death of neurons and glial cells (Zhang et al. 2022) and are related to a variety of neuropathic diseases and disorders (Li et al. 2023). Relevant studies have shown that fluoride could activate microglia, which produced pro-inflammatory cytokines including IL-1β, IL-6, IL-18, and TNF-α, leading to severe oxidative stress and inflammation in brain (Chen et al. 2017; Zhang et al. 2021). The level of neurotransmitters and the function of neurons are changed. It is reported that inflammation, oxidative stress, and subsequent neuronal degeneration are the basic causes of the toxic effect of fluoride on neurons.
Inflammatory reaction is involved in pyroptosis which is a form of inflammatory programmed cell death pathway activated by Caspase-1, Caspase-4, Caspase-5, or Caspase-11. Nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) is a sensitive receptor for inflammatory signaling. Once inflammation appears, the NLRP3 receptor will recruit the adaptor molecule apoptosis-associated speckle-like protein (ASC) to form a macromolecular complex. NLRP3 inflammasome could activate the effector protein Caspase-1. Activated Caspase-1 cleaved pro-IL-18 and pro-IL-1β and led to the release of activated IL-18 and IL-1β and inflammatory response. Activated Caspase-1 also cleaved gasdermin D (GSDMD), which initiated pyroptosis (Shi et al. 2015). Li et al. (2020) reported that sodium fluoride (NaF) could induce pyroptosis in the small intestine of C57BL/6 J male mice, which led to inflammatory reaction. However, there are few reports about whether NaF could induce microglia pyroptosis leading to inflammation. Therefore, we speculated that NLRP3/Caspase-1/GSDMD pathway presumably played an indispensable part in regulating NaF-induced BV-2 pyroptosis. Moreover, disulfiram, an inhibitor of the pyroptosis, was used to explore the role in the activation of BV2 cells induced by fluoride.
This study aimed to investigate the effect of fluoride on the activation of NLRP3 inflammasome in microglia, and the role of Caspase-1 mediated pyroptosis in the damage of microglia caused by fluorosis.
Materials and methods
Chemicals and reagents
Sodium fluoride (NaF) was purchased from Tianjin Damao Chemical Plant (China). DMEM was purchased from Gibco (USA), and fetal bovine serum (FBS) was purchased from BI(Israel). Disulfiram (DSF) was obtained from MCE (USA).
Cell culture
BV2 cells were purchased from the Institute of Materia Medica, Chinese Academy of Medical Sciences (Beijing, China). Cells were maintained in DMEM supplemented with 10% FBS in a humidified atmosphere at 37 °C with 5% CO2 (Thermo Scientific, USA). The growth medium was changed 2–3 times a week. Cells were subcultured during 80–90% cell fusion. Then, cells were treated with NaF and with/without GSDMD inhibitor as indicated.
Cell viability assay
To determine the effects of NaF on BV2 cell viability, the MTT assay was used. Cells were seeded in 96-well plates at 5 × 103 cells/well. After 24 h, cells were treated with varying doses of NaF (0, 0.25, 0.5, 1, 1.5, 2, 2.5, 5 mmol/L) for 24, 48, and 72 h, respectively. Ten microliter of the MTT reagent was added to each well and incubated for 4 h at 37°C when each time point. After incubation, the supernatant was removed. Subsequently, 100 μl dimethyl sulfoxide (DMSO) (Solarbio, Shanghai, China) was added, and the solution was oscillated on a shaking table at room temperature for 10 min to dissolve the blue–violet precipitate. Finally, the absorbance was determined at 490 nm.
Cell morphology observation
Cells were seeded in 6-well plates at 106 cells/well and incubated for 24 h. Then, they were exposed to different doses of NaF (0, 0.5, 1, 2 mmol/L) for 24, 48, and 72 h, respectively. Cells were treated with 0 and 2 mmol/L NaF in the presence or absence of disulfiram for 24 h in another experiment. After cells were washed three times with PBS, the cell morphology of each group added with PBS was observed with the optical inversion phase contrast microscope.
Lactate dehydrogenase (LDH) release detection
Cells in the logarithmic phase were seeded in 96-well plates at 5 × 103 cells/well. After 24 h, cells were treated with varying doses of NaF (0, 0.5, 1, 2 mmol/L) with/without disulfiram (5 μmol/L) for 24 h. Cells were treated with 0 and 2 mmol/L NaF and/or 5 μmol/L disulfiram for 24 h in another experiment, respectively. The cell culture plates were removed and centrifuged at 400 × g for 5 min. Then 120 μl supernatant was removed and transferred to another 96-well plate, and 60 μl LDH detection fluid was added into each well. Finally, the reaction mixture was incubated for 30 min at room temperature in the dark. LDH concentration was quantified by measuring the absorbance at 490 nm.
Total RNA extraction and real-time quantitative PCR (qPCR) assay
Total RNA was extracted from BV2 cells using Trizol reagent (Thermo,15,596,018). Then 3 μg of total RNA was reverse transcribed into cDNA using Reverse Transcription Kit (Thermo, K1622). Finally, qPCR was used for cDNA amplification. Primers obtained from Sangon Biotech (Shanghai, China) were showed as follows: NLRP3-F, 5́-GCCGTCTACGTCTTCTTCCTTTCC-3́, and NLRP3-R, 5́-CATCCGCAGCCAGTGAACAGAG-3́; Caspase-1-F, 5́-AGAGGATTTCTTAACGGATGCA-3́, and Caspase-1-R, 5́-TCACAAGACCAGGCATATTCTT-3́; GSDMD-F, 5́-CGATGGGAACATTCAGGGCAGAG-3́, and GSDMD-R, 5́-ACACATTCATGGAGGCACTGGAAC-3́; IL-1β-F, 5́-CACTACAGGCTCCGAGATGAACAAC-3́, and IL-1β-R, 5́-TGTCGTTGCTTGGTTCTCCTTGTAC-3́; and GAPDH-F, 5́-GGCAAATTCAACGGCACAGTCAAG-3́, and GAPDH-R, 5́-TCGCTCCTGGAAGATGGTGATGG-3́. The relative expression of the target gene was calculated by 2−△△Ct with GAPDH as internal reference control.
Western blotting assay
Cells were incubated and treated as mentioned above. Total proteins were extracted with cell lysis buffer (Solarbio, ROO20) containing protease inhibitors (Solarbio, ROO20). The lysates were incubated on ice for 20 min and cleared by centrifugation at 14,000 rpm for 5 min at 4 °C to the supernatants and were collected and used for evaluation of the relevant protein levels. Protein concentrations were quantified by using a BCA protein kit (Thermo,23,225). Samples (30 μg protein per lane) were loaded on 10% SDS-PAGE gels and transferred to PVDF membranes (Roche). After blocking with 5% non-fat milk for 2 h at room temperature in TBST (TBS with 0.5% Tween 20), the membranes were incubated with appropriate primary antibodies at 4 °C overnight. The primary antibodies included NLRP3 (1:1000, SAB 38679–1), Caspase-1 (1:1000, Abcam, EPR16883), GSDMD (1:1000, SAB, 30,392–1), IL-1β (1:1000, SAB, 41,059–1), and GAPDH (1:1000, SAB, 21,612–2). The next day, the membranes were washed three times with TBST buffer for 10 min and incubated with anti-rabbit horseradish peroxidase (HRP)-conjugated immunoglobulin G (IgG) secondary antibodies diluted in TBST (1:5000) for 2 h. Then, the membranes were washed with TBST buffer three times for 10 min and then treated with enhanced chemiluminescence (ECL, livning, C1006-100). Western blot images were captured by Tanon 4200SF multi-chemiluminescent imaging system (Tanon, China), and bands were quantified by densitometry using ImageJ software.
Statistical analysis
All experiments were performed at least three times, and the values were presented as mean ± SD. The comparison between groups was evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test using GraphPad Prism 5.0 software (GraphPad Software Inc., USA). P values < 0.05 were considered statistically significant.
Results
Effects of fluoride on BV2 cell viability
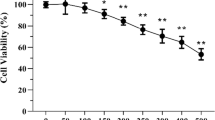
The cell viability of BV2 cells exposed to different doses of fluoride were detected by MTT assay. As shown in Fig. 1, cell viability increased at 0.25 and 0.5 mmol/L NaF-treated groups following 24 h of culture. And cell viability of other NaF-treated groups was lower than the control group after exposure for 24 h (P < 0.05). The survival rate of BV2 cell decreased significantly with the increase of NaF concentration at 48 h and 72 h after exposure of NaF (P < 0.05). The results suggested that NaF inhibited the survival of BV2 cells in a dose-dependent manner.
Effects of fluoride on BV2 cell morphology
The cell morphology was observed with the optical inversion phase contrast microscope. As shown in Fig. 2, the cells of the control group (0 mmol/L NaF) grew with good adhesion and highly branched, and the number was more than other groups. Compared with the control group, the cell morphology of NaF-treated groups was changed. Cells of NaF-treated groups floated up and showed poor adhesion compared to cells of the control group. Protuberances of BV2 cell in NaF-treated groups disappeared. The number of cells in NaF-treated groups decreased significantly. These changes were more obvious with the extension of time and the increase of fluoride dose. These results indicated that NaF changed BV2 cell morphology in a dose- and time-dependent fashion.
Effects of fluoride on lactate dehydrogenase (LDH) release
Normally, LDH existed in the cytoplasm of living cells and could not pass through the cell membrane. But LDH could be leaked from intracellular to extracellular when the cell membrane was compromised. To explore the effect of fluoride on the integrity of cell membrane, the leakage of LDH from the cells in varying doses of NaF for 24 h was detected. As shown in Fig. 3, the leakage of LDH increased significantly in all concentrations of NaF tested (P < 0.05). At 2 mmol/L NaF, the extent of LDH leakage reached the most. Results indicated that the integrity of the cell membrane was damaged after fluoride exposure in a dose-dependent fashion.
Effects of fluoride on the expression of NLRP3/Caspase-1/GSDMD pathway-related mRNAs and proteins
To investigate the potential mechanism involved in the toxicity of fluoride to BV2 cells at gene transcription and protein expression, the levels of NLRP3, Caspase-1, GSDMD, and IL-1β mRNAs and proteins were examined after exposure of NaF for 24 h. As shown in Figs. 4 and 5, compared with the control group, the gene and protein expression levels of NLRP3, Caspase-1, GSDMD, and IL-1β were increased significantly in a dose-dependent manner after NaF treatment for 24 h (P < 0.05). The results suggested that pyroptosis was involved in BV2 cell injury induced by NaF treatment.
Effects of fluoride on the expression of NLRP3/Caspase-1/GSDMD pathway-related gene. Cells were treated with varying doses of NaF for 24 h. A–D: The results of mRNA expression of NLRP3 (A), Caspase-1 (B), GSDMD (C), and IL-1β (D). Data are expressed as mean ± SD (n = 3). *P < 0.05 and **P < 0.01 vs the control group (0 mmol/L NaF)
Effects of fluoride on the expression of NLRP3/Caspase-1/GSDMD pathway-related protein. Cells were treated with varying doses of NaF for 24 h. A: The protein expression levels of NLRP3, Caspase-1, GSDMD, and IL-1β were determined by Western Blotting; B–E: The statistical results of protein expression of NLRP3 (B), Caspase-1(C), GSDMD(D), and IL-1β (E). Data are expressed as mean ± SD (n = 3). *P < 0.05 and **P < 0.01 vs the control group (0 mmol/L NaF)
Effects of fluoride and disulfiram on BV2 cell morphology
To further discuss the role of NLRP3/Caspase-1/GSDMD pathway in NaF-induced BV2 cell injury, BV2 cells were treated with 2 mmol/L NaF in the presence or absence of disulfiram, a GSDMD inhibitor. As shown in Fig. 6, disulfiram could significantly improve the reduction of cells induced by fluorosis. This result suggested that NLRP3/Caspase-1/GSDMD signaling was involved in NaF-induced BV2 microglia damage.
Effects of fluoride and disulfiram on lactate dehydrogenase (LDH) release
The integrity of cell membrane of BV2 cells culturing for 24 h in 2 mmol/L NaF in the presence or absence of 5 μmol/L disulfiram was analyzed by determining LDH release (Fig. 7). LDH released at 2 mmol/L NaF was more than that of the control group (P < 0.05). LDH released after adding disulfiram was statistically decreased (P < 0.05). Results indicated that the integrity of the cell membrane was damaged after fluoride exposure, and disulfiram could prevent membrane damage induced by fluoride.
Effects of fluoride and disulfiram on the expression of NLRP3/Caspase-1/GSDMD pathway-related mRNAs and proteins
To explore the effect of sodium fluoride and disulfiram on pyroptosis, the gene and protein expression of NLRP3, Caspase-1, GSDMD, and IL-1β was detected by qPCR and western blotting. Compared with the control group, the mRNA and protein expression levels of NLRP3, Caspase-1, GSDMD, and IL-1β were markedly promoted after 2 mmol/L NaF treatment for 24 h (P < 0.05). However, the mRNA and protein expression levels of NLRP3, Caspase-1, GSDMD, and IL-1β were significantly decreased with the addition of the disulfiram compared with 2 mmol/L NaF treatment group (P < 0.05) (Figs. 8 and 9). The results suggested that disulfiram could inhibit pyroptosis induced by fluoride.
Effects of fluoride and disulfiram on the expression of NLRP3/Caspase-1/GSDMD pathway-related gene. Cells were treated with 0 and 2 mmol/L NaF in the presence or absence of disulfiram for 24 h. A–D: The results of mRNA expression of NLRP3 (A), Caspase-1 (B), GSDMD(C), and IL-1β (D).Data are expressed as mean ± SD (n = 3). *P < 0.05 vs the control group (0 mmol/L NaF). #P < 0.05 vs the NaF group (2 mmol/L)
Effects of fluoride and disulfiram on the expression of NLRP3/Caspase-1/GSDMD pathway-related protein. Cells were treated with 0 and 2 mmol/L NaF in the presence or absence of disulfiram for 24 h. A: The protein expression levels of NLRP3, Caspase-1, GSDMD, and IL-1β were determined by Western Blotting; B–E: The statistical results of protein expression of NLRP3 (B), Caspase-1 (C), GSDMD (D), and IL-1β (E). Data are expressed as mean ± SD (n = 3). *P < 0.05 vs the control group (0 mmol/L NaF). #P < 0.05 vs the NaF group (2 mmol/L)
Discussion
Fluoride is a necessary micronutrient for the human body, and moderate amounts of fluoride can prevent dental caries and promote bone development. However, a large number of experimental studies have found that fluoride intake beyond the appropriate range will lead to damage to the structure and function of central nervous system (Waugh 2019; Yuan et al.2019; Li et al. 2019; Dec et al. 2017). The pathological changes in the cerebrum and cerebellum of Wistar rats treated with fluoride were severe, including neuronal necrosis, gliosis, spongiform degeneration, vascular cuff, congestion, and hemorrhage (Sharma et al. 2022), and fluoride exposure significantly altered the morphological characteristics of hippocampal CA3 neurons (Ran et al. 2023). There was no clear mechanism in those changes.
In recent years, microglia have become a creative and promising therapeutic target for many neurological and mental diseases. Genome-wide association studies (GWASs) revealed that microglia expressed risk genes for many central nervous system diseases, including Alzheimer’s disease, Parkinson’s disease, schizophrenia, autism, and multiple sclerosis (Prinz et al. 2019). The dose of fluoride used in this study was determined through extensive literature review and preliminary experiments before. BV2 cells were treated with different concentrations of NaF for 24, 48, and 72 h. The inhibitory effects of excessive fluoride on BV2 cell viability were evaluated by the MTT assay. The results indicated that the survival rate of cells decreased significantly with the increase of fluoride concentration and exposure time. Based on this, 0.25 mmol/L NaF was selected as the low-dose group, 1 mmol/L NaF as the mid-dose group, and 2 mmol/L NaF as the high-dose group. Twenty-four hour was selected as the subsequent exposure time. With the increase of NaF concentrations, cells had less protuberances and poor adhesion. Cells presented spherical amoeba-like appearance. The data were consistent with previous studies (Yan et al. 2013; Yang et al. 2018).
Hyperactivation of microglia could lead to chronic inflammation and released large amounts of pro-inflammatory cytokines that aggravated nervous system damage (Subhramanyam et al. 2019). Inflammation resulted in the increase of pro-inflammatory cytokines, including IL-1β, IL-6, IL-18, and TNF-α (Fu et al. 2023). IL-1β levels in school-aged children with low-to-moderate fluoride exposure were higher (Tang et al.2023). Some experiments showed that fluoride exposure induced an increased release of pro-inflammatory cytokines (Yan et al. 2016; Zhang et al. 2022). The increased production of the pro-inflammatory cytokine from microglia led to severe oxidative stress and inflammation inside the brain regions, which led to alterations in the neurotransmitter levels and resulted in neuronal apoptosis induced by fluoride (Ottappilakkil et al. 2023). IL-1β that was a pro-inflammatory cytokine released by activated microglia induced JNK phosphorylation and was involved in NaF-induced apoptosis of hippocampal neurons and cognitive dysfunction (Zhang et al. 2022). Our results showed that NaF significantly increased mRNA and protein expression of IL-1β in BV2. IL-1β was also considered to be released by pyroptosis once induced by the activation of NLRP3 inflammasome (Wang et al. 2022).
Pyroptosis was programmed by an inflammatory caspase. NLRP3/Caspase-1/GSDMD pathway was an important pathway of pyroptosis. Caspase-1 was activated by the NLRP3 inflammasome. Activated Caspase-1 processed pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18 (Gao et al. 2022; Li et al. 2020). Moreover, activated Caspase-1 could cleave GSDMD to GSDMD-N, which formed pores in the plasma membrane and resulted in IL-1β and IL-18 release (Shi et al. 2015; Ding et al. 2016). In addition, water entered cell by those pores led to cell swelling and finally lysis. Therefore, GSDMD controlled the release of pro-inflammatory cytokines such as IL-1β and entering of water performed on inflammatory programmed cell death. GSDMD was an executive protein involved in pyroptosis, which was also known as a perforating protein. Myocardial ischemia/reperfusion injury led to cardiac pyroptosis by increasing the cleavage of Caspase-1 and GSDMD (Shi et al. 2022). It has been found that the NLRP3 inflammasome was activated in primary ovarian dysfunction rats, which triggered the cell death cascade and led to the upregulation of genes and proteins involved in the cell death pathway (Miao et al. 2023). In other experiments, the microglia were injured by cerebral ischemia/reperfusion in vitro or in vivo. The expression levels of NLRP3, Caspase-1, and IL-1β in the microglia of cerebral ischemia rats were significantly increased (Cui et al. 2022; Gong et al. 2018; Liu et al. 2021). Pyroptosis was involved in neuroinflammation and neuronal injury after traumatic brain injury, and ablation of Caspase-1 inhibited pyroptosis induced by traumatic brain injury (Liu et al. 2018). NLRP3 inflammasome and pyroptosis activation might contribute to AD-related neurodegeneration (Moonen et al. 2023). After fluoride exposure, pro-inflammatory cytokines and pyroptosis-related mRNAs and proteins were notably increased in the duodenum, jejunum, and ileum of C57BL/6 J mice model (Li et al. 2020). Our results showed that the protein and gene expression levels of NLRP3, Caspase-1, and GSDMD increased with increasing NaF concentrations, which were correlated with the extent of BV2 cells pyroptosis. It is suggested that pyroptosis mediated by NLRP3/Caspase-1/GSDMD pathway was activated by NaF treatments for 24 h in BV2 cells.
LDH is an intracellular enzyme. Once plasma membrane integrity is destroyed, they enter into the plasma or culture medium. In the present study, NaF significantly increased the levels of LDH, which was consistent with the increased pyroptosis proteins and the increased IL-1β. It was indicated that the plasma membrane was damaged with the increased permeability after exposure of BV2 cells to NaF, and then LDH and IL-1β were released.
It was necessary to select inhibitors of pyroptosis to explore the role of pyroptosis in the activation of BV2 cells induced by fluoride. There existed a large amount of advantages in disulfiram, though there were many inhibitors of pyroptosis, such as MCC950 (Coll et al. 2022) which was a kind of NLRP3 inhibitors. Disulfiram is an active L-cysteine drug that inhibits pyroptosis by blocking gasdermin D pore formation or activation of the NLRP3 inflammasome (Hu et al. 2020). Thereby, it could reduce the release of IL-1β. It has been proved that disulfiram inhibited the formation of GSDMD channels in podocytes to inhibit the occurrence of focal death and thus alleviate membranous nephropathy (Lv et al. 2022). Disulfiram or GSDMD gene knockout could inhibit the occurrence of cell death, which could improve the inflammation of acute lung injury and related intestinal mucosal damage (Zhao et al. 2022). Disulfiram inhibited the cleavage of GSDMD, alleviated acute pancreatitis and related lung injury induced by caerulein, and reduced the expression levels of IL-1β and IL-18 in pro-inflammatory cytokine (Wu et al. 2021). In addition, studies have demonstrated that disulfiram attenuated lung injury and inflammatory response in cytomegalovirus pneumonia-induced mice by inhibiting signal transduction of nuclear factor κB and activation of the NLRP3 inflammasome (Huang et al. 2022). Disulfiram could effectively improve renal function damage in kidney ischemia/reperfusion injury by inhibiting pyroptosis, both in vivo and in vitro (Cai et al. 2022). In our study, disulfiram significantly increased cell survival and cell number of NaF. Protein and mRNA expression levels of NLRP3, Caspase-1, and GMDSD were significantly decreased, and the release of LDH and IL-1β was severely decreased in the group of NaF and disulfiram. These results indicated that disulfiram prevented BV2 microglia death induced by NaF via inhibiting the activation of NLRP3 inflammasome and the conserved L-cysteine modification in GSDMD.
Conclusion
The results from our present data supported the hypothesis that pyroptosis mediated by NLRP3/Caspase-1/GSDMD pathway was the crucial mechanistic event involved in NaF-induced injury of microglia (Fig. 10). Our findings have potential implications for public health policies on the treatment of fluoride neurotoxicity. Depressed pyroptosis can alleviate some of the neuroinflammation and neurological deficits involved in the fluorosis. However, it is important to carry out in vivo research to get more information. Moreover, further experiments are warranted to reveal the details of how fluoride triggers pyroptosis in microglia.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Akinrinade ID, Memudu AE, Ogundele OM, Ajetunmobi OI (2015) Interplay of glia activation and oxidative stress formation in fluoride and aluminium exposure. Pathophysiology 22:39–48. https://doi.org/10.1016/j.pathophys.2014.12.001
Cai Q, Sun Z, Xu S, Jiao X, Guo S, Li Y, Wu H, Yu X (2022) Disulfiram ameliorates ischemia/reperfusion-induced acute kidney injury by suppressing the Caspase-11-GSDMD pathway. Ren Fail 44(1):1169–1181. https://doi.org/10.1080/0886022X.2022.2098764
Chen J, NIU Q, XIA T, Zhou GY, Li P, Zhao Q, Xu CY, Dong LX, Zhang S, Wang AG, (2018) ERK1/2-mediated disruption of BDNF-Trk B signaling causes synaptic impairment contributing to fluoride-induced developmental neurotoxicity. Toxicology 410:222–230. https://doi.org/10.1016/j.tox.2018.08.009
Chen R, Zhao LD, Liu H, Li HH, Ren C, Zhang P, Guo KT, Zhang HX, Geng DQ, Zhang CY (2017) Fluoride induces neuroinflammation and alters Wnt signaling pathway in BV2 microglial cells. Inflammation 40:1123–1130. https://doi.org/10.1007/s10753-017-0556-y
Choi AL, Sun G, Zhang Y, Grandjean P (2012) Developmental fluoride neurotoxicity: a systematic review and meta-analysis. Environ Health Perspect 120:1362–1368. https://doi.org/10.1289/ehp.1104912
Coll RC, Schroder K, Pelegrín P (2022) NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci 43(8):653–668. https://doi.org/10.1016/j.tips.2022.04.003
Cui Y, Zhang NN, Wang D, Meng WH, Chen HS (2022) Modified citrus pectin alleviates cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome activation via TLR4/NF-ĸB signaling pathway in microglia. J Inflamm Res 15:3369–3385. https://doi.org/10.2147/JIR.S366927. (eCollection 2022)
Dec K, Łukomska A, Maciejewska D, Jakubczyk K, Baranowska-Bosiacka I, Chlubek D, Wąsik A, Gutowska I (2017) The influence of fluorine on the disturbances of homeostasis in the central nervous system. Biol Trace Elem Res 177:224–234. https://doi.org/10.1007/s12011-016-0871-4
Dharmaratne RW (2019) Exploring the role of excess fluoride in chronic kidney disease: a review. Hum Exper Toxicol 38:269–279. https://doi.org/10.1177/0960327118814161
Ding JJ, Wang K, Liu W, She Y, Sun Q, Shi JJ, Sun HZ, Wang DC, Shao F (2016) Pore-forming activity and structural auto inhibition of the gasdermin family. Nature 535:111–116. https://doi.org/10.1038/nature18590
Feng F, Jia Y, Yang Y, Huan H, Lian X, XuX XF, Han X, Jiang Y (2020) Hydrogeochemical and statistical analysis of high fluoride groundwater in northern China. Environ Sci Pollut Res 27(28):34840–34861. https://doi.org/10.1007/s11356-020-09784-z
Fu YB, Cao JF, Wei XB, Ge YZ, Su ZDZ, Yu DQ (2023) Klotho alleviates contrast-induced acute kidney injury by suppressing oxidative stress, inflammation, and NF-KappaB/NLRP3-mediated pyroptosis. Int Immunopharmacol 03:110105. https://doi.org/10.1016/j.intimp.2023.110105
Gao WT, Wang XY, Zhou Y, Wang XQ, Yu Y (2022) Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther 7:196. https://doi.org/10.1038/s41392-022-01046-3
Godebo TR, Jeuland M, Tekle-Haimanot R, Shankar A, Aleehu B, Assefa G, Whitford G, Wolfe A (2020) Bone quality in fluoride-exposed populations: a novel application of the ultrasonic method. Bone Rep 12:100235. https://doi.org/10.1016/j.bonr.2019.100235
Gong Z, Pan J, Shen Q, Li M, Peng Y (2018) Mitochondrial dysfunction induces NLRP3 inflammasome activation during cerebral ischemia/reperfusion injury. J Neuroinflammation 15(1):242. https://doi.org/10.1186/s12974-018-1282-6
Grandjean P (2019) Developmental fluoride neurotoxicity: an updated review. Environ Health 18:110. https://doi.org/10.1186/s12940-019-0551-x
Hu JJ, Liu X, Xia SY, Zhang ZB, Zhang Y, Zhao JX, Ruan JB, Luo XM, Lou XW, Bai Y, Wang JH, Hollingsworth LR, Magupalli VG, Zhao L, Luo HR, Kim J, Lieberman J, Wu H (2020) FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol 21:736–745. https://doi.org/10.1038/s41590-020-0669-6
Huang X, Sun P, Qin Y, Wang XJ, Wang M, Lin Y, Zhou R, Hu W, Liu Q, Yu X, Qin A (2022) Disulfiram attenuates MCMV-induced pneumonia by inhibition of NF-κB/NLRP3 signaling pathway in immunocompromised mice. Int Immunopharmacol 103:108453. https://doi.org/10.1016/j.intimp.2021.108453
Kanagaraj VV, Panneerselvam L, Govindarajan V, Ameeramja J, Perumal E (2015) Cafeic acid, a phyto polyphenol mitigates fluoride induced hepatotoxicity in rats: a possible mechanism. BioFactors 41(2):90–100. https://doi.org/10.1002/biof.1203
Li XH, Zhang JM, Niu RY, Manthari RK, Yang KD, Wang JD (2019) Effect of fluoride exposure on anxiety- and depression-like behavior in mouse. Chemosphere 215:454–460. https://doi.org/10.1016/j.chemosphere.2018.10.070
Li MY, Wang JM, Wu PH, Manthari RK, Zhao YF, Li WP, Wang JD (2020) Self-recovery study of the adverse effects of fluoride on small intestine: involvement of pyroptosis induced inflammation. Sci Total Environ 742:140533. https://doi.org/10.1016/j.scitotenv.2020.140533
Li R, He W, Duan J, Feng S, Zhu Z, Zhang Y (2022) Existing form and distribution of fluorine and phosphorus in phosphate rock acid-insoluble residue. Environ SciPollut Res Int 29:7758–7771. https://doi.org/10.1007/s11356-021-16267-2
Li H, Zhou B, Liao P, Liao DQ, Yang LH, Wang J, Liu J, Jiang RT, Chen LM (2023) Prolonged exposure of neonatal mice to sevoflurane leads to hyper-ramification in microglia, reduced contacts between microglia and synapses, and defects in adult behavior. Front Neurol 14:1142739. https://doi.org/10.3389/fneur.2023.1142739
Liu W, Chen Y, Meng J, Wu M, Bi F, Chang C, Li H, Zhang L (2018) Ablation of Caspase-1 protects against TBI-induced pyroptosis in vitro and in vivo. J Neuroinflammation 15(1):48. https://doi.org/10.1186/s12974-018-1083-y
Liu J, Ma W, Zang CH, Wang GD, Zhang SJ, Wu HJ, Zhu KW, Xiang XL, Li CY, Liu KP, Guo JH, Li LY (2021) Salidroside inhibits NLRP3 inflammasome activation and apoptosis in microglia induced by cerebral ischemia/reperfusion injury by inhibiting the TLR4/NF-κB signaling pathway. Ann Transl Med 9(22):1694. https://doi.org/10.21037/atm-21-5752
Lv D, Jiang S, Zhang M, Zhu X, Yang F, Wang H, Li S, Liu F, Zeng C, Qin W, Li L, Liu Z (2022) Treatment of membranous nephropathy by disulfiram through inhibition of podocyte pyroptosis. Kidney Dis (Basel) 8(4):308–318. https://doi.org/10.1159/000524164
Ma Y, Ma Z, Yin S, Yan XY, Wang JD (2017) Arsenic and fluoride induce apoptosis, inflammation and oxidative stress in cultured human umbilical vein endothelial cells. Chemosphere 167:454–461. https://doi.org/10.1016/j.chemosphere.2016.10.025
Miao C, Zhao Y, Chen Y, Wang R, Ren N, Chen B, Dong P, Zhang Q (2023) Investigation of He’s Yang Chao recipe against oxidative stress-related mitophagy and pyroptosis to improve ovarian function. Front Endocrinol (Lausanne) 14:1077315. https://doi.org/10.3389/fendo.2023.1077315
Moonen S, Koper MJ, Van Schoor E, Schaeverbeke JM, Vandenberghe R, von Arnim CAF, Tousseyn T, De Strooper B, Thal DR (2023) Pyroptosis in Alzheimer’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol 145(2):175–195. https://doi.org/10.1007/s00401-022-02528-y
Ottappilakkil H, Babu S, Balasubramanian S, Manoharan S, Perumal E (2023) Fluoride induced neurobehavioral impairments in experimental animals: a brief review. Biol Trace Elem Res 201(3):1214–1236. https://doi.org/10.1007/s12011-022-03242-2
Prinz M, Jung S, Priller J (2019) Microglia biology: one century of evolving concepts. Cell 179(2):292–311. https://doi.org/10.1016/j.cell.2019.08.053
Ran RY, Xiang J, Zeng XX, He W, Dong YT, Yu WF, Qi XL, Xiao Y, Cao K, Zou J, Guan ZZ (2023) The influence of NQO2 on the dysfunctional autophagy and oxidative stress induced in the hippocampus of rats and in SH-SY5Y cells by fluoride. CNS Neurosci Ther 29(4):1129–1141. https://doi.org/10.1111/cns.14090
Rezaee T, Bouxsein ML, Karim L (2020) Increasing fluoride content deteriorates rat bone mechanical properties. Bone 136:115369. https://doi.org/10.1016/j.bone.2020.115369
Sabour S, Ghorbani Z (2013) Developmental fluoride neurotoxicity: clinical importance versus statistical significance. Environ Health Perspect 121:A70. https://doi.org/10.1289/ehp.1206192
Sharma P, Verma PK, Sood S, Singh R, Gupta A, Rastogi A (2022) Distribution of fluoride in plasma, brain, and bones and associated oxidative damage after induced chronic fluorosis in Wistar rats. Biol Trace Elem Res 200:1710–1721. https://doi.org/10.1007/s12011-021-02782-3
Shi J, Zhao Y, Wang K, Shi XY, Wang Y, Huang HW, Zhuang YH, Cai T, Wang FC, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. https://doi.org/10.1038/nature15514
Shi M, Su F, Dong Z, Shi YJ, Tian XL, Cui ZS, Li JX (2022) TRIM16 exerts protective function on myocardial ischemia/reperfusion injury through reducing pyroptosis and inflammation via NLRP3 signaling. Biochem Biophys Res Commun 632:122–128. https://doi.org/10.1016/j.bbrc.2022.09.057
Subhramanyam CS, Wang C, Hu QD, Dheen ST (2019) Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol 94:112–120. https://doi.org/10.1016/j.semcdb.2019.05.004
Tang HY, Wang MR, Li GC, Wang MW, Luo C, Zhou GY, Zhao Q, Dong LX, Liu HL, Cui YS, Liu L, Zhang S, Wang G (2023) Association between dental fluorosis prevalence and inflammation levels in school-aged children with low-to-moderate fluoride exposure. Environ Pollut 320:120995. https://doi.org/10.1016/j.envpol.2022.120995
Wang L, Ren W, Wu Q, Liu T, Wei Y, Ding J, Zhou C, Xu H, Yang S (2022) NLRP3 inflammasome activation: a therapeutic target for cerebral ischemia-reperfusion injury. Front Mol Neurosci 15:847440. https://doi.org/10.3389/fnmol.2022.847440
Waugh DT (2019) Fluoride exposure induces inhibition of sodium/iodide symporter (NIS) contributing to impaired iodine absorption and iodine deficiency: molecular mechanisms of inhibition and implications for public health. I Int J Environ Res Public Health 16:1086. https://doi.org/10.3390/ijerph16061086
WHO (2011) Guideline for drinking-water quality, 4th edn. World Health Organization, Geneva
Wu JX, Zhang JT, Zhao JP, Chen SH, Zhou T, Xu JW (2021) Treatment of severe acute pancreatitis and related lung injury by targeting gasdermin D-mediated pyroptosis. Front Cell Dev Biol 9:780142. https://doi.org/10.3389/fcell.2021.780142
Xie J, Yan X, Xu G, Tian X, Dong N, Feng J, Liu P, Li M, Zhao Y, Wei C, LyuY MG, Song G, Wang T, Yan X (2020) ITRAQ-based proteomics reveals the potential mechanism of fluoride-induced myocardial contraction function damage. Ecotoxicol Environ Saf 197:110605. https://doi.org/10.1016/j.ecoenv.2020.110605
Yan L, Liu SN, Wang C, Wang F, Song YL, Yan N, Xi SH, Liu ZY, Sun GF (2013) JNK and NADPH oxidase involved in fluoride-induced oxidative stress in BV-2 microglia cells. Mediators Inflamm 2013:895975. https://doi.org/10.1155/2013/895975
Yan N, Liu Y, Liu S, Cao S, Wang F, Wang Z, Xi S (2016) Fluoride-induced neuron apoptosis and expressions of inflammatory factors by activating microglia in rat brain. Mol Neurobiol 53(7):4449–4460
Yang L, Jin PY, Wang XY, Zhou Q, Lin XL, Xi SH (2018) Fluoride activates microglia, secretes inflammatory factors and influences synaptic neuron plasticity in the hippocampus of rats. Neurotoxicology 69:108–120. https://doi.org/10.1016/j.neuro.2018.09.006
Yuan JQ, Li Q, Niu RY, Wang JD (2019) Fluoride exposure decreased learning ability and the expressions of the insulin receptor in male mouse hippocampus and olfactory bulb. Chemosphere 224:71–76. https://doi.org/10.1016/j.chemosphere.2019.02.113
Zhang Y, Wu JL, Jiang L, Lu CK, Huang ZW, Liu B (2021) Prospects for the role of ferroptosis in fluorosis. Front Physiol 12:773055. https://doi.org/10.3389/fphys.2021.773055
Zhang C, Yang Y, Gao Y, Sun DJ (2022) NaF-induced neurotoxicity via activation of the IL-1β/JNK signaling pathway. Toxicology 469:153132. https://doi.org/10.1016/j.tox.2022.153132
Zhao J, Wang H, Zhang J, Ou F, Wang J, Liu T, Wu J (2022) Disulfiram alleviates acute lung injury and related intestinal mucosal barrier impairment by targeting GSDMD-dependent pyroptosis. J Inflamm (Lond) 19(1):17. https://doi.org/10.1186/s12950-022-00313-y
Zheng DS, Liu YL, Luo LL, Shahid MZ, Hou DM (2020) Spatial variation and health risk assessment of fluoride in drinking water in the Chongqing urban areas, China. Environ Geochem Health 42:2925–2941. https://doi.org/10.1007/s10653-020-00532-3
Funding
This work was supported by the Natural Science Foundation of Inner Mongolia (2021LHMS08012) and the Graduate Research Innovation Project of Inner Mongolia (S20210172Z).
Author information
Authors and Affiliations
Contributions
Qiuyi Zhang: conceptualization, methodology, software, investigation, and writing—original draft. Tao Li: validation and formal analysis. Ruili Shi: supervision. Ruifang Qi: software. Xiaoqiong Hao: data curation. Baohui Ma: writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All individual participants gave their oral and written informed consent in this work.
Consent for publication
All authors have read the manuscript and agreed to submit it in its current form for consideration for publication in the journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Li, T., Shi, R. et al. Fluoride promotes the secretion of inflammatory factors in microglia through NLRP3/Caspase-1/GSDMD pathway. Environ Sci Pollut Res 31, 19844–19855 (2024). https://doi.org/10.1007/s11356-024-32443-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32443-6