Abstract
Fluoride is a common element in nature and our daily life, and excessive intake of this element can cause fluorosis and irreversible brain damage. The toxic effects of fluoride on the central nervous system may be attributed to the release of inflammatory cytokines and ROS. GSK3β is a key protein that modulates NF-κB activity and inflammatory cytokine levels and plays an important role in the Wnt signaling pathway. In this study, we found that fluoride altered the inflammatory status and oxidative stress by inhibiting Wnt signaling pathway activity. This study thus provides a valid basis for the fluorine-induced neuroinflammation injury theory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Fluoride is a common element in nature and our daily life that is present in drinking water, soil, and the atmosphere. Recently, some reports have shown that excessive fluoride intake can cause fluorosis and irreversible brain damage, with main clinical manifestations in learning and memory and other cognitive dysfunctions [1, 2]. In animals, chronic fluorosis decreases the number of nicotinic acetylcholine receptors (nAChRs), which play an important role in cognitive processes in mice [3]. Some studies have suggested that children living in high-fluoride areas have lower IQ scores than those living in low-fluoride areas [4], and extensive evidence has shown that fluoride can impair cognitive function. However, the mechanism underlying fluoride-induced cognitive impairment is not fully understood.
Some groups have reported that fluoride can induce oxidative stress, characterized by increased intracellular reactive oxygen species (ROS) levels, in brain tissue [5]. The central nervous system (CNS) is sensitive to ROS, which play a key role in the pathogenesis of many diseases, including neuroinflammation [6]. The neuroinflammation process is meditated by microglia: activated microglia release pro-inflammatory cytokines and neurotoxic molecules such as IL-1β, IL-6, and TNF-α, which can induce tissue injury and damage neural cells [6, 7]. In a previous study, we demonstrated that fluorosis causes cognitive impairment in a rat model [2]. Several other studies also found that neuroinflammation and the release of pro-inflammatory cytokines are associated with cognitive impairment, such as that in AD [8].
Neuroinflammatory mechanisms are complex and incompletely understood. Inflammation is characterized by activation of the pro-inflammatory transcription factor nuclear factor-κB (NF-κB) and increased cytokine levels. In the CNS, NF-κB activity and cytokine levels are regulated by glycogen synthase kinase-3β (GSK3β), a key protein that maintains the balance between pro- and anti-inflammatory function [9] and is a critical protein in the Wnt signaling pathway [10]. Previous research has demonstrated that the Wnt signaling pathway can regulate neural stem cell proliferation, differentiation, and migration, thus affecting learning and memory [11, 12]. The Wnt signaling pathway can also regulate neuroinflammation to promote chronic pain. Dickkopf-1 (DKK1) is an antagonist that blocks the Wnt signaling pathway by binding to low-density lipoprotein receptor-related protein 5 and 6 (LRP5/6), which is over expressed in patients with cognitive impairment. Some studies have indicated that persistent inflammation can activate various cell stress response pathways, including DKK1 overexpression [13]. Therefore, we hypothesized that fluoride promotes neuroinflammation by modulating Wnt signaling, leading to cognitive impairment.
MATERIALS AND METHODS
Materials
DMEM/H-G was purchased from HyClone (Logan, UT, USA), and fetal bovine serum (FBS) was purchased from Gemini Bio-products (Mexico). Sodium fluoride (NaF) was purchased from Jena Bioscience (Germany). Lithium chloride (LiCl) was purchased from Sigma-Aldrich (USA).
Cell Culture
BV2 cells were a gift from Dr. Yuan-jian Song (Neurobiology laboratory, Xuzhou Medical University, Xuzhou, China). Cells were cultured in DMEM/H-G containing 10% FBS and antibiotics (100 IU/ml penicillin and 100 mg/ml streptomycin) and maintained at 37 °C and 5% CO2 in an incubator (Heal Force Development LTD, Hong Kong). The growth medium was changed every day, and cells were subcultured when 80–90% confluent.
CCK-8 Cell Viability Assay
Cell viability was detected via the CCK-8 method (Dojindo, Japan). Cells were seeded into 96-well plates at a density of 6000 cells/100 μl. As soon as they became stable (approximately 2 h later), the cells were exposed to various NaF concentrations (0, 250, 500, 1000, or 2000 μm/L) for 6, 12, or 24 h. After treatment, the cells were washed twice with phosphate-buffered saline (PBS), 10% CCK-8 was added to the medium, and the plates were incubated for 30 min. Absorption was measured at 450 nm.
Biochemical Analysis
The oxidation–antioxidation status of the NaF-treated BV2 cells was evaluated via superoxide dismutase (SOD) activity and lipid peroxidation. Lipid peroxidation was determined by testing the malondialdehyde (MDA) level. Briefly, BV2 cells were treated with various concentrations of NaF (0, 250, 500, 1000, or 2000 μm/L) for 24 h, and the cell supernatant was transferred to tubes. SOD activity and MDA levels were determined by following the manufacturer’s instructions for their reagent kits (Nanjing Jiancheng, China).
Measurement of Intracellular ROS Production
A reactive oxygen species assay kit was used to measure intracellular ROS generation with the fluorescent marker DCFH-DA. Briefly, BV2 cells were seeded into 6-well plates and treated with different concentrations of NaF (0, 250, 500, 1000, or 2000 μm/L) for 2 h. We selected 2 h as the treatment period because ROS are generated during the early stages of cell apoptosis [14]. Then, the medium was removed, and the cells were incubated with DCFH-DA for 20 min at 37 °C before washing three times with serum-free medium (SFM). The DCFH-DA was diluted in SFM at a ratio of 1:1000 to a final concentration of 10 μmol/L. ROS generation was observed directly with a fluorescence microscope (Olympus, Japan).
ELISA
BV2 cells were seeded into 24-well plates and treated with various concentrations of NaF (0, 250, 500, 100, 1000, or 2000 μmol/L) for 24 h. The IL-6 and TNF-α levels in the culture medium were measured with an ELISA kit (KeyGen Biotech, Nanjing, China) according to the manufacturer’s instructions.
Immunofluorescence Labeling Analysis
BV2 microglial cells were seeded into 48-well plates and treated with various concentrations of NaF (0, 250, 500, 1000, or 2000 μmol/L). NF-κB p52 localization (Santa Cruz, USA) was visualized in BV2 microglial cells pretreated with NaF for 24 h. First, the cells were fixed with 4% paraformaldehyde, permeabilized with 0.3% Triton X-100, blocked with 10% goat serum for 40 min, and incubated with an anti-NF-κB p52 antibody (1:250) overnight at 4 °C. Then, the cells were stained with an anti-rabbit IgG secondary antibody (1:100, VICMED Life Science, China) for 2 h at room temperature. Finally, the cells were counterstained with DAPI (Beyotime Institute of Biotechnology, Jiangsu, China) for 5 min after being washed with PBS. Images were captured with a fluorescence microscope (Olympus, Japan).
Western Blot Analysis
BV2 cells exposed to different concentrations of NaF (0 to 2000 μM) for 24 h were washed twice in ice-cold PBS and lysed in RIPA lysis buffer for 30 min on ice. The lysates were centrifuged at 12,000 rpm for 20 min at 4 °C, and the supernatants were collected and used for evaluation of the relevant protein levels. The protein concentration in each sample was measured using a BCA Protein Assay Kit. Western blots were performed according to standard protocols. Rabbit oligoclonal antibodies (anti-p-GSK3β and anti-GSK3β, 1:1000, Cell Signaling Technology, USA; anti-β-catenin and anti-DKK1, 1:1000, Abcam, UK; anti-β-actin, 1:1000, Santa Cruz Biotechnology, USA) and anti-rabbit IgG secondary antibodies (1:200, Santa Cruz Biotechnology, USA) were used in this study.
Statistical Analysis
All results are expressed as the mean ± SD. Significant differences between different groups were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s test. Statistical significance was set at P < 0.05.
RESULTS
NaF Decreases BV2 Cell Viability
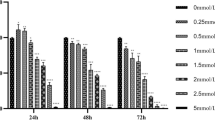
BV2 cell viability was evaluated with a CCK-8 assay. To exclude the possibility that NaF is directly toxic to microglia, different concentrations of NaF (ranging from 0 to 2000 μm/L) were added to BV2 cells, and cell viability was evaluated at 6, 12, and 24 h. As shown in Fig. 1a, BV2 cell viability increased in the 250 and 500 μm/L NaF-treated groups following 6 h of culture and then returned to the control level at higher NaF concentrations. As shown in Fig. 1b, c, NaF showed no cytotoxicity on BV2 cells at low concentrations after 12 or 24 h of incubation. However, cell viability decreased significantly after treatment with high NaF concentrations. Thus, 2000 μmol/L NaF significantly reduced BV2 cell viability relative to the control group (P < 0.001).
The Effect of NaF on MDA Levels, T-SOD Activity, and Intracellular ROS Production
MDA is a common indicator of lipid peroxidation in the cell membrane. The MDA level reflects the degree of lipid peroxidation and indirectly reflects the degree of cell damage. SOD is a vital enzyme that maintains the balance between oxidation and antioxidation and protects cells from damage by removing superoxide anion-free radicals. ROS, including oxygen ions, peroxide, and oxygen-free radicals, are a by-product of aerobic metabolism. High ROS levels can damage cells and DNA. MDA levels and T-SOD activity were measured with relevant assay kits, and intracellular ROS production was measured with an ROS assay kit. As shown in Fig. 2a, b, the MDA level increased in the NaF group, although there were no significant differences between the NaF group and the control group. SOD activity was significantly lower in the 2000 μmol/L NaF group than the control group (P < 0.001). As shown in Fig. 2c, the DCFH-DA fluorescence intensity gradually increased as the NaF concentration increased, indicating that intracellular ROS levels increased gradually relative to the control group.
The effect of NaF on MDA levels, SOD activity, and intracellular ROS production. a The MDA level in BV2 cells increased in the NaF group, although there was no significant difference between the NaF group and the control group. b SOD activity was significantly lower in the 2000 μmol/L NaF group than the control group (P < 0.001). The data are presented as the mean ± SD (n = 3) of three independent experiments.
NaF Stimulates the Production of IL-6 and TNF-α in BV2 Cells
Activated microglia secrete pro-inflammatory cytokines such as IL-6 and TNF-α. These cytokines are considered inflammation biomarkers and were measured by ELISA. As shown in Fig. 3a, the IL-6 concentration was significantly higher in the 1000 and 2000 μmol/L NaF groups than in the control group after 24 h (P < 0.001). As shown in Fig. 3b, only 2000 μmol/L NaF induced a significant increase in TNF-α levels (P < 0.05).
NaF Induces NF-κB Activation in BV2 Cells
NF-κB plays a crucial role in the neuroinflammatory response and promotes the production of pro-inflammatory mediators such as iNOS, IL-1β, TNF-α, and IL-6. NF-κB activity is attributed to the Rel/NF-κB protein family, the members of which form homodimers and heterodimers consisting of the p65 (or RelA), p50, p52, c-Rel, and RelB subunits. IL-1β, TNF-α, and ROS can induce NF-κB by activating IκB kinases, which phosphorylate IκBα, leading to its polyubiquitination and degradation by the 26S proteasome [15]. This allows NF-κB to translocate to the nucleus and modulate the transcription of its target genes. Immunofluorescence labeling was used to determine the effect of NaF on NF-κB activity. As shown in Fig. 4, NF-κB p52 translocated into the nucleus of microglial cells after NaF exposure, possibly causing the increase in IL-6 and TNF-α levels.
The Effect of NaF on the Expression of DKK1 and Wnt Signaling Pathway Components in BV2 Cells
To determine whether NaF-induced neuroinflammation changed the levels of proteins in the canonical Wnt signaling pathway, western blots were used to measure the DKK1, p-GSK3β, GSK3β, and β-catenin levels. As shown in Fig. 5a, NaF increased DKK1 expression and the p-GSK3β/GSK3β ratio. β-Catenin expression clearly decreased as the NaF concentration increased, indicating that NaF inhibited the canonical Wnt signaling pathway in BV2 cells by increasing the DKK1 level and suggesting that this pathway might be involved in NaF-induced neuroinflammation. To further understand the possible role of the Wnt signaling pathway in fluoride-induced neuroinflammation, BV2 cells were treated with 2000 μmol/L NaF in the presence or absence of the Wnt inhibitor LiCl (20 nM). As shown in Fig. 5b, c, the GSK3β inhibitor LiCl reduced the GSK3β activity and increased β-catenin expression. LiCl also markedly reduced the TNF-α and IL-6 levels in the cell culture medium. This suggests that fluoride inhibits the Wnt signaling pathway, which is involved in NaF-induced neuroinflammationin BV2 cells.
DISCUSSION
Fluorosis is very common in developing countries. According to the literature, higher fluoride intake may result in dental and skeletal fluorosis as well as learning and memory impairment [1, 2, 16]. A previous report suggested that almost all elementary and junior high school students 10–15 years of age suffered dental fluorosis (99.5%), and the prevalence of skeletal fluorosis was 42.1% in the fluorosis area of China [17]. Choi et al. performed a meta-analysis and found that children in high-fluoride areas had significantly low IQ scores than these who lived in low-fluoride areas. This result supports the possibility of an adverse effect of high-fluoride exposure on children’s neurodevelopment [18]. In another project of our team, cognitive function of elder people was investigated in the high-fluoride area. The results indicated that the cognitive impairment is more serious in high-fluoride areas than in low-fluoride areas (the data unpublished). These studies demonstrated that the high-fluoride drinking water is a risk factor to our health.
Fluoride exposure increases IL-1β and TNF-α production and changes the cellular oxidation and antioxidation status [5, 19]. Neuroinflammation seems to be an important mediator of the effects of fluoride. Neuroinflammation, which is mediated by microglia, contributes to most pathological neurologic processes, including CNS infections, ischemic stroke, neurodegenerative disease, and anesthetic neurotoxicity. Increased pro-inflammatory mediator production is thought to cause neuronal cell death. As previously mentioned, inflammation is characterized by activation of the pro-inflammatory transcription factor NF-κB, a process mediated by GSK3β, a critical protein in the Wnt signaling pathway [9]. Therefore, we conducted the present study to investigate the changes in the Wnt signaling pathway in BV2 cells exposed to NaF.
Microglia are the resident macrophages in the brain and play critical roles in the development and maintenance of the neural environment [20]. To exclude the possibility that NaF directly affects microglial viability and proliferation, we examined the effects of different NaF concentrations on BV2 viability at different time points. As shown in Fig. 1, BV2 cell viability increased after treatment with lower concentrations of NaF for 6 h. This increase in cell viability was likely caused by a stress response to NaF. However, cell viability decreased significantly when the NaF concentration increased, indicating that high NaF concentrations are toxic to cells.
Microglia can be activated in response to perturbations in the brain microenvironment or changes in neuronal structure. Activated microglia cells break the balance between oxidation and antioxidation. MDA levels reflect the degree of lipid peroxidation and indirectly reflect the degree of cell damage. SOD, an antioxidant, can defend against ROS activity and reduce the MDA level. ROS can cause oxidative damage to cells and DNA structure [2]. As shown in Fig. 2, the MDA levels increased in the NaF groups. SOD activity decreased significantly at higher NaF concentrations. The intracellular ROS levels increased as the NaF concentration increased. Activated microglia also produce various inflammatory mediators, including IL-6 and TNF-α, which are the two main pro-inflammatory cytokines produced by these cells. As shown in Fig. 3, higher NaF concentrations stimulated IL-6 and TNF-α production in BV2 cells. This result could indicate that NaF-induced oxidative stress and inflammation play an important role in fluorosis.
Previous studies have shown that the Wnt signaling pathway can affect cognitive function by regulating the proliferation, differentiation, and migration of neural stem cells. This pathway includes several members of the Frizzled protein family and LRP5/6, the activation of which leads to the downregulation of glycogen synthase kinase-3β (GSK3β) activity. GSK3β inactivity increases the β-catenin level in the cytosol; β-catenin then translocates into the nucleus. β-Catenin and T cell factor/lymphoid enhancer factor (TCF/LEF) form complexes that modulate the transcription of target genes involved in apoptosis, proliferation, migration, differentiation, and metabolism [21].GSK3β is an important protein in the Wnt signaling pathway. In addition to its role in the Wnt pathway, increased GSK3β activity could be related to the presence of neuroinflammation. GSK3β has been linked to the modulation of several transcription factors, including NF-κB activation and β-catenin inhibition [22]. Some studies have indicated a possible crosstalk between the NF-κB signaling pathway and the Wnt/β-catenin pathway. It is well known that both pathways play complex roles in the pathogenesis of certain age-related diseases; for example, while both β-catenin and NF-κB activate iNOS expression, β-catenin also has an inhibitory effect on NF-κB-mediated transcriptional activation, including for iNOS [23]. It is unclear whether there is functional cross regulation between these two pathways (NF-κB and β-catenin) in NaF-induced neuroinflammation. In the present study, we found that NaF activated microglia and increased GSK3βand NF-κB activity, while inhibiting β-catenin expression (Figs. 4 and 5). This result is consistent with reports investigating age-related neuroinflammation, Akt-GSK3β, and Wnt/β-catenin signaling in the rat hippocampus [9].
DKK1 has been described as an extracellular antagonist of the Wnt pathway, and studies have shown that DKK1 is required for the pathogenesis of many neurodegenerative diseases, including AD in patients and animal models [24], frontotemporal dementia in transgenic mice [25], ischemic insults [26], and mesial temporal lobe epilepsy with hippocampal sclerosis [27]. However, the relationship between DKK1 level and fluorosis remains unknown. In this study, we established a model of NaF-induced neuroinflammation to measure DKK1 expression and found increased DKK1 levels in BV2 cells treated with various concentrations of NaF. This result suggested that DKK1, an inhibitor of the canonical Wnt signaling pathway, might be involved in NaF-induced neuroinflammation.
In summary, our study showed that NaF increases the production of the pro-inflammatory mediators IL-6, TNF-α, and ROS and disrupts the oxidation–antioxidation balance. These inflammatory processes may result from Wnt/β-catenin signaling pathway inhibition and NF-κB activation, and these pathways may represent good targets for protecting the CNS against fluorosis. The crosstalk between the NF-κB and Wnt/β-catenin signaling pathways should be investigated in future studies of neuroinflammation.
References
Liu, F., et al. 2014. Fluoride exposure during development affects both cognition and emotion in mice. Physiology & Behavior 124: 1–7.
Zhang, C., et al. 2013. The analog of Ginkgo biloba extract 761 is a protective factor of cognitive impairment induced by chronic fluorosis. Biological Trace Element Research 153 (1–3): 229–236.
Long, Y.G., et al. 2002. Chronic fluoride toxicity decreases the number of nicotinic acetylcholine receptors in rat brain. Neurotoxicology and Teratology 24 (6): 751–757.
Khan, S.A., et al. 2015. Relationship between dental fluorosis and intelligence quotient of school going children in and around Lucknow District: a cross-sectional study. Journal of Clinical and Diagnostic Research 9 (11): ZC10–ZC15.
Shuhua, X., et al. 2012. A role of fluoride on free radical generation and oxidative stress in BV-2 microglia cells. Mediators of Inflammation 2012: 102954.
Abeti, R., and M.R. Duchen. 2012. Activation of PARP by oxidative stress induced by beta-amyloid: implications for Alzheimer’s disease. Neurochemical Research 37 (11): 2589–2596.
Zhou, J., et al. 2008. Complement C3 and C4 expression in C1q sufficient and deficient mouse models of Alzheimer’s disease. Journal of Neurochemistry 106 (5): 2080–2092.
Cortese, G.P. and C. Burger, Neuroinflammatory challenges compromise neuronal function in the aging brain: postoperative cognitive delirium and Alzheimer’s disease. Behav Brain Res, 2016.
Orellana, A.M., et al. 2015. Age-related neuroinflammation and changes in AKT-GSK-3beta and WNT/ beta-CATENIN signaling in rat hippocampus. Aging (Albany NY) 7 (12): 1094–1111.
Ajmone-Cat, M.A., et al. 2016. Glycogen synthase kinase 3 is part of the molecular machinery regulating the adaptive response to LPS stimulation in microglial cells. Brain, Behavior, and Immunity 55: 225–235.
Chen, J., C.S. Park, and S.J. Tang. 2006. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. The Journal of Biological Chemistry 281 (17): 11910–11916.
Margarit, C., et al. 1998. Efficacy and safety of oral low-dose tacrolimus treatment in liver transplantation. Transplant International 11 (Suppl 1): S260–S266.
Esposito, G., et al. 2008. S100B induces tau protein hyperphosphorylation via Dickopff-1 up-regulation and disrupts the Wnt pathway in human neural stem cells. Journal of Cellular and Molecular Medicine 12 (3): 914–927.
Ke, L., et al. 2016. Effects of sodium fluoride on lipid peroxidation and PARP, XBP-1 expression in PC12 cell. Biological Trace Element Research 173 (1): 161–167.
Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-kappaB puzzle. Cell 109 (Suppl): S81–S96.
Chioca, L.R., et al. 2008. Subchronic fluoride intake induces impairment in habituation and active avoidance tasks in rats. European Journal of Pharmacology 579 (1–3): 196–201.
Ando, M., et al. 1998. Health effects of indoor fluoride pollution from coal burning in China. Environmental Health Perspectives 106 (5): 239–244.
Choi, A.L., et al. 2012. Developmental fluoride neurotoxicity: a systematic review and meta-analysis. Environmental Health Perspectives 120 (10): 1362–1368.
Yan, L., et al. 2013. JNK and NADPH oxidase involved in fluoride-induced oxidative stress in BV-2 microglia cells. Mediators of Inflammation 2013: 895975.
Kraft, A.D., and G.J. Harry. 2011. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. International Journal of Environmental Research and Public Health 8 (7): 2980–3018.
Teo, J.L., and M. Kahn. 2010. The Wnt signaling pathway in cellular proliferation and differentiation: a tale of two coactivators. Advanced Drug Delivery Reviews 62 (12): 1149–1155.
Die, L., et al. 2012. Glycogen synthase kinase-3 beta inhibitor suppresses Porphyromonas gingivalis lipopolysaccharide-induced CD40 expression by inhibiting nuclear factor-kappa B activation in mouse osteoblasts. Molecular Immunology 52 (1): 38–49.
Du, Q., and D.A. Geller. 2010. Cross-regulation between Wnt and NF-kappaB signaling pathways. For Immunopathol Dis Therap 1 (3): 155–181.
Caricasole, A., et al. 2004. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. The Journal of Neuroscience 24 (26): 6021–6027.
Rosi, M.C., et al. 2010. Increased Dickkopf-1 expression in transgenic mouse models of neurodegenerative disease. Journal of Neurochemistry 112 (6): 1539–1551.
Cappuccio, I., et al. 2005. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. The Journal of Neuroscience 25 (10): 2647–2657.
Busceti, C.L., et al. 2007. Induction of the Wnt inhibitor, Dickkopf-1, is associated with neurodegeneration related to temporal lobe epilepsy. Epilepsia 48 (4): 694–705.
Acknowledgements
This study was supported by BK20151159 from the Natural Science Foundation of Jiangsu Province, China, Project 81501185 of National Natural Science Foundation of China, Jiangsu Provincial Medical Youth Talent No.QNRC2016369, Xuzhou Medical Talents Project and Xuzhou technological and scientific project No. KC14SH050.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Rui Chen, Lian-Dong Zhao, and Hong Liu are the authors who contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, R., Zhao, LD., Liu, H. et al. Fluoride Induces Neuroinflammation and Alters Wnt Signaling Pathway in BV2 Microglial Cells. Inflammation 40, 1123–1130 (2017). https://doi.org/10.1007/s10753-017-0556-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0556-y