Abstract
Sulfides are usually associated with deposits of metals and coal. The reactive wastes from their exploitation, typically stored in piles and tailings dams, are often the mining sector’s primary source of environmental problems. The surrounding river waters can present signs of acid mine drainage, responsible for aquatic ecosystem degradation. So, the main target of the present study is to investigate the impact of this process on the water’s environmental quality and potential ecological risk. The study area is located at the Iberian Pyrite Belt, in an old sulfide exploitation, closed without environmental rehabilitation measures. The results exhibit high sulfate concentrations (410,601 mg/L) and potentially toxic elements, with prominence of Fe (134,000 mg/L), overcoming many other extreme cases of AMD pollution. The Ficklin diagram exposes that most samples are classified as “high-acid, high-metal.” Two of them have extreme classifications (high-acid, extreme-metal). The pH value is well below the acceptable range for the environmental quality of superficial waters (5–7), measuring at a minimum of 0.84. Regarding seasonal variability, the study showed a higher degree of contamination in dry conditions (e.g., 4,420 mg/L of Cu), while the rainy month had lower concentrations of PTE (186.8 mg/L of Cu for the same sampling point). In addition, the water does not accomplish the environmental objectives established by the EU Water Framework Directive. According to the new approach developed based on a scale adjustment, the potential ecological risk index studied indicates that most sampled sites present strong, very strong, and even extremely potential ecological risk. With a typical Mediterranean climate, the region suffers from water scarcity, predicting increasingly in the future more degrading scenarios for water environmental quality. Consequently, urgent mitigation and remediation measures are necessary to improve and preserve water quality and fulfill the objectives of the United Nations Sustainability Development Goals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfides are the most common minerals, stable under reducing circumstances (Nordstrom and Alpers 1999). Lottermoser (2010) highlights the role of pyrite, marcasite, pyrrhotite, and chalcopyrite due to their abundance and environmental relevance under oxidative dissolution conditions. They are usually associated with coal and metallic mining (e.g., Cu, Zn, and Pb). However, exploitation of these elements can generate large amounts of waste that are typically stored in specific infrastructures, such as piles around the mine and tailings dams, constituting the main environmental focus of the mining sector (e.g., Sánchez España et al. 2008). The problem centers on mineral–water, mineral-atmosphere, and mineral-biosphere interactions, producing acid mine drainage (AMD) (Nordstrom et al. 2015). Classic mining landscapes start to present waters with typical ochre coloration, supergenic minerals in various bright colors (Alpers et al. 1994; Wolkersdorfer et al. 2020), and crustification of streambeds (Valente et al. 2012). Also, the receiving water systems are characterized by low pH, high concentrations of acidity, sulfate, and potentially toxic elements (PTE) (Gomes et al. 2018), which often behave as permanent pollutants (Kicińska et al. 2021). From an ecological point of view, the specific impact of sulfide-rich wastes is often manifested by the appearance of extremophiles, where acidophilic algae and other microorganisms essentially proliferate (e.g., Aguilera et al. 2006; Amils et al. 2011; Levings et al. 2005; Schneider et al. 2018; Gomes et al. 2021).
As water is an essential resource for the maintenance of biota, its environmental quality is a critical factor influencing ecosystems and human health (Zhang et al. 2012). Therefore, water availability with a suitable value for specific uses, like human consumption or maintenance of ecological quality, is a crucial issue, especially in semi-arid climates (Tiri et al. 2014). Nevertheless, water scarcity associated with frequent drought generates deep concern about the quantity and quality of this vital resource (Bonnail et al. 2019). According to the same authors, the environmental quality standards in aquatic ecosystems have been increasingly prioritized by the EU Water Framework Directive (WFD, Directive, 2000/60/EU, and modifications such as Directives, 2008/105/EU and 2013/39/EU). “Good chemical status,” such as PTE, and “ecological status” are two fundamental environmental quality standards for surface waters. According to Zhou et al. (2022) and Zhang et al. (2019), it is necessary to identify factors, such as land use, meteorology, and hydrology, that may contribute to degradation and explore their effects on water quality to protect riverine water resources scientifically. In this context, according to knowledge, no study has been carried out that allows the assessment of surface waters in the old mining area of São Domingos about current EU requirements. Furthermore, it developed and proposed an addition of new classes of potential ecological risk index. So, this work intends to investigate the fulfillment of quality environmental objectives for surface water, the impact that water scarcity, related to seasonality, may have in the aquatic medium, and the potential ecological risk inherent in current conditions. The present study focuses on the hydrochemical properties of two streams that run through an old sulfide mine, representing a unique scenario of AMD contamination. Downstream, the reservoir (The Chança River) is used for drinking water production. So, the study contemplated 12 sampling points, including a pit lake and water dams along the streams, over a complete hydrological year. Thus, this investigation intends to contribute to the knowledge about the available water bodies, warning to their appropriate management — in terms of improving ecological and functional quality — in a traditional mining region with a typical Mediterranean climate that faces water shortages.
Materials and methods
Study area and sampling sites
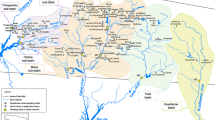
The Iberian Pyrite Belt (IPB) is a large metallogenic province in Portugal and Spain known as one of the major in the world (Fig. 1). Mining activities in the region have resulted in important contamination due to the exploitation of sulfide deposits. The São Domingos mine (Fig. 1), situated in the Portuguese sector of IPB, has a long history of mining activity dating back to pre-Roman times, with operations continuing until 1966. It was closed without environmental remediation, having recently started a rehabilitation project (EDM 2021; www.edm.pt.). Several authors (e.g., Abreu et al. 2008; Tavares et al. 2009; Pérez-López et al. 2008) characterized the study area as exhibiting low pH and abundant elements with potential implications for the environment and human health, such as Fe, Cu, Zn, Sb, As, Hg, and Pb. Numerous old infrastructures (ore-processing plants and machinery), waste dumps, and tailings dams are disseminated along the mining complex (Cordeiro et al. 2017). The pit lake (PAT2 in Figs. 1 and 2) reached a depth of 120 m and a perimeter of 2 km. PAT1, the Tapada Grande, was initially constructed for mining activities and is now a reservoir for recreational use. The remaining sampling points are located within the mining complex along the two main streams that drain the area: São Domingos and Mosteirão (Fig. 1). The waters have an intense red–orange color associated with high iron concentrations and ochre-precipitates deposition (Fig. 2a, b) (Gomes et al. 2017). Regarding aquatic biodiversity, blooms of acidophilic algae are frequently reported (Fig. 2d) (Wolowski et al. 2008; Luís et al. 2019) but with low diversity (Gomes and Valente 2019).

Adapted from Gomes et al. 2022
Sketch with the location of the São Domingos mine in the Portuguese sector of the Iberian Pyrite Belt and respective sampling sites (PAT1 to PAT12).
Samples were collected monthly from October 2016 to September 2017 in a complete hydrological year. The sampling sites can be categorized according to their hydrological conditions related to the flow regime. PAT1, PAT2, PAT3, PAT4, PAT5, PAT7, and PAT12 generally indicate lentic behavior, while the remaining PATs indicate lotic conditions (Fig. 2) (Gomes et al. 2022). The final receptor of the acidic discharges is the Chança River, here entitled with PAT12, which presently works as a reservoir for human supply.
Climate conditions
Concerning average monthly precipitation, the 30 hydrological years (1936/37–1966/67) demonstrated that precipitation varied between 1.1 and 85.9 mm, in July and January, respectively. For the same period, the average air temperature varied between 10.4 °C in January and 25.7 °C in July, with the hottest period occurring from May to October, while November to April is the coldest period. According to the United Nations Framework Convention on Climate Change (ENAAC, 2010), Portugal is one of the European countries that is most vulnerable to the impacts of climate change. In this sense, risk situations such as precipitation peaks, heat waves, and storms associated with strong winds can occur more frequently (APA, 2016). The year of the present sampling was, especially in Portugal, atypical of high temperatures. The country suffered heat waves that led to fires of magnitudes and proportions never before reported, affecting numerous populations. Thus, the period corresponding to the sampling (2016 to 2018) was analyzed. So, the average monthly temperature for this period reveals that the driest months are also the hottest. The coldest month is January, with the lowest temperature recorded (7.7 °C) in 2017. July 2016 was the hottest month, with an average temperature of 26.6 °C. These data demonstrate higher thermal amplitude and temperature increase. Figure 3 presents the rainy season from October to March, with an average rainfall of 54 to 72 mm, and the dry period comprises the months between April and September, with rainfall ranging from 17.6 to 0.2 mm. Figure 3 also shows February as having the highest rainfall, 72.6 mm. July is the driest month, with 0.2 mm, showing less precipitation during the study period.
Analytical methods
The pH, electrical conductivity (EC at 25 °C), and potential redox (Eh) were recorded in situ with a portable meter Thermo Scientific Orion. Surface water was collected for further analyses: 500 mL was used for sulfate determination, and 100 mL was filtered (0.45 µm) and acidified with nitric acid (75%) to maintain a pH below 2. The samples were immediately transported to the laboratory in polyethylene containers and refrigerated conditions (4 °C).
Sulfate was determined by turbidimetric method (Standard Methods 4500 E; APHA 2012). Selected element concentrations (Al, As, Cu, Fe, Ni, Co, Zn, Pb, and Cd) were obtained by inductively coupled plasma optical or mass spectrometry (ICP-OES/MS). These analyses were performed by Activation Laboratory, Lda—Actlabs, Canada, including duplicate samples and blanks to check precision, whereas accuracy was obtained by using certified standards (IV-STOCK-1643 (ICP/MS) Cert). All the reagents used were of analytical grade or Suprapur quality (Merck, Darmstadt, Germany). The standard solution was the Merck AA Certificate. Milli-Q water was utilized in all the experiments.
The SPSS Release 25.0 software was used to treat the results statistically. Ficklin diagram (Ficklin et al. 1992) was applied to distinguish and classify the different water samples.
Potential ecological risk — a new approach
According to different authors (e.g., Ojekunle et al. 2016; Withanachchi et al. 2018), the metal index (MI) and the potential ecological risk index (RI) provide the bigger picture of water quality. Based on the work of Ojekunle et al. (2016), the RI represents different classification categories. The MI is a general index applied for different types of water uses, e.g., river waters (Bakan et al. 2010; Khoshnam et al. 2017). The RI was proposed by Håkanson (1980) for sediments and aims to assess PTE’s characteristics and environmental behavior for basins/lakes. Although, according to more recent studies, several authors have applied similar indexes to evaluate the potential ecological risk for water (e.g., Karunanidhi et al. 2022).
The first is calculated using the following equation:
According to Tamasi and Cini (2004) and Withanachchi et al. (2018), \({C}_{i}\) is each PTE concentration in each sample, and MAV is the standard maximum allowed concentration, as defined by the European and Portuguese legal framework for water quality (Decree Law No. 236/98).
The second one was achieved through:
where \({T}_{r}^{i}\) is the toxic response factor (Håkanson 1980) and the \({C}_{f}^{i}\) is the contamination coefficient of a specific PTE, calculated by measured value obtained in the field sample, divided by the reference value, which is the MAV \(({C}_{f}^{i}={C}_{sl}^{i}/{MAV}_{n}^{i})\).
However, because the index results may be out of adjustment for different types of water, this investigation proposes an adaptation and addition of new classes of potential ecological risk and Metal Index (MI). This new organization is presented in Table 1.
Results and discussion
Hydrochemistry in the mining area
Table 2 presents the statistical summary of in situ parameters and sulfate. The box and whisker plots regarding the PTE selected are shown in Fig. 4. Both were analyzed monthly in the hydrological year of 2016/2017 and are crucial AMD indicators.
Box and whisker plots of selected PTE analyzed during a completed hydrological (12 campaigns) in the respective sampled points. The box plot displays the interquartile range with the median represented by the line inside the box. The whiskers are lines extending from the box to the highest and lowest, excluding outliers, representing extreme (star symbol) or mild outliers (circle symbol)
The highest pH value was detected in the dam upstream of the mining area, PAT1, with 8.13. In the opposite situation, there is PAT7, with the lowest pH recorded (0.4). EC is also highlighted in PAT7, as it has the highest registered value, with 27,300 μS/cm, in May 2017. Comparing specifically with other sites in the IPB, González et al. (2020) refer to EC values of around 11,000 μS/cm in the Tharsis mines. So, in the present study, São Domingos (PAT7) presents an even superior EC. As expected, higher values for Eh (500–600 mV) are observed in environments that are presumed to be more oxidizing, associated with the evolution of AMD. This parameter generally behaves similarly to the EC along the sampled path. PAT7, PAT2, and PAT5 exhibit higher sulfate concentrations (410,601; 6,699; and 6,565 mg/L, respectively).
The results of PTE demonstrate that PAT7 and PAT2, followed by PAT5, are the most contaminated sites, and Fe and Al have higher concentrations. The results obtained for Al can be explained by the abundant dissolution of felsic host rocks, increased by the medium's strong acidity, as suggested by Soyol-Erdene et al. (2018)On the other hand, high Fe concentrations may be related to the paragenesis of the study area, very rich in pyrite, whose availability is associated with AMD. For example, Fe presents a concentration of 134,000 mg/L in PAT7. These values agree with others registered in different parts of the world, corresponding to extreme AMD pollution cases. According to Giloteaux et al. (2013), Carnoulés, in France, also exhibits extreme sulfate concentrations, up to 30,000 mg/L, and low values for pH (down to 1.2). A paradigmatic example is the Richmond Mine at Iron Mountain, which presents 111,000 mg/L of Fe, 23,500 mg/L of Zn, 340 mg/L of As, and 760,000 mg/L of sulfate (Nordstrom and Alpers 1999). According to Migaszewski et al. (2014), Wisniówka tailing pile pools in Poland show 66,000 mg/L of Fe, 1500 mg/L of As, pH values of 1.2, and 330,000 mg/L of sulfate concentrations.
The most contaminated samples revealed higher range values for almost all the PTEs analyzed, indicating a stronger seasonality impact (PAT5 and PAT7). According to Zhou et al. (2022), Cu and As are more associated with the precipitation, and they might decrease abruptly when the precipitation is above 4.68 mm. However, the results referring to PAT2 (also a very contaminated site) do not reveal the same behavior, that is, a large variation in the concentration of these elements throughout the hydrological year. This event may be because PAT2 is a pit lake whose hydrochemical component appears to remain more stable (Gomes and Valente 2019). Despite presenting with a lower degree of contamination, the remaining points, such as PAT6, PAT10, and PAT11, also appear to reflect some variation in the Cu, Fe, Zn, and Al concentrations. One factor contributing to these changes is that these sampling points may vary from lotic to lentic environments in the dry season. So, with the decrease in precipitation (in the driest months), these points adopt lentic characteristics. Thus, seasonality appears to have a higher impact on the concentration of PTE, revealed in bigger or less water contamination and inevitably in its quality. PAT1 and PAT12 do not show ample variation throughout the hydrological year. These sampling points reveal low concentrations of analyzed PTE.
Trends of fluvial system-Ficklin diagram
Ficklin diagram (Ficklin et al. 1992), in Fig. 5, reveals the result of 12 sampling points projected according to the sum of metals and their respective pH. It is possible to observe a clear distinction among samples. Thus, PAT7 and PAT1 are at opposite extremes, with “high-acid, extreme-metal” classifications and “near-neutral, low-metal”, respectively. In the same range as PAT7, however, with a higher pH and lower concentration of metals, is PAT2. These results are in accordance with the obtained by Gonzalez (2020) for Tharsis mine. In the previous study, the projection in the Ficklin diagram revealed that samples are mostly classified as high acid-extreme metal and high acid-high metal, like PAT2. However, PAT7 exhibits more extreme values in the São Domingos mine. Another study (Sarmiento et al. 2018) focuses on a lagoon designated by “radical point” and located in a small mining leachate dam (IPB, Spanish side, in Cobica River watershed), revealing a negative pH, and that seems similar to PAT7, studied here. PAT7 seems to have extreme contamination characteristics, being an acidic lake in the industrial area of the complex. The more considerable degree of contamination seems to be strongly associated with the presence of the most reactive wastes, such as accumulations of washed ore, that is, materials highly enriched in fine-grained pyrite disposed of, for example, around the most acidic lagoon — PAT7 (e.g., Sarmineto et al. 2018; Tavares et al. 2009). The digital surface model obtained by Gomes et al. (2021) indicates the influence of drainage pathways on water chemistry in this area. This information confirms the direct relationship between the nature of the materials in the surrounding area and the degree of impact on the water environment. Cordeiro et al. (2017) identified the waste dumps and landfills with the highest contamination potential. These are located in the North sector (near the pit lake) and in the industrial area of Achada do Gamo (PAT7), in line with the results obtained in the present study. The very fine accumulations of washed ore had high reactivity index (pH paste < 3.0). So, according to the results, and similar to what was investigated by other authors (Pérez-López et al. 2008; Sarmiento et al. 2018; Grande et al. 2015), the finest mining wastes are responsible for more harmful effects on the environment. PAT11 and PAT12 are arranged separately in the “acid, high-metal” and “near-neutral, high-metal” ranges. All other samples are grouped in the same classification: “high-acid, high-metal,” emphasizing the PAT5 point, located at the interface with the most concentrated range of metals and the lowest pH. The results can be related to the disposition, proximity, and quantity of waste in mining areas. PAT2 is highly contaminated in this case due to low pH and extreme metal concentration. Additionally, surrounding wastes (reactive slags) can contribute to runoff phenomena in the pit lake. After points PAT3, PAT4, PAT5, and PAT6, there appears to be a tendency towards a reduction in contamination along the sampling line. In addition to reducing the amount of waste disposed along the watercourse and its reactivity, another aspect that can contribute to the reduction of contamination after point PAT8 is the confluence of the Mosteirão River. This clean stream without metallic contamination promotes an increase in the hydrological load, which in turn is reflected in the dilution effect.
Ficklin diagram (1992) showing different classifications for water sampled at the São Domingos mine
Furthermore, from PAT11 onwards, there tends to be decreased contamination, mainly because of dilution and higher distance from the waste accumulation. Also, mineralogical controls related to the precipitation of jarosite and other secondary phases contribute to this attenuation, as Alpers et al. (1994) referred to. PAT12, water for human consumption, was revealed to be a point of clean water, as indicated by lower concentrations of the selected elements.
Seasonal variability and environmental quality
Figure 6 reveals the maximum allowed values (MAV), considered as standards for environmental objectives of quality for surface waters (pH, Cu, Zn, SO4, Pb, As, and Cd) (Decree Law No. 236/98, of August 1) in different periods: the wettest (02/17) and driest month (07/17). Only PAT1 and PAT12 proved to comply with MAV (water dam for recreational use and water dam for human consumption, respectively). As regards the other points, it is possible to notice that all elements suffered notable variations, with higher concentrations in the dry month (July 2017 campaign). The MVA was largely exceeded. The pH value at PAT7 is exceptional compared to the other analyzed data, with a value of 0.84 in the July campaign, well below the range presented as admissible (between 5 and 9). Despite not reporting extreme events in their study, Sarmiento et al. (2018) have found, between October 2003 and January 2007, negative pH values on the other side of the border, also in a lagoon of an old IPB mine. Such results are justified by the low dilution in the context of lesser water availability, leading to the intense water–rock interaction processes (Cánovas et al. 2007).
Hydrochemical variations referring to the rainiest month (02/17) and the driest month (07/17), considering the quality environmental objectives for surface waters (Decree Law No. 236/98, of August 1st – Annex XXI). The x-axis represents each MAV value: pH (5–7); Cu = 0.1; Zn = 0.5; S04 = 250; Pb = 0.05; As = 0.1; Cd = 0.01
Nevertheless, some elements showed minimum concentrations in the dry period, probably due to the intense precipitation of Fe oxyhydroxysulfate and sulfates that incorporated some elements in their structure (González et al. 2020). These lower concentrations occur for Pb and Cd in PAT11. According to Farkas et al. (2007); Duodu et al. 2016), after some metals are introduced into the aquatic ecosystem, most can be attached to fine-grained particles and accumulate in bottom sediments through settling. Even when water quality criteria are not exceeded, these substances can harm biological systems (Bibi et al. 2007). In this regard, during field sampling, it was possible to visually confirm the presence of ochre precipitates, which were more intense at the end of the channel, specifically in locations PAT10 and PAT11. These precipitates formed long, thick pastes deposited on the watercourse bed. Furthermore, Rao et al. (2021) reported that the metals with the highest potential eco-risk were Pb and Cd. These were retained in the sediments, presenting high concentrations. Also, concerning Pb, Gomes et al. (2021) made it possible to verify in their study of clusters an intrinsic association of this element with algae in the aquatic environment. These algal mats could be accumulating these metals in their cells. Moreover, Yu and Wang (2004) and Yuan et al. (2015) found that eutrophication can stimulate the absorption and accumulation of toxic metals in freshwater phytoplankton cells, which can affect their mobility in the aquatic ecosystem.
Several authors have reported for decades (e.g., Johnson and Hallberg 2005; Gomes et al. 2018) that AMD from abandoned mines can be the main limiting factor for water use in the river basins in which they are located, as well as the primary source of ecological degradation of river systems. Thus, the São Domingos and Mosterirão streams are outside the stipulated environmental quality objectives. It should be noted that according to the EU Commission (2000), the main goal of the EU Water Framework Directive (WFD) is to achieve good ecological and chemical quality for all European rivers. In this sense, the results obtained for this mining site are clearly out of the scope of the legal framework and the United Nations Sustainability Development Goals (SDG-UN, Agenda, 2030).
Drought episodes: potential ecological risk
PTE’s implications for the ecosystem were analyzed in the driest conditions, as they present more extreme values and, therefore, higher risk. In this way, Table 3 exposes the results of the MI and RI.
The analysis indicates that PAT1, PAT11, and PAT12 have low potential ecological risk. PAT4, PAT6, and PAT9 belong to class 3, exhibiting strong risk. PAT3, PAT8, and PAT10 reveal one of the proposed new classes (class 4), evidencing very strong risk. As revealed throughout the study, PAT2, PAT5, and PAT7 are classified in the highest proposed class (> 5000), indicating potential extremely strong ecological risk. In this way, it is possible to order the sampling points by groups, depending on the ecological risk of contamination: PAT1, PAT11, PAT12 < PAT4, PAT6, PAT9 < PAT3, PAT8, PAT10 < PAT2, PAT5, PAT7. No samples are in the moderate class (> 150 < 300). The index thus reveals extreme classification values. According to Bakan et al. (2010), the results of MI are even more pessimistic, indicating that all sampled points are representative of a warning limit (MI > 1). According to Batty et al. (2010) and Reyes-Becerril et al. (2019), elements such as Cu, Zn, Pb, As, or Cd are directly toxic to the metabolism of aquatic organisms, and their release poses an environmental and health threat.
Conclusion
The AMD-studied waters have very high EC, low pH, and very high concentrations of dissolved PTE. They are classified mostly as high-acid, extreme-metal, and high-acid, high-metal. A seasonal pattern in AMD hydrochemistry can be observed: the rainy month has lower PTE. In contrast, the driest month is the most contaminated, revealing the impact of extreme drought episodes on the water medium. In addition to not complying with the environmental objectives in the legislation, the water quality is not in line with the United Nations Sustainability Development Goals. Only PAT1 (recreation dam) and PAT12 (dam for human consumption) agree with the minimum quality environmental objectives for surface water in both studied months: dry and rainy. However, the MI reveals that all samples are representative of a warning limit (> 1). The potential ecological risk shows extreme classes, and the risks presented in this study may have major implications in the future, considering the current water scarcity scenario. According to the preliminary document of the Intergovernmental Panel on Climate Change (IPCC, ONU), published in 2021, the region is in a “hot spot,” being subject to extreme climate changes, implying a strong probability of heat waves and extreme risk of drought. Thus, the new classifications can alert competent authorities to implement preventive and even remedial measures in line with different risks determined.
References
Abreu M, Tavares MT, Batista MJ (2008) Potential use of Erica andevalensis and Erica australis in phytoremediation of sulphide mine environments: São Domingos. Portugal J Geochem Explor 96:210–222
Aguilera A, Manrubia SC, Gomez F, Rodriguez N, Amils R (2006) Eukaryotic community distribution and its relationship to water physicochemical parameters in an extreme acidic environment Rio Tinto Southwestern Spain. Appl Environ Microbiol 72:5325-e5330
Alpers CN, Blowes DW, Nordstrom DK, Jambor JL (1994) Secondary minerals and acid mine-water chemistry. In: Jambor JL, Blowes DW (eds) Short course handbook on environmental geochemistry of sulfide mine-wastes, vol 22. Mineralogical Assoc of Canada. Waterloo, Ontario, pp 247–270
Amils R, Gonzalez-Toril E, Aguilera A, Rodríguez N, Fernandez-Remolar D, Gomez F, García-Moyano A, Malki M, Oggerin M, Sanchez-Andrea I, Sanz JL 2011 From Río Tinto to mars. The terrestrial and extraterrestrial ecology of acidophiles. Adv Appl Microbiol:. https://doi.org/10.1016/B978-0-12-387044-5.00002-9
APA (2016) Agência portuguesa do Ambiente, I.P.- Plano de Gestão da Região Hidrográfica do Sado e Mira RH6. Parte 2 – Caracterização e diagnostico. Available in https://www.apambiente.pt/_zdata/Politicas/Agua/PlaneamentoeGestao/PGRH/2016-2021/PTRH6/PGRH6_Parte2.pdf
APHA, (2012) Standard methods for the examination of water and wastewater, twentieth ed. American Public Health Association, American Water Works Association, Water Environment Federation, Washington DC
Bakan G, Özkoç HB, Tülek S, Cüce H (2010) Integrated environmental quality assessment of the Kızılırmak River and its coastal environment. Turk J Fish Aquat Sci 10:453–462
Batty LC, Auladell M, Sadler J (2010) The impacts of metalliferous drainage on aquatic communities in streams and rivers. In: Batty LC, Hallberg KB (eds) Ecology of Industrial Pollution. Cambridge University Press, British Ecological Society, pp 71–100
Bibi MH, Ahmed F, Ishiga H (2007) Assessment of metal concentrations in lake sediments of Southwest Japan based on sediment quality guidelines. Environ Geol (Heidelberg, Ger.) 52:625–639
Bonnail E, Macías F, Osta V (2019) Ecological improvement assessment of a passive remediation technology for acid mine drainage: water quality biomonitoring using bivalves. Chemosphere 219:695–703. https://doi.org/10.1016/j.chemosphere.2018.12.037
Cánovas CR, Olías M, Nieto JM, Sarmiento AM, Cerón JC (2007) Hydrogeochemical characteristics of the Odiel and Tinto rivers (SW Spain). Factors controlling metal contents. Sci Total Environ 373:363–382
ENAAC (2010) Estratégia Nacional de Adaptação às Alterações Climáticas - Resolução do Conselho de Ministros nº24/2010. Diário da República 1ª série—Nº 64—1 de Abril de 2010
EU Commission (2000) Directive 2000/60/EC of the European Parliament and of the Council, of 23 October 2000, establishing a framework for Community action in the field of water policy. Off. J. Eur. Econ. L 327/1, 22.12.2000. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri1⁄4CELEX:32000L0060. (Accessed 18 January 2022).
Cordeiro M, Valente T, Grande JA, Gomes P. 2017. Mapping mining wastes and analyzing affected areas through expeditious physico-chemical parameters. XII Congreso Nacional de Geoquímica/XI Congreso Ibérico de Geoquímica, Linares, 26 a 28 de Setembro, 178–183p.
Dong J, Bian Z, Wang H (2007) Comparison of heavy metal contents between different reclaimed soils and the control soil. J China Univ Min Technol 36(4):531–536
Duodu GO, Goonetilleke A, Ayoko G (2016) Comparison of pollution indices for the assessment of heavy metal in Brisbane River sediment. Environ Pollut 219:1077–1091
EDM (2021) Empresa de desenvolvimento mineiro, S.A. (www.edm.pt). https://edm.pt/projetos/recuperacao-do-sistema-de-canais-de-recolha-de-aguas-de-escorrencia/ (consulted in 2021)
Farkas A, Erratico C, Viganò L (2007) Assessment of the environmental significance of heavy metal pollution in surficial sediments of the River Po. Chemosphere 68(4):761–768
Ficklin WH, Plumlee GS, Smith KS, McHugh JB (1992) Geochemical classification of mine drainages and natural drainages in mineralized areas. In: Kharaka YK, Maest, AS (eds) Proceedings of water-rock interaction, Balkema, Rotterdan, no7, pp 381–384
Giloteaux L, Duran R, Casiot C, Bruneel O, Elbaz-Poulichet F, Goni-Urriza M (2013) Three-year survey of sulfate-reducing bacteria community structure in Carnoules acid mine drainage (France), highly contaminated by arsenic. FEMS Microbiol Ecol 83(3):724–737
Gomes P, Valente T, Pereira P (2018) Addressing quality and usability of surface water bodies in semi-arid regions with mining influences. Environ Process 5:707. https://doi.org/10.1007/s40710-018-0329-0
Gomes P, Valente T, Albuquerque T, Henriques R, Flor-Arnau N, Pamplona J, Macías F (2021) Algae in acid mine drainage and relationships with pollutants in a degraded mining ecosystem. Minerals 11:110. https://doi.org/10.3390/min11020110
Gomes P, Valente T, Marques R, Prudêncio MI, Pamplona J (2022) Rare earth elements — source and evolution in an aquatic system dominated by mine-Influenced waters. J Environ Manage 322(2022):116125. https://doi.org/10.1016/j.jenvman.2022.116125
Gomes P, Valente T (2019) Physical and chemical conditions for colonization by Euglena mutabilis: case studies in two acid mine drainage sites. In: Wolkersdorfer C, Khayrulina E, Polyakova S, Bogush A (eds) Proceedings of the IMWA: mine water: technological and ecological challenges, Perm, Russia. International Mine Water Association, Wendelstein, Germany, pp 419–424
Gomes P, Valente T, Grande JA, Cordeiro M (2017) Occurrence of sulphate efflorescences in São Domingos mine. Comunicações Geológicas 104(1):83–89
González R, Cánovas C, Olías M, Macías F (2020) Rare earth elements in a historical mining district (south-west Spain): hydrogeochemical behaviour and seasonal variability. Chemosphere 253:126742. https://doi.org/10.1016/j.chemosphere.2020.126742
Grande JA, de la Torre ML, Valente T, Borrego J, Santisteban M, Cerón JC, Sánchez-Rodas D (2015) Stratification of metal and sulphate loads in acid mine drainage receiving water dams — variables regionalization by cluster analysis. Water Environ Res 87:626–634. https://doi.org/10.2175/106143015x14212658614793
Håkanson L (1980) An ecological risk index for aquatic pollution control A Sedimentological Approach. Water Res 14:975–1001
Yuan H, Liu E, Shen J (2015) The accumulation and potential ecological risk of heavy metals in microalgae from a eutrophic lake (Taihu Lake, China). Environ Sci Pollut Res 22:17123–17134. https://doi.org/10.1007/s11356-015-4891-y
Jiao B, Xu G, Li D, Luo J, Yang K (2012) Hazards of heavy metals in coal. Disaster Adv 5(4):1812–1818
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14. https://doi.org/10.1016/j.scitotenv.2004.09.002
Karunanidhi D, Aravinthasamy P, Subramani T, Rohana Chandrajith; Janardhana Raju, N., Antunes, IMHR., (2022) Provincial and seasonal influences on heavy metals in the Noyyal River of South India and their human health hazards. Environ Res 204:111998. https://doi.org/10.1016/j.envres.2021.111998
Khoshnam Z, Sarikhani R, Ahmadnejad Z (2017) Evaluation of water quality using heavy metal index and multivariate statistical analysis in Lorestan Province. Iran J Adv Environ Health Res 5:29–37
Kicińska A, Pomykała R, Izquierdo-Diaz M (2021) Changes in Soil pH and Mobility of Heavy Metals in Contaminated Soils Eur J Soil Sci 73:e13203. https://doi.org/10.1111/ejss.13203
Levings CD, Varela DE, Mehlenbacher NM, Barry KL, Piercey GE, Guo M, Harrison PJ (2005) Effect of an acid mine drainage effluent on phytoplankton biomass and primary production at Britannia Beach, Howe Sound, British Columbia. Mar Pollut Bul 50:1585-e1594. https://doi.org/10.1016/j.marpolbul.2005.06.032
Lottermoser B (2010) Mine wastes — characterization, treatment and environmental impacts, 3rd edition. Springer, Berlin, p 400
Luís AT, Teixeira M, Durães N, Pinto R, Almeida S, Ferreira da Siva E, Figueira E (2019) Extremely acidic environment: biogeochemical effects on algal biofilms. Ecotoxicol Environ Saf 177:124-e132. https://doi.org/10.1016/j.ecoenv.2019.04.001
Migaszewski ZM, Gałuszka A, Migaszewski A (2014) The study of rare earth elements in farmer’s well waters of the Podwiśniówka acid mine drainage area (south-central Poland. Environ Monit Assess 186:1609–1622
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc Natl Acad Sci USA 96:3455–3462
Nordstrom K, Blowes DW, Ptacek CJ (2015) Hydrogeochemistry and microbiology of mine drainage: an update. J Appl Geochem 57:3–16
Ojekunle OZ, Ojekunle OV, Adeyemi AA, Taiwo AG, Sangowusi OR, Taiwo AM, Adekitan AA (2016) Evaluation of surface water quality indices and ecological risk assessment for heavy metals in scrap yard neighbourhood. Springerplus 5:560
Pérez-López R, Álvarez-Valero AM, Nieto JM, Sáez R (2008) Use of sequential extraction procedure for assessing the environmental impact at regional scale of the São Domingos Mine (Iberian Pyrite Belt). J Appl Geochem 23:3452–3463. https://doi.org/10.1016/j.apgeochem.2008.08.005
Rao K, Tang T, Zhang X, Wang M, Liu J, Wu B, Wang P, Ma Y (2021) Spatial-temporal dynamics, ecological risk assessment, source identification and interactions with internal nutrients release of heavy metals in surface sediments from a large Chinese shallow lake. Chemosphere 282:131041. https://doi.org/10.1016/j.chemosphere.2021.131041
Reyes-Becerril M, Angulo C, Sanchez V, Cuesta A, Cruz A (2019) Methylmercury, cadmium and arsenic (III)-induced toxicity, oxidative stress and apoptosis in Pacific red snapper leukocytes. Aquat Toxicol 213:105223
Sánchez España J, López-Pamo E, Santofimia E, Diez M (2008) The acidic mine pit lakes of the Iberian Pyrite Belt: an approach to their physical limnology and hydrogeochemistry. Appl Geochem 23:1260–1287
Sarmiento AM, Grande JA, Luís AT, Dávila JM, Fortes JC, Santisteban M, Curiel J, de la Torre ML, da Silva EF (2018) Negative pH values in an open-air radical environment affected by acid mine drainage. Characterization and proposal of a hydrogeochemical model. Sci Total Environ 644:1244–1253. https://doi.org/10.1016/j.scitotenv.2018.06.381
Schneider SC, Oulehle F, Kra P, Hruska J (2018) Recovery of benthic algal assemblages from acidification: how long does it take, and is there a link to eutrophication? Hydrobiologia 805:33-e47. https://doi.org/10.1007/s10750-017-3254-8
Soyol-Erdene TO, Valente T, Grande JA, de la Torre ML (2018) Mineralogical controls on mobility of rare earth elements in acid mine drainage environments. Chemosphere 205:317–327. https://doi.org/10.1016/j.chemosphere.2018.04.095
Tamasi G, Cini R (2004) Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci Total Environ 327:41–51
Tavares MT, Abreu MM, Vairinho MM, Sousa AJ, Quental L (2009) Comportamento geoquímico de alguns elementos vestigiais na envolvente das Minas de S. Domingos, Alentejo: Áreas da Tapada e do Telheiro. Rev Ciências Agrárias 32:182–194
Tiri A, Lahbari N, Boudoukha A (2014) Hydrochemical characterization of surface water in the Timgad watershed. East Algeria Desalin Water Treat 57:562–571
Valente T, Gomes P, Pamplona J, de la Torre ML (2012) Natural stabilization of mine waste-dumps — evolution of the vegetation cover in distinctive geochemical and mineralogical environments. J Geochem Explor 123:152–161. https://doi.org/10.1016/j.gexplo.2012.05.005
Withanachchi SS, Ghambashidze G, Kunchulia I, Urushadze T, Ploeger A (2018) Water quality in surface water: a preliminary assessment of heavy metal contamination of the Mashavera River, Georgia. Int J Environ Res Public Health 15(4):621. https://doi.org/10.3390/ijerph15040621
Wolkersdorfer C, Nordstrom DK, Beckie RD, Cicerone DS, Elliot T, Edraki M, Valente T, França SC, Kumar P, Oyarzún Lucero RA, Soler I, Gil A (2020) Guidance for the integrated use of hydrological, geochemical, and isotopic tools in mining operations. Mine Water Environ 39:204–228. https://doi.org/10.1007/s10230-020-00666-x
Wolowski K, Turnau K, Henriques FS (2008) The algal fora of an extremely acidic, metal-rich drainage pond of São Domingos pyrite mine (Portugal). Cryptogam. Algol 29:313–324
Yu RQ, Wang WX (2004) Biokinetics of cadmium, selenium, and zinc in freshwater alga Scenedesmus obliquus under different phosphorus and nitrogen conditions and metal transfer to Daphnia magna. Environ Pollut 129:443–456
Zhang B, Song X, Zhang Y, Han D, Tang C, Yu Y, Ma Y (2012) Hydrochemical characteristics and water quality assessment of surface water and groundwater in Songnen plain. Northeast China Water Res 46:2737–2748. https://doi.org/10.1016/j.watres.2012.02.033
Zhang J, Li S, Dong R, Jiang C, Ni M (2019) Influences of land use metrics at multi- spatial scales on seasonal water quality: a case study of river systems in the Three Gorges Reservoir Area. China J Clean Prod 206:76–85
Zhou H, Rao K, Yao M, Xiong Y, Wang Y, Yin Y (2022) Effects of land use, meteorology, and hydrology on nutrients, biochemical indexes, and heavy metals in Qingjiang River Basin. China J Clean Prod 370:133416. https://doi.org/10.1016/j.jclepro.2022.133416
Acknowledgements
The authors are deeply grateful to the anonymous reviewers for their contribution to the improvement of the manuscript.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was supported by the European Social Fund and National Funds of MCTES (Ministério da Ciência, Tecnologia e Ensino Superior) with reference SFRH/BD/108887/2015. This work was co-funded by the FCT (Fundação para a Ciência e a Tecnologia – Portugal) through projects UIDB/04683/2020, UIDP/04683/2020, and Nano-MINENV 029259 (PTDC/CTAAMB/0.29259/2017).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Patricia Gomes and Teresa Valente. The first draft of the manuscript was written by Patricia Gomes, and both authors commented on previous versions of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Research does not involve humans and/or animals, and the authors declare compliance with ethical standards.
Consent to participate
All authors agreed with the content and gave explicit consent to submit before submission.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gomes, P., Valente, T. Seasonal impact of acid mine drainage on water quality and potential ecological risk in an old sulfide exploitation. Environ Sci Pollut Res 31, 21124–21135 (2024). https://doi.org/10.1007/s11356-024-32367-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32367-1