Abstract
Acidification has adversely affected freshwater ecosystems in many areas, and recovery from acidification is often interrupted by acidic events. We lack detailed information about how benthic algae react to short-term acidic events and long-term recovery from acidification. We sampled 15 stream sites in the Czech Republic to study the effects of (a) water pH, aluminium and lead concentrations, (b) short-term acidic events, (c) 20 years of recovery from acidification and (d) high phosphorus concentrations combined with low pH on soft-bodied benthic algae. Water pH and aluminium concentrations affected benthic algal assemblages, but the acidification index periphyton (AIP) mainly reflected pH. Benthic algal assemblages reflected recent acidic events more closely than maximum or average pH. Our results indicate that the reaction of benthic algae to pH results from a fast effect of pH minima that cause sensitive species to disappear within a few months, and a slower process of dispersal and competition for resources during periods of higher pH. After an acidic event, recolonization of stream sites by sensitive species had started within 2 years, and was largely completed after 9 years. Our data indicate that acidification may mask the effect of enhanced phosphorus concentrations on benthic algal eutrophication indices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater ecosystems have long been affected by various types of human impacts, with negative consequences on water quality and biota (Søndergaard & Jeppesen, 2007). Acidification, caused by acidic precipitation, has adversely affected freshwater ecosystems in many areas, for example, in North America (Clair et al., 2011), Asia (Duan et al., 2016), Scandinavia (Skjelkvåle et al., 2005) and parts of Central Europe (Hruška et al., 2002; Oulehle et al., 2017). In Europe, emissions of acidifying gases peaked in the 1970s/1980s (Schöpp et al., 2003). Since the early 1990s, water chemistry has shown recovery from acidification, manifested by increasing pH and reductions in aluminium concentrations (Stoddard et al., 1999; Skjelkvåle et al., 2005; Garmo et al., 2014). Given lower sulphur deposition, improvements in surface water pH should continue to occur, but deposition of nitrogen compounds will continue to contribute to freshwater acidification (Stoddard et al., 2001; Oulehle et al., 2015).
Chemical recovery is an essential precondition for biological recovery, but biological recovery from acidification often lags behind chemical recovery. This is likely due to both physical and biotic interactions (Monteith et al., 2005), dispersal constraints (Gray & Arnott, 2012) and short-term acidic episodes (i.e. periods of reduced pH generated during rainstorms or snow melt over hours to weeks), which may set back the slowly recovering biota to an acid-tolerant assemblage (Kowalik et al., 2007). Algae are sensitive to acidification, and have long been used to infer surface water pH and reduced levels of acid deposition (Battarbee, 1984; Kovacs et al., 2006; Burns et al., 2008). In Norway, the acidification index periphyton (AIP), which is based on non-diatom benthic algae, is used to monitor stream acidification and the effects of liming (Schneider & Lindstrøm, 2009).
Acidification and eutrophication (i.e. the excessive growth of plants and algae caused by overenrichment of waters with nutrients) are among the major stressors on freshwater ecosystems (Malmqvist & Rundle, 2002). However, areas that are most sensitive to acidification often have nutrient-poor soils, which makes them poorly suited for agriculture, such that they are less at risk for eutrophication (Chuman et al., 2013). As a consequence, there are few instances in which low pH and clearly elevated phosphorus concentrations are combined (Schneider et al., 2013). On the other hand, field evidence in Scandinavia and elsewhere shows that settlements and agriculture exist in areas affected by acidification. Situations where freshwater ecosystems simultaneously are exposed to both eutrophication and acidification, may therefore occur. Also, streams affected by acid mine drainage may have low pH and high phosphorus concentrations (Niyogi et al., 2013). However, we have insufficient information about benthic algal assemblages at sites where low pH and elevated phosphorus are combined. We simply do not know if acidification and eutrophication indices give a “correct” signal at such sites.
Acidification effects on fishes and macroinvertebrates are mainly caused by elevated hydrogen, aluminium and heavy metal concentrations (Herrmann et al., 1993; Herrmann, 2001). For benthic algae, water pH, aluminium, as well as changed grazing pressure from macroinvertebrates at low pH may play a role (Genter & Amyot, 1994; Planas, 1996). Water pH may have a direct effect via hydrogen ions, but may also affect benthic algae via inorganic carbon acquisition, since shifts in benthic algal assemblages often occur in the pH range where the carbonic acid equilibrium shifts between HCO3 − and CO2 being the dominant carbon fraction (Schneider & Lindstrøm, 2009). Flume experiments demonstrated that both pH and aluminium may affect the growth of benthic algae (Kinross et al., 2000), but to our knowledge the importance of pH versus aluminium has never been verified with field studies, likely due to a lack of data. The mechanism of aluminium toxicity on algae is explained by an aluminium-induced reduction in the biologically available phosphorus fraction (Exley et al., 1993). This suggests that acidification may limit possible effects of enhanced phosphorus loads on the growth of algae. In other words, acidification may mask effects of eutrophication. In contrast to acidification, the effect of increased nutrient concentrations on benthic algae seems relatively straightforward: additional input of phosphorus, but also nitrogen, causes increased growth of macrophytes and algae, which eventually leads to shifts in their species assemblages via competition (Schindler, 2006).
The Czech Republic belongs to a region that is heavily affected by acid deposition (Hruška et al., 2002; Kolář et al., 2015), and regional decreases in SO2 emissions are among the most pronounced examples of pollution reduction in Europe (Vestreng et al., 2007; Kopáček et al., 2012). In order to monitor the effects of pollution reduction on freshwater ecosystems, water chemistry in 15 streams across the Czech Republic has been sampled monthly since 1995 (Oulehle et al., 2017). These stream sites (called the GEOMON network) represent a lithological, climatic and acidic deposition gradient typical for Central Europe. One of these sites has low pH combined with high phosphorus concentrations due to the occurrence of P-rich minerals in the bedrock (Oulehle et al., 2017). The monitoring includes monthly measurements of water pH, aluminium, nitrogen and phosphorus concentrations, i.e. parameters related to acidification and eutrophication which are important for benthic algae.
We sampled non-diatom benthic algae at the 15 sites of the GEOMON network, in order to study (1) the importance of acidic events and long-term (20 years) recovery from acidification on benthic algal assemblages and the acidification index periphyton (AIP), (2) the relationship between parameters commonly associated with acidification (pH, aluminium, heavy metals) and benthic algal assemblages as well as the AIP and (3) the combined effect of low pH and high phosphorus concentrations on commonly used acidification and eutrophication indices. Our hypotheses were: (1) benthic algal assemblages are more closely related to recent acidic events than to long-term stream acidity, because they respond rapidly to acidic episodes, but slowly to recovery after pH is elevated (Hirst et al., 2004); (2) both aluminium concentrations and pH affect algal assemblages (Kinross et al., 2000); and (3) low pH overrides the effect of high phosphorus concentrations because low pH is a disturbance to which benthic algae respond rapidly (Hirst et al., 2004), while high phosphorus concentrations act as a long-term nutrient subsidy and do not have immediate negative effects on algae (Blindow, 1988); in other words, we hypothesized that a site with low pH and high phosphorus concentrations would have an acidic, not a eutrophic, benthic algal assemblage.
Materials and methods
Sampling sites
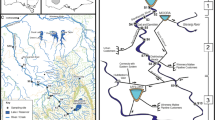
The GEOMON network consists of 15 catchments located across the Czech Republic (Fig. 1; Table 1). All catchments are small forested areas (median size of 85 ha), situated in rural mountainous landscapes (median elevation of 773 m a.s.l., median temperature 6°C, median precipitation 861 mm year−1). Vegetation cover consists mostly of managed Norway spruce (Picea abies, L.), which is typical for central Europe. However, some of the catchments contain natural alpine grasslands (MOD) and substantial areas of broadleaved forests (mostly European beech, Fagus sylvatica, L.; JEZ, LES, POM). Forests in all catchments, except MOD, are managed for timber production. During the peak of acidification, forest dieback in some of the catchments was substantial (JEZ, UHL, UDL). Monthly sampling of stream water started in November 1993. Not all parameters, however, were consistently measured at all sites. For 13 sites (Table 1), we were able to assemble a complete dataset for water pH, calcium, NH4 +, NO3 − and aluminium concentrations over 20 years (1995–2014). In 2015, monthly measurements of 15 water chemical parameters (Table 2) were available for all 15 sites. One site (LYS) has the unusual combination of naturally high phosphorus concentrations while at the same time being acidic.
Map of sampling sites; see Table 1 for detailed information
Data collection
Water chemistry
Water pH was measured using a Radiometer TTT-85 pH meter with a combination electrode, and conductivity with a Radiometer CDM 83 Conductivity Meter. The concentrations of Ca, Na, K, Al and Si were analysed by atomic absorption spectrophotometry (AAS, AAnalyst Perkin Elmer 100 and 200). SO4 2−, NO3 − and Cl− were analysed by high-performance liquid chromatography (Knauer 1000). Low-concentrated total Al and Pb were measured by ETA-AAS (electrothermal AAS, AAnalyst Perkin Elmer 700). NH4 + was determined by indophenol blue colorimetry, and alkalinity was measured by Gran titration to pH < 4.3. Dissolved organic carbon (DOC) was determined by a non-dispersive infrared (NDIR) detector after sample conversion to CO2 in a combustion furnace (Tekmar–Dohrmann Apollo 9000). Samples for total phosphorus were digested with perchloric acid, and analysed manually using the molybdate method and a Perkin Elmer Lambda 25 spectrophotometer. Water samples were not filtered, except for analysing DOC (0.45-µm glass filters).
Benthic algae
Samples were taken on June 24–29, 2015. At each site, samples of non-diatom benthic algae were collected according to European standard procedures (EN 15708:2009) along an approximately 10-m length of stream bottom using an aquascope (i.e. a bucket with a transparent bottom). Percent cover of each form of macroscopically visible benthic algae was recorded, and samples were collected and stored separately in vials for species determination. In addition, microscopic algae were collected from ten cobbles/stones with diameters ranging between approximately 10 and 20 cm, taken from each site. An area of about 8 × 8 cm from the upper side of each cobble/stone was brushed with a toothbrush to transfer the algae into a beaker containing approximately 1 L of river water from which a subsample was taken. All samples were preserved with a few drops of formaldehyde. The preserved benthic algae samples were later examined in a microscope (200–600 × magnification) and all non-diatom algae identified to species, when possible. Diatoms were not included due to the great differences in methodology for sample preparation and enumeration between diatom and non-diatom benthic algae. For some genera of filamentous green algae that could not be determined to species level (e.g. Spirogyra Link or Mougeotia C. Agardh), categories based mainly on filament width were used [see Schneider & Lindstrøm (2009, 2011) for further details]. The primary identification keys used were Komarek & Anagnostidis (2007), Gutowski & Förster (2009), John et al. (2011) and Komarek (2013). Abundance of each microscopic taxon was estimated in the laboratory as “rare”, “common” and “abundant”. For data analysis, these estimates were later translated into % cover as 0.001, 0.01 and 0.1%, respectively. Macroscopic algae whose cover was recorded as “<1%” in the field were noted as “0.1%” for data analysis. For all other taxa, the cover that was estimated in the field was used.
Data treatment and statistics
Water chemistry
After exploratory analysis, data were log-transformed when necessary to improve normality and homoscedasticity (data from 2015: Na, K, NO3 −, SO4 2−, Al, alkalinity, DOC, Pb; data for 1994–2014: Ca, slope in Ca, Al). For pH, Ca, NH4 +, NO3 − and Al at each site, we calculated (1) average values for the period between 1995 and 2014 in order to characterize long-term average water chemical characteristics and (2) the slopes of linear regressions of each parameter against time (year), in order to characterize the average change in water chemical conditions between 1995 and 2014. Prior to calculating slopes, we inspected scatter plots to check for possible non-linear changes over time. No such relationships were found. In order to study within which time period benthic algal assemblages react to water pH, we calculated minimum, maximum, mean and median pH for the year before benthic algal sampling, as well as for the period of 2, 3, 4 years, …etc. … up to 20 years before sampling, separately for each site. For the data measured in 2015, we calculated mean, minimum and maximum values from the 6 monthly measurements taken between January and June 2015, separately for each parameter and each site. Data were normalized to a mean of zero and a standard deviation of one to allow comparison of values among different parameters. In order to characterize overall water chemistry at each site, a PCA (principal component analysis) was conducted, using the vegan package in R (Oksanen et al., 2012). We used Pearson correlations to test the strength of linear relationships among explanatory and response parameters, using Statistica version 13.1.
Benthic algae
To explore species composition and abundance of the benthic algal assemblages, an NMDS (non-metric multidimensional scaling) was computed on the square root transformed data. NMDS was used because, in contrast to other ordination methods, it can also handle non-linear responses. The NMDS was computed using the meta MDS function in R version 2.14.2 (R Development Core Team, 2012), extended with the “vegan” package 2.0-4. Bray–Curtis was used as the dissimilarity measure because it is less dominated by single large differences than many other dissimilarity measures (Quinn & Keough, 2002). In addition to NMDS scores, the following response parameters were calculated from the benthic algal taxon list: (1) taxon richness (total benthic algal taxon richness, as well as richness of green algae and cyanobacteria); (2) the PIT index (Periphyton Index of Trophic Status; Schneider & Lindstrøm, 2011) because it provides a link to eutrophication and ecological status assessment (PIT ranges from 2 to 69, where high values indicate that the algal assemblage is dominated by eutrophic taxa); (3) the AIP index (Acidification Index Periphyton; Schneider & Lindstrøm, 2009) because it provides a link to the acidity tolerance of the benthic algal assemblage (AIP ranges from 5.1 to 7.5, where low values indicate that the benthic algal assemblage is dominated by acidic taxa); for a reliable calculation of the AIP index at a particular site, at least 3 indicator taxa are necessary; however, this was the case at only two sites; we therefore included uncertain AIP indices in the analysis, i.e. indices which were based on only one or two indicator taxa; at three sites, however, no indicator taxa were found, such that calculation of an AIP index was not possible. The AIP index is based on the presence/absence of sensitive species only, while NMDS scores were calculated based on % cover of all taxa.
Results
Site characteristics
The water chemistry in 2015 showed that the sites cover a gradient from acidic (the lowest mean pH was 4.2) to circumneutral (the highest mean pH was 7.5; Table 2). Low values of pH, alkalinity and conductivity and low concentrations of Cl, SO4 2−, Ca, Na, K, as well as high concentrations of Al, DOC and Pb were characteristic for mountain catchments with slowly weathering bedrock, soils rich in organic matter and a high amount of precipitation (MOD, UHL, LYS, UDL). The opposite pattern, i.e. mostly high values of pH, and high concentrations of SO4 2− and Ca as well as low concentrations of Al and DOC, occurred at sites situated at low elevation (LES) or in well-buffered catchments (CER). Phosphorus concentration was the highest at LYS (62.8 µg L−1), caused by the P-rich granite that occurs in the catchment (Štědrá et al., 2016) coupled with high DOC concentrations in the stream water. The lowest phosphorus concentrations were observed at the sites LIT (16 µg l−1), which has a bedrock composed of nutrient-poor arkose and greywacke, and MOD (14.4 µg l−1), a subalpine catchment with high water runoff underlain by phyllite and mica schist.
Between 1995 and 2014, water pH increased at most sites (by a median value of 0.02 pH units per year; Table 2), likely as a result of declining acid deposition. In the well-buffered catchments (ANE, LIZ, LKV, POM, SAL), pH did not increase significantly (data not shown). The median reduction in total Al concentration across all catchments was 5.4 µg l−1 per year, representing a decline of about 20% during the period 1995–2014 (Table 2). Total Al increased only at PLB (data not shown), probably due to dissolution of secondary Al-bearing minerals containing SO4 2− (Krám et al., 2009). Likewise, water Ca and NO3 − concentrations declined at most sites (Table 2). Detailed results on water chemistry at each site are presented elsewhere (Oulehle et al., 2017), but some important results (e.g. pH and total phosphorus) are given in Table S1.
We found 49 non-diatom benthic algal taxa at the 15 sampling sites (Table S1), with an average of six taxa per site (range 3–11 taxa per site). The most common taxa were the cyanobacterium Heteroleibleinia sp., the green algae Microspora palustris var. minor Wichmann and Closterium sp., and the red alga Audouinella pygmaea (Kützing) Weber-van Bosse. The green algae Microspora palustris var. minor, Klebsormidium flaccidum (Kützing) P. C. Silva, K. R. Mattox & W. H. Blackwell and Stigeoclonium sp. reached the highest abundances at individual sites (Table S1). The AIP indicated acidic (minimum 5.5 at site UHL) to circumneutral (maximum 7.3 at site POM) conditions, while the PIT indicated nutrient-poor (minimum 4 at UDL) to slightly nutrient-enriched conditions (maximum 23 at LKV).
To remove autocorrelations among environmental variables, we summarized the water chemical data into principal components (Table 3). To ensure comparability of the relative strength of correlations, only the 13 sites for which we had both recent and long-term water chemical data were used (Table 1). The first three components of the PCA explained 74% of the variation in the water chemical data (Table 3). PC1 represented a gradient of non-nutrient salts (Na, K, Ca, Cl, SO4 2−, conductivity). PC2 not only represented a gradient of organic matter and phosphorus, but also correlated with aluminium concentrations, while PC3 represented a gradient in acid conditions (Table 3; Fig. S1). Higher PC axes explained less variation (no axis explained more than 10%), and no strong correlations occurred with any of the water chemical variables, such that these axes could not be meaningfully interpreted.
Relationships between environmental parameters and benthic algal assemblages
We then tested the strength of correlations between explanatory and response variables. Catchment characteristics generally were not related to algal response variables, with the exception of a correlation between longitude and total algal richness, indicating more algal taxa in the west of the Czech Republic (Table 4). The AIP index was strongly correlated with average pH, with a slightly stronger correlation with long-term (1995–2014) than recent (2015) pH (Table 4). Surprisingly, the eutrophication index PIT was not correlated with P concentrations, but correlated weakly negatively with DOC concentrations, indicating that high DOC concentrations were associated with a low (=oligotrophic) PIT. Taxon richness of cyanobacteria was most closely related to pH (with a low number of taxa at acidic conditions), while taxon richness of green algae showed the opposite pattern. Total algal taxon richness (and richness of green algae) was strongly negatively correlated with SiO2 concentrations. Algal assemblages (reflected as NMDS scores) were most closely related to PC3, which represented a gradient in acid conditions (Table 3). Al concentrations and PC2 (which reflected a gradient in aluminium, organic matter and phosphorus), however, were also influential (Table 4).
In order to further explore within which time period benthic algal assemblages reacted to changes in pH, and if average water chemistry or extreme values were more closely related to benthic algae, we calculated mean, median, minimum and maximum pH, separately for the 6 months before sampling as well as for the time periods of 1, 2, 3, 4 years, …etc. … up to 20 years before sampling. We then calculated a correlation matrix, separately for the AIP index and NMDS1 scores (Table S2); the results (Pearson correlation coefficients) are shown in Fig. 2. In order to ensure comparability of the strength of the relationships, we only used the 10 sites from which we had a complete dataset (i.e. including the AIP index as well as monthly pH measurements since January 1995; Table 1).
The AIP index was most closely related to recent minimum pH, i.e. to recent acidic events (Figs. 2, 3; Table S2; Fig. S2). There occurred two steps in the correlation coefficients between the AIP and minimum pH; firstly, the correlation became notably poorer for the time period of two or more years before sampling, and secondly, it became insignificant for the time period of nine or more years before sampling. These results indicate that pH minima that occurred two or more years before sampling had less effect on benthic algae than recent minima, and that minima that occurred nine or more years before sampling apparently were unrelated to benthic algal assemblages. Maximum pH was correlated with the AIP index only in the time interval between 5 and 12 years before benthic algal sampling (Table S2, Fig. 2). The correlation between mean pH and the AIP index was not significant for the first years before benthic algal sampling, but became stronger when 3 or more years of water chemistry were included (Fig. 2). Median pH followed a similar pattern as mean pH, but was generally less closely related to the AIP index than mean pH (Table S2, Fig. 2). These results indicate that the AIP index reacts quickly (within less than 6 months) and closely to acidic episodes, while pH maxima are less influential and do not have an immediate effect.
Correlation coefficients (Pearson r) between AIP (acidification index periphyton; upper panels) as well as NMDS1 scores (non-metric multidimensional scaling scores of algal assemblages; lower panels) and minimum, maximum, mean and median pH, calculated for the time intervals from 6 months (indicated as 0 in the figures), as well as 1, 2, 3, 4 years, etc., up to 20 years before the benthic algal sampling (x-axis). For better comparison, correlation coefficients for NMDS scores were inverted (from minus to plus)
NMDS1 scores are calculated from the abundances of all taxa present at a site, whereas the AIP index is calculated on the presence of sensitive taxa only. Nevertheless, the correlation coefficients between pH and NMDS1 scores by and large followed a similar pattern as the AIP index, but generally were weaker than those between pH and the AIP (Fig. 2; Table S2). This is not surprising, since tolerant species, which are included in NMDS1 scores, but not in the AIP, are less affected by changes in pH than sensitive species.
Effect of low pH combined with high phosphorus conditions on benthic algae
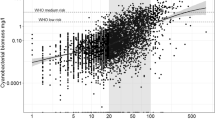
We then tested for correlations between pH and aluminium with phosphorus concentrations. If one outlier (LIT) was removed, TP concentrations were correlated with Al concentrations (Pearson r = 0.77; P = 0.001; data not shown). If the outlier LYS was removed, TP concentrations correlated with pH (Pearson r = 0.57; P = 0.034; Fig. 4). However, LYS had high phosphorus concentrations while pH at the same time was low (TP slightly above 60 µg l−1, pH slightly below 4.5; Fig. 3). At LYS, the AIP index was low, indicating acidic conditions, but the PIT index also was low, indicating oligotrophic conditions, in spite of high phosphorus concentrations (Fig. 4).
Scatter plots of mean pH and TP in 2015 (at all 15 sites), as well as AIP and PIT indices (at the 10 sites at which sufficient taxa for calculating the indices were found). The site marked with a triangle is LYS, which is unusual due to its high P concentrations, while pH at the same time is low. AIP acidification index periphyton, PIT periphyton index of trophic status
Discussion
Several lines of evidence together indicate that, in our dataset, benthic algal assemblages were more strongly affected by acid conditions than by nutrient concentrations: (1) the AIP, calculated based on species composition of benthic algae, was strongly related to pH (Table 4); (2) in contrast, the PIT, which indicates eutrophication, did not strongly correlate with any of the explanatory parameters, including nutrient concentrations; (3) the similarity in algal species assemblages (as characterized by NMDS values) was most closely related to PC3, which represented a gradient in acid conditions (Table 3). The AIP index was developed from data on algal assemblages and stream water pH from Norway, and includes only taxa which have relatively narrow ranges with respect to pH (Schneider & Lindstrøm, 2009). However, when calibrating the index, no data were available that allowed testing if parameters which typically correlate with pH, such as concentrations of aluminium or heavy metals (Stockdale et al. 2014), could explain more of the variation in benthic algal assemblages than pH. Also in our data, concentrations of total aluminium and Pb were related to pH (Fig. S1). Neither the AIP index nor similarity of algal assemblages (NMDS values), however, were significantly correlated with water Pb concentrations (Table 4). This indicates that the heavy metal Pb likely did not strongly affect algal species assemblages. In contrast, aluminium concentrations as well as pH correlated with the AIP, although the correlation with pH was stronger (Table 4). Aluminium concentrations were most closely related to PC2, while pH values were most closely related to PC3 (Table 3). NMDS scores correlated with both PC2 and PC3, although the correlation with PC3 was the strongest. Taken together, our results indicate that both aluminium and pH may affect algal assemblages. This lends support to hypothesis 2, which stated that both aluminium concentrations and pH would affect algal assemblages, and agrees with growth experiments performed by Kinross et al. (2000).
The AIP index, however, first and foremost reflected pH rather than total aluminium concentrations (Table 4). This is in accordance with expectations that pH affects sensitive algal species only (which is reflected in the AIP), while aluminium, via its effects on the biologically available phosphorus fraction (Exley et al., 1993), may be expected to affect all algal taxa (reflected in NMDS1 scores). An alternative explanation for the closer correlation of the AIP with pH than with Al may be that our data are for total aluminium concentrations, which comprise both organic and inorganic monomeric Al species. It is often assumed that inorganic monomeric Al most affects biota (Baldigo et al., 2007), and inorganic Al concentrations are usually highly correlated with pH (Driscoll, 1985). Although we cannot exclude the alternative explanation, we believe that the different responses of AIP and NMDS scores indicate that entire algal assemblages are affected by pH and aluminium, but that the AIP is mainly affected by pH. The AIP index was developed in Norway, and it is reasonable to assume that taxa which are acid-sensitive in Norway also are acid-sensitive in the Czech Republic. It is, however, possible that there occur acid-sensitive taxa in the Czech Republic which are not included in the AIP. An adaptation of the AIP index to the Czech Republic was beyond the scope of our manuscript, but would likely have increased the number of indicator taxa per site, and lead to an improved correlation with pH.
In spite of a relatively wide gradient in TP concentrations (ranging from 15 to 63 µg P l−1; Table 2), we were unable to detect an effect of TP on benthic algal assemblages (Table 3). This contradicts well-established relationships (Rott et al., 1999; Porter et al., 2008; Schneider & Lindstrøm, 2011), but may be explained by light limitation, or high Al concentrations. If light was limiting, an increase in a non-limiting resource (phosphorus) may not have affected competition among benthic algal taxa. This would lead to the absence of a correlation between species assemblages and TP [because phosphorus is not directly toxic for algae (Blindow, 1988), but affects algal assemblages via growth and competition (Schneider et al., 2016)]. High Al concentrations reduce the availability of phosphorus to algae (Exley et al., 1993). With one exception (LIT, which has naturally high Al and low P due to a bedrock chemistry which largely consists of arkose), sites that had high TP concentrations also had high Al concentrations. Therefore, part of the phosphorus likely was inaccessible to the algae. This should mean that high Al concentrations may mask the effect of enhanced phosphorus concentrations on the growth of benthic algae.
At LYS, i.e. the site with low pH and also high phosphorus concentrations, algal assemblages were acid-tolerant (the AIP index was low), correctly indicating low pH. The PIT index, however, also was low, seemingly at odds with the high TP concentrations measured at this site. This lends cautious support to hypothesis 3, i.e. that low pH apparently overrides the effect of high phosphorus concentrations on benthic algae. This may be caused by enhanced Al concentrations, which often are associated with acidification (Driscoll, 1985). Alternatively, it may simply result from the fact that the eutrophication index PIT was developed from a dataset that did not cover the full range of possible combinations of pH and phosphorus concentrations (e.g. we simply did not know that acid-tolerant taxa like Microspora palustris var. minor also would tolerate high phosphorus concentrations; Schneider et al., 2013). Next to Microspora, the dominating taxon at LYS was Stigeoclonium, and this taxon has been found before in acidic, P-rich mine drainage (Niyogi et al., 2002). Compiling the sparse information from acidic P-rich sites could reveal valuable information on algal assemblages that are characteristic of these combined stressors.
The interaction between acidification and eutrophication, however, is even more complicated. Exley et al. (1993) have shown that the presence of silicic acid protects against aluminium toxicity by preferentially binding aluminium in competition with phosphorus. This would suggest a three-way interaction between phosphorus, SiO2 and Al. Unfortunately, our dataset contains too few sites to confidently test so many interactions. However, the strongest correlation of algal taxon richness occurred with water SiO2 concentrations, and lowest richness occurred at high SiO2 concentrations (Table 4). Schneider et al. (2013) have shown that, in Norwegian rivers, non-diatom benthic algal taxon richness decreases with increasing TP concentration, and the same trend has been observed in a German dataset (unpublished). If we assume that increasing SiO2 in our Czech dataset correlates with increased bioavailability of phosphorus, then our observation of reduced taxon richness at high SiO2 concentrations would mirror the observation of reduced taxon richness at high phosphorus concentrations in Norway and Germany. An alternative explanation, however, could be competition with diatoms, a group of algae which we did not analyse. Silicate is an essential nutrient for diatoms (Tilman & Kilham, 1976). While diatom blooms can lead to a decrease in water SiO2 concentrations (Opfergelt et al., 2011), growth conditions for diatoms obviously are better when SiO2 reaches comparatively high, non-limiting levels. This suggests a stronger competition between diatoms and non-diatom benthic algae at sites with high SiO2 concentrations, and may thus explain the lower non-diatom taxon richness observed at sites with high SiO2 concentrations. While indices for ecological status assessment (both with respect to acidification and eutrophication) between diatoms and non-diatom benthic algae generally agree with each other (Kelly et al., 2008; Schneider et al., 2013), competition between diatom and non-diatom benthic algae may be an important driver of diversity patterns. Contrasting species richness patterns between diatom and non-diatom benthic algae have also been observed in relation to water TP concentrations (Schneider et al., 2013).
Our results were consistent with hypothesis 1, which stated that benthic algae are more closely related to recent acidic events than to long-term average pH (Fig. 2). Acidic events caused the disappearance of sensitive taxa within 6 months (because pH minima for 2015 were calculated from a period of 6 months prior to algal sampling, and the AIP index is based on the presence of sensitive taxa only). Naturally, the entire benthic algal assemblage (as characterized by NMDS values) was affected less by pH minima than sensitive species (as characterized by AIP). pH minima that occurred 2 years (or more) before sampling had less effect on the presence of sensitive species (the correlation coefficient between AIP and pH minima dropped markedly when a period of 2 or more years were included for calculating pH minima; Fig. 2). This indicates that recolonization by sensitive species had already started (but was not complete) 2 years after an acidic event. In 2006, severe acidic events affected 9 out of the 10 sites for which we had a complete dataset. At this point in time (9 years before sampling), there occurred a major drop in the correlation coefficients between pH minima and AIP as well as NMDS1 scores (Fig. 2). This indicates that pH minima that occurred 9 or more years before sampling apparently no longer affected present-day benthic algal assemblages. Taken together, our results indicate that recolonization of stream sites after an acidic event starts within two years after its occurrence, and is largely completed after 9 years (or less).
pH maxima were poorly related to algal assemblages. This indicates that pH maxima, in contrast to pH minima, do not have an immediate effect on benthic algal assemblages. While the disappearance of sensitive taxa after extreme acidic events is quick (Hirst et al., 2004), the process of colonization by sensitive species during periods of high pH is governed by slow dispersal, followed by competition with other taxa for resources. The correlation between mean pH and the AIP index was not significant for the first 2 years before benthic algal sampling, but became stronger when 3 or more years of water chemistry were included (Fig. 2). This is consistent with the assumption that the reaction of benthic algae to pH is a result of two processes: (1) a quick disturbance effect of pH minima on sensitive species and (2) a slow process of dispersal and competition for resources during periods when pH is higher.
These results have two consequences for stream monitoring and management: (1) indices such as the AIP may have even greater explanatory power if they were calibrated on recent pH minima instead of averages. This requires, however, frequent water chemical monitoring, and such data often are not available; (2) it explains the observation of obvious “mismatches” between stream chemistry and biology (Burns et al., 2008), because water chemistry, even if samples are taken frequently, may not capture short-term acidic events which nevertheless will affect biotic indices such as the AIP.
Correlation coefficients between average (and median) pH and the AIP index continued to become stronger if more years were included in calculating the average (Fig. 2). This indicates that “old” (up to 20 years) pH values (which in most cases were more acidic; Table 2) still affected benthic algae, and is a sign that recovery from acidification in the Czech Republic is still ongoing. This is supported by the observation that the slope in pH values over 20 years did not explain additional variation in the AIP index (tested by using linear models including pH minima/means and slopes; data not shown). This presumably indicates that biological recovery from acidification in the Czech Republic operates at time scales of more than two decades. Although acid deposition has decreased sharply since the 1980s, there remains significant deposition of both sulphur and nitrogen (Stoddard et al., 2001; Oulehle et al., 2015). Chemical recovery is therefore still ongoing, and consequently also biological recovery, which additionally may be put off by the occurrence of acidic events. Acidic events are an important stressor for benthic algal assemblages, and add complexity to the cocktail of multiple stressors that affect river ecosystems.
We are aware that our inferences are based on correlations only, and that our field data may not be used as causal evidence. However, our results indicate that acidification may have masked potential effects of phosphorus in stream ecosystems. Present-day eutrophication indices are not suitable to detect an increased P-load at acidic sites. Although areas that are most sensitive to acidification usually are not intensively used for agriculture, other potential sources of nutrients do exist, such as residences in mountainous areas. Increasing water DOC may be related to recovery from acidification (e.g. Hruška et al., 2009), and the organic-bound phosphorus may be available for benthic algae via phosphatase enzymes (Whitton et al., 1991). Consequently, there is a risk that recovery from acidification may result in increased eutrophication. This may potentially become an issue in many previously and presently acidified regions around the world. Therefore, we advocate that this aspect is given attention in future research.
References
Baldigo, B. P., G. Lawrence & H. A. Simonin, 2007. Persistent mortality of brook trout in episodically acidified streams of the southwestern Adirondack Mountains, New York. Transactions of the American Fisheries Society 136: 121–134.
Battarbee, R. W., 1984. Diatom analysis and the acidification of lakes. Philosophical Transactions of the Royal Society B-Biological Sciences 305: 451–477.
Blindow, I., 1988. Phosphorus toxicity in Chara. Aquatic Botany 32: 393–395.
Burns, D. A., K. Riva-Murray, R. W. Bode & S. Passy, 2008. Changes in stream chemistry and biology in response to reduced levels of acid deposition during 1987–2003 in the Neversink River Basin. Catskill Mountains. Ecological Indicators 8: 191–203.
Chuman, T., J. Hruška, F. Oulehle, P. Gürtlerová & V. Majer, 2013. Does stream water chemistry reflect watershed characteristics? Environmental Monitoring and Assessment 185: 5683–5701.
Clair, T. A., I. F. Dennis & R. Vet, 2011. Water chemistry and dissolved organic carbon trends in lakes from Canada’s Atlantic Provinces: no recovery from acidification measured after 25 years of lake monitoring. Canadian Journal of Fishery and Aquatic Sciences 68: 663–674.
Driscoll, C. T., 1985. Aluminum in acidic surface waters - chemistry, transport, and effects. Environmental Health Perspectives 63: 93–104.
Duan, L., Q. Yu, Z. Zhang, Z. Wang, Y. Pan, T. Larssen, J. Tang & J. Mulder, 2016. Acid deposition in Asia: emissions, deposition and ecosystem effect. Atmospheric Environment 146: 55–69.
Exley, C., A. Tollervey, G. Gray, S. Roberts & J. D. Birchall, 1993. Silicon, aluminium and the biological availability of phosphorus in algae. Proceedings of the Royal Society B-Biological Sciences 253: 93–99.
Garmo, Ø. A., B. L. Skjelkvåle, H. A. de Wit, L. Colombo, C. Curtis, J. Folster, A. Hoffmann, J. Hruska, T. Hogasen, D. S. Jeffries, W. B. Keller, P. Kram, V. Majer, D. T. Monteith, A. M. Paterson, M. Rogora, D. Rzychon, S. Steingruber, J. L. Stoddard, J. Vuorenmaa & A. Worsztynowicz, 2014. Trends in surface water chemistry in acidified areas in Europe and North America from 1990 to 2008. Air and Soil Pollution 225: 1880.
Genter, R. B. & D. J. Amyot, 1994. Fresh-water benthic algal population and community changes due to acidity and aluminum-acid mixtures in artificial streams. Environmental Toxicology and Chemistry 13: 369–380.
Gray, D. K. & S. E. Arnott, 2012. The role of dispersal levels, allee effects and community resistance as zooplankton communities respond to environmental change. Journal of Applied Ecology 49: 1216–1224.
Gutowski A. & J. Förster, 2009. Benthische Algen ohne Diatomeen und Characeen. LANUV-Arbeitsblatt 9. In German.
Herrmann, J., 2001. Aluminium is harmful to benthic invertebrates in acidified waters, but at what threshold(s)? Water, Air and Soil Pollution 130: 837–842.
Herrmann, J., E. Degerman, A. Gerhardt, C. Johansson, P. E. Lingdell & I. P. Muniz, 1993. Acid-stress effects on stream biology. Ambio 22: 298–307.
Hirst, H., F. Chaud, C. Delabie, I. Juttner & S. J. Ormerod, 2004. Assessing the short-term response of stream diatoms to acidity using inter-basin transplantations and chemical diffusing substrates. Freshwater Biology 49: 1072–1088.
Hruška, J., F. Moldan & P. Krám, 2002. Recovery from acidification in Central Europe - observed and predicted changes of soil and streamwater chemistry in the Lysina catchment, Czech Republic. Environmental Pollution 120: 261–274.
Hruška, J., P. Krám, W. H. McDowell & F. Oulehle, 2009. Increased dissolved organic carbon (DOC) in Central Europe streams is driven by reduction in ionic strength rather than climate change or decreasing acidity. Environmental Science and Technology 43: 4320–4326.
John, D. M., B. A. Whitton & A. J. Brook (eds), 2011. The freshwater algal flora of the British Isles. Cambridge University Press, London.
Kelly, M. G., L. King, R. I. Jones, P. A. Barker & B. J. Jamieson, 2008. Validation of diatoms as proxies for phytobenthos when assessing ecological status in lakes. Hydrobiologia 610: 125–129.
Kinross, J. H., P. A. Read & N. Christofi, 2000. The influence of pH and aluminium on the growth of filamentous algae in artificial streams. Archiv fuer Hydrobiologie 149: 67–86.
Kolář, T., P. Čermák, F. Oulehle, M. Trnka, P. Štěpánek, P. Cudlín, J. Hruška, U. Buntgen & P. Rybníček, 2015. Pollution control enhanced spruce growth in the “Black Triangle” near the Czech-Polish border. Science of the Total Environment 538: 703–711.
Komarek J., 2013. Cyanoprokaryota. In: Büdel G., Gärtner G., Krienitz L. & Schagerl M. (eds) Süßwasserflora von Mitteleuropa, Bd. 19/3. Spektrum, Heidelberg.
Komarek J. & K. Anagnostidis, 2007. Cyanoprokaryota. In: Büdel G., Gärtner G., Krienitz L. & Schagerl M. (eds) Süßwasserflora von Mitteleuropa, Bd. 19/1 and 19/2. Spektrum, Heidelberg.
Kopáček, J., M. Posch, J. Hejzlar, F. Oulehle & A. Volková, 2012. An elevation-based regional model for interpolating sulphur and nitrogen deposition. Atmospheric Environment 50: 287–296.
Kovacs, C., M. Kahlert & J. Padisak, 2006. Benthic diatom communities along pH and TP gradients in Hungarian and Swedish streams. Journal of Applied Phycology 18: 105–117.
Kowalik, R. A., D. M. Cooper, C. D. Evans & S. J. Ormerod, 2007. Acidic episodes retard the biological recovery of upland British streams from chronic acidification. Global Change Biology 13: 2439–2452.
Krám, P., J. Hruška, C. T. Driscoll, C. E. Johnson & F. Oulehle, 2009. Long-term changes in aluminum fractions of drainage waters in two forest catchments with contrasting lithology. Journal of Inorganic Biochemistry 103: 1465–1472.
Malmqvist, B. & S. Rundle, 2002. Threats to the running water ecosystems of the world. Environmental Conservation 29: 134–153.
Monteith, D. T., A. G. Hildrew, R. J. Flower, P. J. Raven, W. R. B. Beaumont, P. Collen, A. M. Kreiser, E. M. Shilland & J. H. Winterbottom, 2005. Biological responses to the chemical recovery of acidified fresh waters in the UK. Environmental Pollution 137: 83–101.
Niyogi, D. K., W. M. Lewis & D. M. McKnight, 2002. Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems 5: 554–567.
Niyogi, D. K., J. S. Harding & K. S. Simon, 2013. Organic matter breakdown as a measure of stream health in New Zealand streams affected by acid mine drainage. Ecological Indicators 24: 510–517.
Oksanen J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, et al., 2012. Vegan: Community Ecology Package: R Package Version 2.0-4.
Opfergelt, S., E. S. Eiriksdottir, K. W. Burton, A. Einarsson, C. Siebert, S. R. Gislason & A. N. Halliday, 2011. Quantifying the impact of freshwater diatom productivity on silicon isotopes and silicon fluxes: Lake Myvatn, Iceland. Earth and Planetary Science Letters 305: 73–82.
Oulehle, F., B. J. Cosby, K. Austnes, C. D. Evans, J. Hruška, J. Kopáček, F. Moldan & R. F. Wright, 2015. Modelling inorganic nitrogen in runoff: Seasonal dynamics at four European catchments as simulated by the MAGIC model. Science of the Total Environment 536: 1019–1028.
Oulehle, F., T. Chuman, J. Hruška, P. Krám, W. H. McDowell, O. Myška, T. Navrátil & M. Tesař, 2017. Recovery from acidification alters concentrations and fluxes of solutes from Czech catchments. Biogeochemistry 132: 251–272.
Planas, D., 1996. Acidification effects. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology. Academic Press, San Diego.
Porter, S. D., D. K. Mueller, N. E. Spahr, M. D. Munn & N. M. Dubrovsky, 2008. Efficacy of algal metrics for assessing nutrient and organic enrichment in flowing waters. Freshwater Biology 53: 1036–1054.
Quinn, G. P. & M. F. Keough, 2002. Experimental Design and Data Analysis for Biologists. Cambridge University Press, Cambridge.
R Development Core Team, 2012. A language and environment for statistical computing. In: R Foundation for Statistical Computing. R Development Core Team, Vienna, Austria.
Rott E., H. van Dam, P. Pfister, E. Pipp, K. Pall, N. Binder & K. Ortler, 1999. Indikationslisten für Aufwuchsalgen. Teil 2: Trophieindikation, geochemische Reaktion, toxikologische und taxonomische Anmerkungen. Wasserwirtschaftskataster; Bundesministerium für Land- und Forstwirtschaft, Vienna, Austria.
Schindler, D. W., 2006. Recent advances in the understanding and management of eutrophication. Limnology and Oceanography 51: 356–363.
Schneider, S. & E. A. Lindstrøm, 2009. Bioindication in Norwegian rivers using non-diatomaceous benthic algae: The acidification index periphyton (AIP). Ecological Indicators 9: 1206–1211.
Schneider, S. & E. A. Lindstrøm, 2011. The periphyton index of trophic status PIT: a new eutrophication metric based on non-diatomaceous benthic algae in Nordic rivers. Hydrobiologia 665: 143–155.
Schneider, S. C., M. Kahlert & M. G. Kelly, 2013. Interactions between pH and nutrients on benthic algae in streams and consequences for ecological status assessment and species richness patterns. Science of the Total Environment 444: 73–84.
Schneider, S. C., S. Hilt, J. E. Vermaat & M. Kelly, 2016. The “forgotten” ecology behind ecological status evaluation: re-assessing the roles of aquatic plants and benthic algae in ecosystem functioning. Progress in Botany 78: 285–304.
Schöpp, W., M. Posch, S. Mylona & M. Johansson, 2003. Long-term development of acid deposition (1880–2030) in sensitive freshwater regions in Europe. Hydrology and Earth System Sciences 7: 436–446.
Skjelkvåle, B. L., J. L. Stoddard, D. S. Jeffries, K. Torseth, T. Hogasen, J. Bowman, J. Mannio, D. T. Monteith, R. Mosello, M. Rogora, D. Rzychon, J. Vesely, J. Wieting, A. Wilander & A. Worsztynowicz, 2005. Regional scale evidence for improvements in surface water chemistry 1990–2001. Environmental Pollution 137: 165–176.
Søndergaard, M. & E. Jeppesen, 2007. Anthropogenic impacts on lake and stream ecosystems, and approaches to restoration. Journal of Applied Ecology 44: 1089–1094.
Štědrá, V., T. Jarchovský & P. Krám, 2016. Lithium-rich granite in the Lysina-V1 borehole in the southern part of the Slavkov Forest, western Bohemia (in Czech, English abstract & captions). Geoscience Research Reports 49: 137–142.
Stockdale, A., E. Tipping, S. Lofts, J. Fott, O. A. Garmo, J. Hruška, B. Keller, S. Loefgren, S. C. Maberlyh, V. Majer, S. A. Nierzwicki-Bauer, G. Persson, A. K. Schartau, S. J. Thackeray, A. Valois, J. Vrba, B. Walseng & N. Yan, 2014. Metal and proton toxicity to lake zooplankton: application of a chemical speciation based modelling approach. Environmental Pollution 186: 115–125.
Stoddard, J. L., D. S. Jeffries, A. Lükewille, T. A. Clair, P. J. Dillon, C. T. Driscoll, M. Forsius, M. Johannessen, J. S. Kahl, J. H. Kellogg, A. Kemp, J. Mannio, D. T. Monteith, P. S. Murdoch, S. Patrick, A. Rebsdorf, B. L. Skjelkvale, M. P. Stainton, T. Traaen, H. van Dam, K. E. Webster, J. Wieting & A. Wilander, 1999. Regional trends in aquatic recovery from acidification in North America and Europe 1980–1995. Nature 401: 575–578.
Stoddard, J. L., T. S. Traaen & B. L. Skjelkvale, 2001. Assessment of nitrogen leaching ICP-Waters sites (Europe and North America). Water Air and Soil Pollution 130: 781–786.
Tilman, D. & S. S. Kilham, 1976. Phosphate and silicate growth and uptake kinetics of diatoms Asterionella formosa and Cyclotella meneghiniana in batch and semicontinuous culture. Journal of Phycology 12: 375–383.
Vestreng, V., G. Myhre, H. Fagerli, S. Reis & T. Tarrasón, 2007. Twenty-five years of continuous sulphur dioxide emission reduction in Europe. Atmospheric Chemistry and Physics 7: 3663–3681.
Whitton, B. A., S. L. J. Grainger, G. R. W. Hawley & J. W. Simon, 1991. Cell-bound and extracellular phosphatase-activities of cyanobacterial isolates. Microbial Ecology 21: 85–98.
Acknowledgements
Dick Wright is gratefully acknowledged for critically reading the manuscript. We thank many colleagues for help with long-term sample collections, namely Miroslav Tesař, Institute of Hydrodynamics of the Czech Academy of Sciences (LIZ), Vladimír Černohous (UDL) and Zdeněk Vícha (CER) from Forestry and Game Management Research Institute, Alena Kulasová from T.G. Masaryk Water Research Institute (UHL), Tomáš Navrátil from the Institute of Geology of the Czech Academy of Sciences (LES), Evžen Stuchlík from Charles University (LIT) and Milan Váňa from the Czech Hydrometeorological Institute (ANE). We also thank the staff of the Czech Geological Survey (CGS) Central laboratories, who provided all long-term water analyses, and Tomáš Chuman (CGS) for help with the database and Fig. 1. We thank two reviewers for helpful comments on an earlier version of the manuscript. The project was funded by EAA Grants and Norway Grants (EHP-CZ02-OV-1-048-01-2014) and the Czech Science Foundation grant GA15-08124S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Verónica Jacinta Lopes Ferreira
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schneider, S.C., Oulehle, F., Krám, P. et al. Recovery of benthic algal assemblages from acidification: how long does it take, and is there a link to eutrophication?. Hydrobiologia 805, 33–47 (2018). https://doi.org/10.1007/s10750-017-3254-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3254-8