Abstract
The Sepetiba Bay (Southeast Brazil) is a known Cd- and Zn-contaminated site that received spills of a large slag pile leachate from a Zn smelter. With important harbors, Sepetiba Bay demands periodic dredging operations which affect the mobility of the metals. The main goal of this work was to assess metal mobility in sediments and its associated toxicity in a fictive dredging area, to evaluate the risks of the operation. To achieve this goal, 18 superficial sediment samples were collected and characterized for pH and Eh. Sediments were analyzed for grain size, organic carbon, and total nitrogen, and metal mobility was evaluated with a sequential extraction procedure, proposed by the European Community Bureau of Reference (BCR). The results demonstrate that Cd and Zn are mainly associated with the exchangeable fraction (mean concentrations 1.4 mg kg−1 and 149.4 mg kg−1, respectively) and reducible fractions (mean concentrations 0.3 mg kg−1 and 65.5 mg kg−1, respectively), while Fe, Cr, Cu, Ni, and Al were associated with the residual fraction. Metals in the residual fraction are probably associated with the mineral lattice of the sediment and should not represent an environmental risk for the biota. The application of the enrichment factor and three risk assessment indexes (Risk Assessment Code, Risky Pollution Index, and Bioavailability Risk Assessment Index) show that the sediments are considerably enriched in metals that constitute a relevant risk for the sediment biota. In the case of dredging operations, Cd and Zn should be released to the overlying waters and be available to organisms, threatening the whole ecosystem. The proposed approach was shown to be much more precise than what is frequently presented in the Environmental Impact Assessments that only consider the threshold limits of the legislation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal areas are the most populated places in the world; however, human activities have been changing these areas into important receptacles of all sorts of pollutants (Felizardo et al. 2021; Zhang et al. 2021). Urbanization, industrialization, and mining are the main responsible for the disposal of trace metals in coastal areas (Chifflet et al. 2019; Di Marzio et al. 2019). After the Minamata disaster in the 1950s, trace metal pollution in the coastal environment became a matter of concern due to toxicity, diffuse sources, persistency, complex, and expensive remediation (Wang et al. 2013). During the last decades, the number of polluted sites related with trace metals increased significantly because the metals accumulate in the sediments that constitute their final fate (Cordeiro et al. 2015).
Trace metals persist in the sediment in different forms and mobilities. The residence time of metals in sediments is linked with the bonding with other compounds (Cáceres-Choque et al. 2013), determining geochemical fractions in the sediment in distinct physicochemical conditions. In other words, the strength of bonds in these fractions determines the potential mobility of the metal. When associated with mobile fractions, metals can be considered of large concern, because any change in physical–chemical conditions may increase bioavailability to organisms, leading them to reach higher levels of the trophic chain, severely threatening the environment and generating health problems in human groups (Ali et al. 2019). These bioavailable metals can be remobilized to the interstitial water and to the water column under many conditions, including resuspension of the sediments due to the incidence of wind in shallow water bodies (Dang et al. 2020). However, more intense resuspension processes like dredging operations can release large quantities of metals to the water, threatening organisms which can bioconcentrate or biomagnify contaminants (Yu et al. 2019).
The impacts of dredging operations on metal bioavailability in sediments can be estimated with sequential extraction methods (Wasserman et al. 2013), in which their geochemical fractions are sequentially separated according to their mobility (Chifflet et al. 2019; Hamdoun et al. 2015; Yu et al. 2019). After Wasserman et al. (2016), the procedure allowed to predict the amount of metals released to the water column during the operation. The authors considered the bioavailable metal fraction in the sediment (obtained from partial extractions) to predict concentrations in the water column. Although during dredging all geochemical fractions are resuspended in the water column, only those metals which are loosely bound or sensitive to oxidation processes can be considered harmful because they are going to be released in the water column.
The development of risk indexes for metals (Cesar et al. 2015; Nasrabadi 2015; Zheng et al. 2010) constituted a significant evolution for the management of the ecosystem impact of metals because total concentrations alone give a distorted idea of their hazard that must be based on the toxicity and bioavailability. When analyzing the impact of resuspension of dredging operations, besides total concentrations of the sediments (as established in dredging regulations all over the world), a consistent risk assessment should be applied, considering geochemical fractionation and toxicity of the metals (Ferronato et al. 2015).
In the present research, a fictive dredging area was simulated in the metal-contaminated Sepetiba Bay, where surface sediment samples were collected and analyzed. The aim of the procedure was to analyze geochemical fractionation (BCR method) of Al, Cd, Cr, Cu, Fe, Ni, Pb, and Zn in the sediments, together with the results of a risk assessment that allowed a comparison with current environmental control of dredging operation, applying local and global legislations.
Materials and methods
Study area
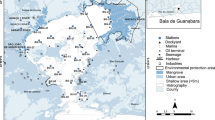
The Sepetiba Bay is located approximately 60 km south of Rio de Janeiro City (Fig. 1). It is a strategic region for sea trade and economic development in Brazil. The drainage basin of the bay possesses a relevant industrial activity accounting over 600 industrial plants and three ports (Itaguaí, Sudeste, and CSA Maritime Terminal). The industrial complex contributes with many industrial diffuse sources of contaminants, including from metal smelting, paper and pulp, chemicals, plastics, and rubbers. Domestic and commercial sewage, domestic wastes, harbor, and navigation contaminations also constitute relevant sources (Trevisan et al. 2020). Since the 1980s, this region is subject to environmental concern, mainly because of the identification of Cd and Zn contamination in the sediments (Lacerda and Molisani 2006), originated from a metal smelting industry (Ingá Metais) which disposed large amounts of these contaminants every year in the Sepetiba Bay (Molisani et al. 2004).
As mentioned above, Sepetiba Bay has three large maritime terminals that are subject to frequent dredging operations, remobilizing sediments to the water column and distributing contaminated materials. For this research, an area close to the mouth of the São Francisco Canal was chosen and delimited to simulate a fictive environmental impact assessment (EIA) of a dredging operation. Like in an EIA, a large number of stations were defined, with a density of 1 station per 3 ha. Considering it was a mere simulation, differently from EIAs, only surface sediments were collected.
Sampling
The fictive dredging polygon was defined in the mouth of the São Francisco Canal in a contaminated region of the Sepetiba Bay. Eighteen representative (of the simulated area) surface sediment samples (a layer of 10-cm depth) were collected with a Van Veen grab. The sediment samples were placed in air-sealed bags and arranged in a cooler, maintained at − 4 °C during transportion to the laboratory, and stored in the freezer at − 20 °C until further analyses.
Physical and chemical characterization
For each sampling station, pH and redox potential of the interstitial water were directly measured in the freshly sampled sediment using a multiparameter Hanna probe. Total organic carbon and total nitrogen elementary concentrations were determined using a flash combustion organic elemental analyzer from thermo scientific. Carbon and nitrogen analyses were carried out after the removal of carbonates from sediment subsamples (0.1 g) by acidification with HCl 0.1 mol L−1. For grain size analysis, the sample preparation included three steps: freeze-drying, loss on ignition (550 C). Acidification with hydrochloric acid (HCl) was carried out with the aim of removing carbonate shells that are abundant in this type of environment and because they are autochthonous, they interfere with granulometry results. After those steps, the samples were three times washed with Milli-Q water and lyophilized for 24 h. A 15 mL of hexametaphosphate deflocculant was added and agitated in centrifuge tubes for 16 h, and particle-size distribution was determined in a laser analyzer (Malvern Instruments Mastersizer 2000) for samples < 1 mm.
Sequential extraction procedure
The BCR sequential extraction procedure was adopted, a widely used method for geochemical fractionation for eight metals (Al, Cd, Cr, Cu, Fe, Ni, Pb, and Zn). Four geochemical fractions could be identified, which are exchangeable (fraction 1), reducible (fraction 2), oxidizable (fraction 3), and residual (fraction 4). The fractions indicate availability, therefore metals’ mobility decreases in the following sequence F1 > F2 > F3 > F4 (Yang et al. 2021).
Approximately 1 g of lyophilized sample was used in the sequential extraction procedure (methods steps are described in Supplementary Material – SM 1), and the extracts were analyzed by optical emission spectrometry with induced coupled plasma in a Varian 720 ES ICP-OES.
All materials used in the sequential extraction were thoroughly washed and soaked in dilute HCl (3%) for 3 days and rinsed with Milli-Q water (Millipore). The reagents used were ultrapure grade. Duplicates of samples BCR reference material and triplicates of blanks were analyzed simultaneously. For each extraction step, recoveries of each element were calculated as percentages of the measure, related to the certified concentration values (BCR-701 reference material was used). The recovery concentration of each metal in each fraction varied between 81.48 and 115.45% (details are shown in Supplementary Material — SM 2). Detection (3 times standard deviation) and quantification (5 times standard deviation) limits were also calculated based on the values of at least three blanks for each fraction (SM 2).
The sum of the four geochemical fractions were considered for the total metal contents, corresponding to the pseudo-total concentrations of metals, as established by US-EPA (2007).
Enrichment factor and ecological risk assessment
The pollution extent and the risk-associated were assessed through widely used indexes. Enrichment factor (EF; Salomons and Förstner (1984)) was applied to distinguishing natural from anthropogenic pollution. The Risk Assessment Code (RAC; Nasrabadi (2015)) and Risky Pollution Index (Ir) were applied to assess the ecological risk (Perin et al. 1985). EF represents the relative trace metal concentration abundance, normalized by a conservative metal, normally Fe and Al, which act as a “proxy” for the clay content (Abrahim and Parker 2008; Windom et al. 1989). In this work, Al was chosen as the proxy for clay mineral contents. The EF was determined by Eq. (1).
where Mx and Alx are the trace metal and Alx concentrations in the sediment sample, while MB and AlB are the trace metal and Al concentrations in the background material. The background values used in EF formula were obtained from bottom layers (4-m depth) of a core next to São Francisco Canal and Sepetiba shore (Pinto et al. 2019). EF values are classified according to enrichment levels given in Table 1.
The Risk Assessment Code (RAC) considers only the exchangeable and carbonate-bound metal fractions of the sediment as proxies. In this geochemical fraction (F1 from the BCR sequential extraction), the trace metal bindings are weak and tend to diffuse from sediment to the pore water and them to the overlying water, constituting a possible risk for the benthonic community (Cáceres-Choque et al. 2013). On the other hand, the Risky Pollution Index (Ir) is based on the Igeo (Müller 1979) principle and additionally, considers the first three geochemical fractions (exchangeable, reducible, and oxidizable), attributing weights, according to the potential metal mobility for each extraction phase. The RAC and Ir are defined, respectively, as Eq. (2) and (3):
where F1 is the concentration of the metals in the fraction 1 (exchangeable); F4 is the metal concentration in the fraction 4 (residual).
where R, As, Re, and Ox are residual, exchangeable, reducible, and oxidizable fractions, respectively. The constants a, b, and c intend to enhance the role of metal release potential of each fraction and were set as 6, 3.5, and 2.5, respectively (after the work of Nasrabadi (2015)). The ecological risk assessement indexes (RAC and Ir) are also interpretated according to different contamination levels (Table 2).
Bioavailability Risk Assessment Index
The Bioavailability Risk Assessment Index (BRAI) was developed by Jamshidi-Zanjani et al. (2015) and constitute an index that evaluates the bioavailability, associated with toxicity of a certain number of metals in the sediment. In the original research, the BRAI was applied with a single extraction with EDTA, corresponding to water soluble metals, exchangeable metals, and metals associated with carbonates (Gismera et al. 2004). In the present research, instead of an EDTA extraction, the fraction 1 of the BCR extraction was used. Alan and Kara (2019) showed that the first fraction of the BCR procedure corresponds to soluble metals, exchangeable metals, and metals associated with carbonates. The calculation can be done after Eq. (4).
where Bdi is a source effect (SEi), which is the potential release of the bioaccessible fraction (percent of fraction 1 of the total concentration) expressed as a categorized value (see Table 3), divided by the individual effect range median (ERM) of the metals Cd, Cr, Cu, Ni, Pb, and Zn (Long et al. 1995). TEi is the summation of ERMs of the considered metals.
Table 4 indicates the BRAI values and their levels of risk. Although the BRAI can also be calculated as a human bioaccessibility risk assessment index, in the present work we did not apply this variation of the index.
Results and discussion
The physical and chemical characteristics of the sediment
The São Francisco Canal mouth is a low energy system, resulting in reduced wind, tide currents, and small wave energy (Carvalho et al. 2021). Nonetheless, the São Francisco Canal is the outlet of an industrial and populated drainage basin and may carry a considerable load of organic matter discharging into the bay (Trevisan et al. 2020). These characteristics promote the settling of large amounts of organic matter and the accumulation of metals. The accumulation of the organic matter associated with a limited oxygen provisions favors the organic matter preservation, resulting in sediments with severely reduced conditions. The redox potential observed in the sediment samples varied between − 115 and − 385 mV (Table 5), which is an expected range for these environmental conditions.
The sediment samples presented a very low variation in the pH because the high ionic potential exerts a strong buffering effect (Jiang et al. 2019). The pH was nearly neutral, varying between 7.33 and 7.88, and the temperature varied between 22.6 and 25.2 °C. The carbon and nitrogen concentrations indicate that organic matter is preserved in the reducing sediment, possibly related to the São Francisco canal inputs settling and anaerobic respiration conditions (Wang et al. 2018).
The highest total organic carbon and total nitrogen concentrations were found in the S-8 (2.07 and 0.23, respectively; Table 5), the neighbor samples of the S-8 presented similar values, however, slightly lower. The sampling points near the coasts apparently tend to present lower levels of carbon and nitrogen due to aerobic degradation associated with wave energy (Scofield et al. 2015). This trend could be confirmed through the values of samples S-2, S-3, and S-4 (located close to the littoral); however, S-1 presents higher values and S-15/S16 similar values. The N/C ratio varied between 0.09 and 0.11, indicating a preferred algal source (authigenic) for the organic matter in this region (Bernardes et al. 2004). In a more recent work, Carvalho et al. (2020) observed slightly lower values of N/C ratios (0.02–0.11), but their stations were closer to the littoral and probably receiving a more intense influence of the continent.
The grain size followed the expected pattern for a low-energy system associated with the canal mouth (Farhat and Aly 2018). In this case, coarse grains and flocculated colloidal particles tend to settle in higher energy areas before it reaches the low energy systems. In deeper areas, farther from the canal mouth, the sediment tends to present an increase of silt and clay particles. In Fig. 2, the triangular diagram of Folk (1954) shows that samples are organized in three distinct groups of grain size, a proximal area with sediments classified as silty-sand, associated with a shallower sediment, closer to the shore and subject to higher wave energy. Transitional area sediments are classified as sandy-silt, in an intermediary environment, and a distal area, classified as silt, is subject to less energy, deeper, and farther from the shore.
The samples’ grain size in a triangular diagram (Folk 1954)
Total metal concentration in the sediment
The metal concentration (Table 6) demonstrated large spatial variability and a trend of more intense accumulation in the center of the fictive dredging area. This area could represent a preferable condition for particle settling, related with the São Francisco Canal input of materials, like organic matter and colloidal particles, enriched with metals (Felizardo et al. 2021). As expected, Fe and Al presented elevated concentrations because these elements are important rock constituent and in tropical environment weathering induces their enrichment in the sediments and waters (Wasserman et al. 2019). Zinc and cadmium are reported as the metals deserving attention, with concentrations varying between 125.9 and 604.9 mg kg−1 and 1.0 and 3.9 mg kg−1, respectively. The other metals’ present concentrations are quite close to their background levels, showing a similar spatial distribution within the simulation area. Stations S-11, S-12, and S-13 presented particularly high total concentrations for Cd, Cu, Ni, and Zn, which can be partially explained by granulometry (but not fully explained). Further discussion on the relationship between samples and parameters will be done farther with the results of the statistical analyses.
As it can be observed from Table 6, Cd concentrations exceed the limit of level 1 (associated with low adverse effects) of the Brazilian regulation in all stations except S-2. No concentrations exceed level 2. For Zn, the situation is closely the same with values exceeding level 1 in all stations except S-2. Stations S-11, S-12, and S-13 also exceed level 2 concentrations for Zn, which is associated with significant biological effects. These stations were mentioned above, located in the center of the fictive dredging area. Ni and Pb also display a few samples with concentrations above level 1 of the legislation, but no value above level 2. This discussion is important because as far as sediment quality criteria (for dredging operations) is considered, only stations S-11, S-12, and S-13 would be considered associated with significant biological effect.
Geochemical fractionation of metals in the sediments
Table 7 presents the average and standard deviation (n = 18) values for the metal concentrations in each fraction of the sequential extraction for all sampling stations (raw data was presented in Supplementary Materials SM 3a–d). Figure 3 shows stacked bar graphs with the percentages of metals in each of the 4 fractions.
Fraction 1 is assumed as representing the amounts of metals readily available for the benthonic fauna uptake, which is immediately susceptible to be released to the water column during dredging operations (Di Nanno et al. 2007). Therefore, the metals accumulated in this geochemical fraction tend to be highly toxic (Salazar et al. 2013). In the sediments of the dredging simulation area, Cd and Zn were shown to be rather present in fraction 1 (average 64.8% and 48.9%, respectively; Fig. 3), which are probably associated with the inputs from a disactivated zinc melting industry (Ingá Metais) that was closed in the early 1990s, but left a slag pile of several meters tall, highly enriched with these metals (Ribeiro et al. 2013). Cu and Ni presented average percentages of 4.5% and 5.0%, respectively, in the F1, which could be linked to diffuse sources in the watershed, mainly domestic wastes, land-filling, and various industries. However, these contributions are insignificant, when compared with the contamination of Zn and Cd. The metals Al, Cr, Fe, and Pb presented the lowest concentration proportions in the F1 fraction. It is noteworthy that Zn and Cd, with such a high percentage in the F1 probably constitute a higher risk of contamination during dredging operations than states regulations (as shown in the discussion of the previous item).
The association of metals with Fe and Mn oxi-hydroxides corresponds to the F2 fraction (also known as reducible fraction), which can be easily dissolved in reducing conditions (Kumari and Paul 2017). Despite the severe negative potential redox in the fictive dredging area (Table 5), it is possible to observe a relevant role of the F2, especially for Pb and expectedly for Fe (average 31.2%), a particularly redox-sensitive element (Chifflet et al. 2019; Guo et al. 2006). The fictive dredging area is located a few hundred meters away from the Port of Itaguai that exports large quantities of iron ore. Although the boarding process is presently improved, a few years ago, a brownish cloud of ore powder could be periodically seen. The inputs of iron oxi-hydroxides, as described by Araujo et al. (2017), may be reflected in the sequential extraction of the sediment. Pb presented the higher percentage in F2 (average 50.3%), and the highest values were observed in S-14, where Pb association with iron ore or in the Fe and Mn oxy-hydroxides mediated by bacterial activity could be adequate explanations (Hnaťuková 2011). Fe and Mn oxi-hydroxides were shown to be a more important binding and adsorption sites for Zn than organic matter in the sediment (Guo et al. 2006), so it also presented a high concentration in F2 (average 22.8%). Cadmium and copper also presented relevant amounts associated in F2 (averages of 13.7% and 17.4%, respectively). The aluminum, chromium, and nickel presented the lower associations with F2. As shown in in situ experiments, it is not expected that metals strongly associated with reducible fraction are released in the solution during dredging operations (Yu et al. 2019).
The anaerobic bacterial metabolism is responsible for organic matter degradation in a reducing environment, promoting reduction of sulfates to sulfides and preserving the bulk organic matter (Bernardes et al. 2004; Mora et al. 2014; Sabadini-Santos et al. 2014). In these conditions, metals associate with organic matter or sulfur through the formation of mono and bisulfides (Machado et al. 2010). The metals associated with the organic matter and sulfides represent fraction 3, also known as oxidizable fraction (He et al. 2017). The percentages of metals associated in fraction 3 are relatively small with averages for Cr, Fe, Ni, and Cu of 29.2%, 19.4%, 19.4%, and 17.0%, respectively. While Cr can be associated with organic matter, the formation of FeS, NiS, and CuS are probably the main reasons for these percentages (Machado et al. 2010). Al, Pb, Zn, and Cd presented percentages below 10% in F3. Therefore, the oxidation process that is intense on dredging operation probably does not significantly affect the release of metals to the water column. Furthermore, regardless of various studies reporting in vitro oxidation experiments (e.g., Cappuyns and Swennen (2005)), their kinetics in dredging operations is still poorly known.
An interesting comparison of the results of the present research with those of Ribeiro et al. (2013), who applied the AVS/SEM model (Allen et al. 1993) to this same Sepetiba Bay is unavoidable, because it was carried out to preview the behavior of metals during a real dredging operation. Total metal concentrations were in the same order of magnitude, showing elevated levels of contamination for zinc and cadmium (twice as much as we found in average in the present study). Regardless of the fact that redox potential was also reducing in those authors’ work, concentrations of acid volatile sulfides were low in the dredging simulation area, and the percentages of metals associated with sulfides (SEM) were also low, corroborating the low availability of the oxidizable (F3) observed in the present study.
Al is a refractory element originated from continental weathering process, presented most of the total concentration in F4, probably associated with clay mineral crystalline structures (Kluczka et al. 2017). This finding shows the reliability of the application of aluminum as a normalizing element. Cr, Ni, and Cu concentrations were mostly associated with F4, followed by Fe. Cd and Zn, also Pb in F4 presented the lowest concentrations in average, indicating that the geologic influence on these elements is relatively small when compared to their anthropogenic proportion. It must be underlined that pseudo-total extractions (applied in F4 extraction procedure) do not attain metals associated with stronger lattice in the sediment. None of the metals present in F4 are intended to be made available to the organisms during dredging operations.
Statistical analyses
The geochemical fractionation of the metals was further assessed applying a principal component analysis (PCA) multivariate test (Fig. 4; individual loadings of each variable were presented in Supplementary Material SM 4). In order to evaluate the influence of natural conditions over metal accumulation, the statistical calculations were performed with the labile fraction concentration (F1 + F2 + F3). The most relevant factor (Ft 1) accounted for 40.45% of the total variance. The main feature related to Ft 1 is a significant approximation between Cd, Cr, Cu, Pb, Zn, organic carbon, and a separated group gathering grain size (clay + silt), Fe, Ni and total nitrogen. Although organic matter and fine sediment are relevant factors for retention of contaminants, the first group seems to be submitted to a closer control from organic matter, while the second group was rather controlled by granulometry. It is interesting to note that Fe is not controlled by Eh, regardless of the fact it is a redox sensitive element (Eggleton and Thomas 2004). Probably most of this element is rather geogenic (as discussed in the results of sequential extractions). Therefore, Ft 1 is substantially controlled by the environmental energy and the associated processes, generating smaller grain-size and accumulation of organic matter associated with metals.
Principal component analyses performed with sediment samples (n = 18), mode R (Klovan 1975)
Considering the conspicuous separation of the variable redox potential (Eh), the Barletts sphericity test, together with Kaiser–Meyer–Olkin test were applied to verify the validity of the PCA. It was observed that although values of correlation between Eh and other elements are frequently low (except silt–clay), the results show that they do not significantly differ from zero (chi-square 190.434; p < 0.0001; α = 0.05). Considering that the calculated p is smaller than the assumed significance level (0.05), it is concluded that at least one of the correlations is different from zero and the PCA is valid. The calculated Kaiser–Meyer–Olkin value was 0.525.
The second factor (Ft 2) contributing with a further 21.70% variation out of the total provides information concerning metals without relation with anthropic contributions. The principal feature related to the first factor showed significant separation between the group pH, Al, Fe, and Ni, corroborating the reduced influence of the physico-chemical variable over the considered lithogenic metals. On the other hand, other metals Cd, Cr, Cu, Pb, and Zn seem to have a consistent contribution from anthropogenic sources and although organic matter and granulometry (smaller extent) showed to control their behavior in Factor 1, in Factor 2 these metals were rather associated with redox potential. Loadings of each variable in components 1 and 2 (Ft 1 and Ft 2) are presented in the SM 4.
The cluster analysis further corroborates the formation of two distinct groups based on sediment variables and metal accumulation (Fig. 5) observed in principal component analyses (PCA). These groups could be divided in metals with and without anthropogenic relation. Al, Fe, and Ni would be associated with a lithogenic origin, while the other metals represent an anthropogenic pollution in different levels for each metal. Clearly, this separation of metals may determine the risk of dredging operations.
Assessment of sediment contamination
Cd and Zn presented EF values above 2 (SM 5), constituting evidence of anthropogenic sources (Cáceres-Choque et al. 2013). EF values demonstrated absent or very low enrichments for Cr and Ni, which can be considered natural. Nonetheless, Pb reaches moderate values in S-8, S-12, and S14, while Cu is moderately enriched in S-5 (Table 8). The index indicates relevant enrichment of Cd and Zn in the fictive dredging sediments in Sepetiba Bay. In areas with such concentrations, regardless of the fact that many stations do not overcome the threshold limits of the legislation, resuspension and release of metals constitute a hazard for the environment. In these areas dredging should apply techniques that reduce resuspension or oxidation of the sediments.
Ecological risk assessment of trace metal pollution
The Risk Assessment Code (RAC) results presented very low risk for Cu, Cr, Ni, and Pb in all sampling stations (Fig. 6A). However, the risk of Cd is significant, varying between medium and very high, reaching more than 70% in seven stations. Zn presented low to very high risk, however more frequent in high risk, reaching 72% at the S-11. Cd and Zn constitute significant risks, mainly for the benthonic community, as well as in dredging operations, where sediments are resuspended. Furthermore, in complex estuarine ecosystem, where trophic chains are composed of many levels, there is a strong risk that dredging promotes intensification of the biomagnification in the top consumers (Dong et al. 2019). It is noteworthy from Fig. 6A that the RAC was able to steeply differentiate Cd and Zn from other metals, which can be considered a consistent statement, based on the previous approaches.
The Risky Pollution Index (Ir) considers the possibility of trace metal release from exchangeable, reducible, and oxidizable fractions, however, in variable proportions. The trace metal release of these geochemical fractions demands relevant physicochemical changes, in this case; only an intense resuspension event (like dredging) could be capable to promote those changes (Fremion et al. 2016). In general, all metals showed significantly higher risk comparing with the RAC (Fig. 6B). Ni presented the lowest values, classified as low to medium risk. Cr and Cu also displayed medium risk, regardless of station S-8 that presented considerable risk. It is interesting to note that in the Ir risk analyses, Pb presented a considerable risk in most of sampling sites, at the S-13, and S-15 reached high range and at S-14 very high range. It is interesting to note that EF for Pb shows low enrichment, while Ir value is related mainly with the F2. Perhaps this element hazard is being overestimated. Considering the RAC and Ir risk analyses, the Zn is the most dangerous pollutant, closely followed by Cd, reaching the highest Ir values and representing a severe risk for the biota.
Figure 7 shows the values of the BRAI calculated in the sediments for the stations in the Sepetiba Bay fictive dredging area. Regardless of the fact that the conception of the indexes applied in the present research, their results did not differ considerably. High values were observed in stations S-2, S-7, S-8, S-11, S-12, S-13, and S-18, while station S-9 showed lower values. In fact, this last station is the only one that presented medium risk of bioavailability. The Index BRAI is differentiated from the others because it integrates the cumulative risks of all metals (except Fe and Al) in one overall index. Considering that the risks are mainly associated to Cd and Zn (Fig. 6), regardless of the different calculations, the responses of all indexes are redundant.
Conclusion
In the present work, the metallic contamination of a fictive dredging area was presented, applying various techniques to assess its hazard. Dredging control legislation considers limiting total concentrations (sediment quality criteria), disregarding chemical fractionation, an approach that is not able to determine the real environmental risk. The metals’ geochemical fractionation in the sediments of the dredging simulation area (mouth of São Francisco Canal in the Sepetiba Bay) demonstrated the influence of anthropogenic sources in the sediment. Cd, Zn and to a lesser extent Pb are the most prominent pollutants, with a relevant part of their concentrations associated with the exchangeable fraction, which can promptly release in the environment. Pb concentrations were also important in the reducible fraction (Pb), which is less important in dredging operations. The other metals (Al, Fe, Cr, Cu, and Ni) presented low concentrations in the labile fractions (F1, F2, and F3). Al, Fe, and Ni were related with lithogenic (natural) sources and do not constitute any risk in dredging operations, while Cr, Pb, and Cu are originated from mixed sources.
The oxidizable fraction is important in dredging because the operation tends to promote an intensive oxidation of the sediments. Regardless of the fact that the sediment in the study area was a reducing environment, most of the metals were not present in this fraction. The normal associations of metals with sulfides in reducing environment were shown not to be relevant in the study area. Therefore, dredging operations would release the contaminants by desorption (fraction 1), rather than by intense oxidation.
Enrichment factor indicates a significant enrichment in Cd and Zn, but also a moderate enrichment in Pb and Cu. The Risk Assessment Code (RAC) presented a negligible risk for Cu, Cr, Ni, and Pb. However, Cd and Zn could represent a very high risk to the biota, mainly during dredging operations or in complex ecosystems (trophic chain with numerous levels). The Risky Pollution Index (Ir) demonstrated that Zn and Pb represent in some samples a severe risk for the environment and Cd a very high risk. Therefore, Cd and Zn represent an eminent environmental risk, although Pb could also represent a risk for the benthonic community. On the other hand, Bioavailability Risk Assessment Index (BRAI) is a cumulative index that integrates the risks of each metal as a function of their toxicity and regardless of the significant different conceptions from the others, it still provides similar information.
Dredging in the area delimited in the present research demands the application of techniques like submarine-confined disposal facilities, controlled dredging velocity procedures, and purposefully designed equipment to reduce resuspension. It is finally noteworthy that many of the evaluated sites presented concentrations below the threshold limits of the legislation, however still presenting severe risk; therefore, we conclude that the evaluation of total (or pseudo-total) concentrations alone is not enough to estimate the risk of a dredging operation. Although the international literature shows that the establishment of sediment quality criteria should be abandoned, the legislations all over the world still consider this approach in dredging operations.
Data availability
Raw data is available under request.
References
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Eviron Monitor Assess 136:227–238. https://doi.org/10.1007/s10661-007-9678-2
Alan M, Kara D (2019) Comparison of a new sequential extraction method and the BCR sequential extraction method for mobility assessment of elements around boron mines in Turkey. Talanta 194:189–198. https://doi.org/10.1016/j.talanta.2018.10.030
Ali H, Khan E, Ilahi I (2019) Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J Chem 2019:6730305. https://doi.org/10.1155/2019/6730305
Allen HE, Fu G, Deng B (1993) Analysis of acid-volatile sulfide (AVS) and simultaneously extracted metals (SEM) for the estimation of potential toxicity in aquatic sediments. Environ Toxicol Chem 12:1–13. https://doi.org/10.1002/etc.5620120812
Araujo DF, Peres LGM, Yepez S, Mulholland DS, Machado W, Tonha M, Garnier J (2017) Assessing man-induced environmental changes in the Sepetiba Bay (Southeastern Brazil) with geochemical and satellite data. C r Geosci 349:290–298. https://doi.org/10.1016/j.crte.2017.09.007
Bernardes MC, Martinelli LA, Krusche AV, Gudeman J, Moreira M, Victoria RL, Ometto J, Ballester MVR, Aufdenkampe AK, Richey JE, Hedges JI (2004) Riverine organic matter composition as a function of land use changes, Southwest Amazon. Ecol Appl 14:S263–S279. https://doi.org/10.1890/01-6028
Cáceres-Choque LF, Ramos Ramos OE, Valdez Castro SN, Choque Aspiazu RR, Choque Mamani RG, Fernández Alcazar SG, Sracek O, Bhattacharya P (2013) Fractionation of heavy metals and assessment of contamination of the sediments of Lake Titicaca. Eviron Monitor Assess 185:9979–9994. https://doi.org/10.1007/s10661-013-3306-0
Cappuyns V, Swennen R (2005) Kinetics of element release during combined oxidation and pH(stat) leaching of anoxic river sediments. Appl Geochem 20:1169–1179. https://doi.org/10.1016/j.apgeochem.2005.02.004
Carvalho ACB, Silva LJ, Dick DP, Moreira VD, Vicente MD, de Andrade AFO, Bidone ED, Sabadini-Santos E (2020) Spectroscopic analyses of organic matter in surface sediments from Sepetiba Bay, Rio de Janeiro, Brazil. Quimica Nova 43:552–557. https://doi.org/10.21577/0100-4042.20170518
Carvalho ACB, Moreira VdA, Vicente MdC, Bernardes MC, Bidone ED, Sabadini-Santos E (2021) Evolution of the sources contribution of aliphatic hydrocarbons and their fate in Sepetiba Bay, Rio De Janeiro, Brazil. Est Coastal Shelf Sci 261:107548. https://doi.org/10.1016/j.ecss.2021.107548
Cesar R, Colonese J, Bidone E, Castilhos Z, Egler S, Polivanov H (2015) Disposal of dredged sediments in tropical soils: Metal ecological risk assessment based on bioassays with aquatic and edaphic organisms. Engenharia Sanitaria e Ambiental 20:181–189. https://doi.org/10.1590/S1413-41522015020000121030
Chifflet S, Tedetti M, Zouch H, Fourati R, Zaghden H, Elleuch B, Quéméneur M, Karray F, Sayadi S (2019) Dynamics of trace metals in a shallow coastal ecosystem: insights from the Gulf of Gabès (southern Mediterranean Sea). AIMS Environ Sci 6:277–297. https://doi.org/10.3934/environsci.2019.4.277
Cordeiro RC, Machado W, Santelli RE, Figueiredo AG, Seoane JCS, Oliveira EP, Freire AS, Bidone ED, Monteiro FF, Silva FT, Meniconi MFG (2015) Geochemical fractionation of metals and semimetals in surface sediments from tropical impacted estuary (Guanabara Bay, Brazil). Environ Earth Sci 74:1363–1378. https://doi.org/10.1007/s12665-015-4127-y
Dang DH, Layglon N, Ferretto N, Omanovic D, Mullot JU, Lenoble V, Mounier S, Garnier C (2020) Kinetic processes of copper and lead remobilization during sediment resuspension of marine polluted sediments. Sci. Total Environ 698. https://doi.org/10.1016/j.scitotenv.2019.134120
Di Marzio A, Lambertucci SA, Fernandez AJG, Martinez-Lopez E (2019) From Mexico to the Beagle Channel: A review of metal and metalloid pollution studies on wildlife species in Latin America. Environ Res 176:12. https://doi.org/10.1016/j.envres.2019.04.029
Di Nanno MP, Curutchet G, Ratto S (2007) Anaerobic sediment potential acidification and metal release risk assessment by chemical characterization and batch resuspension experiments. J Soils Seds 7:187–194. https://doi.org/10.1065/jss2007.04.220
Dong J, Xia X, Liu Z, Zhang X, Chen Q (2019) Variations in concentrations and bioavailability of heavy metals in rivers during sediment suspension-deposition event induced by dams: insights from sediment regulation of the Xiaolangdi Reservoir in the Yellow River. J Soils Seds 19:403–414. https://doi.org/10.1007/s11368-018-2016-1
Eggleton J, Thomas KV (2004) A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ Intern 30:973–980. https://doi.org/10.1016/j.envint.2004.03.001
Environment Canada and Ministère du Développement durable, de l’Environnement et des Parcs du Québec (2007) Criteria for the assessment of sediment quality in Quebec and application frameworks: prevention, dredging and remediation, p 39. https://www.planstlaurent.qc.ca/fileadmin/publications/diverses/Qualite_criteres_sediments_e.pdf
Farhat HI, Aly W (2018) Effect of site on sedimentological characteristics and metal pollution in two semi-enclosed embayments of great freshwater reservoir: Lake Nasser, Egypt. J Afr Earth Sci 141:194–206. https://doi.org/10.1016/j.jafrearsci.2018.02.012
Felizardo JP, Muniz MC, Vezzone M, Cardoso RP, Wasserman J, Padilla R, Migliori A, Anjos RM (2021) Sources of sedimentary organic matter and assessment of heavy-metal levels in estuarine sediments after Fundão dam breach. Estuar Coast Shelf Sci 261:107507. https://doi.org/10.1016/j.ecss.2021.107507
Ferronato C, Vianello G, Antisari LV (2015) Heavy metal risk assessment after oxidation of dredged sediments through speciation and availability studies in the Reno river basin, Northern Italy. J Soils Sediments 15:1235–1245. https://doi.org/10.1007/s11368-015-1096-4
Folk RL (1954) The distinction between grain size and mineral composition in sedimentary-rock nomenclature. J Geol 62:344–359. https://doi.org/10.1086/626171
Fremion F, Courtin-Nomade A, Bordas F, Lenain JF, Juge P, Kestens T, Mourier B (2016) Impact of sediments resuspension on metal solubilization and water quality during recurrent reservoir sluicing management. Sci Total Environ 562:201–215. https://doi.org/10.1016/j.scitotenv.2016.03.178
Gismera MJ, Lacal J, da Silva P, Garcı́a R, Teresa Sevilla M, Procopio JR (2004) Study of metal fractionation in river sediments. a comparison between kinetic and sequential extraction procedures. Environ Pollut 127:175–182. https://doi.org/10.1016/j.envpol.2003.08.004
Guo S-h, Wang X-l, Li Y, Chen J-j, Yang J-c (2006) Investigation on Fe, Mn, Zn, Cu, Pb and Cd fractions in the natural surface coating samples and surficial sediments in the Songhua River, China. J Environ Sci 18:1193–1198. https://doi.org/10.1016/S1001-0742(06)60061-2
Hamdoun H, Van-Veen E, Basset B, Lemoine M, Coggan J, Leleyter L, Baraud F (2015) Characterization of harbor sediments from the English Channel: assessment of heavy metal enrichment, biological effect and mobility. Mar Poll Bull 90:273–280. https://doi.org/10.1016/j.marpolbul.2014.10.030
He Y, Men B, Yang XF, Li YX, Xu H, Wang DS (2017) Investigation of heavy metals release from sediment with bioturbation/bioirrigation. Chemosph 184:235–243. https://doi.org/10.1016/j.chemosphere.2017.05.177
Hnaťuková P (2011) Geochemical distribution and mobility of heavy metals in sediments of urban streams affected by combined sewer overflows. J Hydrol Hydromech 59:85–94. https://doi.org/10.2478/v10098-011-0007-7
Jamshidi-Zanjani A, Saeedi M, Li LY (2015) A risk assessment index for bioavailability of metals in sediments: Anzali International Wetland case study. Environ Earth Sci 73:2115–2126. https://doi.org/10.1007/s12665-014-3562-5
Jiang LQ, Carter BR, Feely RA, Lauvset SK, Olsen A (2019) Surface ocean pH and buffer capacity: past, present and future. Sci Rep 9:11. https://doi.org/10.1038/s41598-019-55039-4
Klovan JE (1975) R- and Q-mode factor analysis. In: McCammon RB (ed) Concepts in geostatistics. Springer Berlin Heidelberg, Berlin, pp 21–69. https://doi.org/10.1007/978-3-642-85976-2_2
Kluczka J, Zolotajkin M, Ciba J, Staron M (2017) Assessment of aluminum bioavailability in alum sludge for agricultural utilization. Eviron Monitor Assess 189:8. https://doi.org/10.1007/s10661-017-6133-x
Kumari J, Paul B (2017) Geochemical and environmental risk assessment of hazardous elements in river sediment. Environ Earth Sci 76:731. https://doi.org/10.1007/s12665-017-7073-z
Lacerda LD, Molisani MM (2006) Three decades of Cd and Zn contamination in Sepetiba Bay, SE Brazil: evidence from the mangrove oyster Crassostraea rhizophorae. Mar Poll Bull 52:974–977. https://doi.org/10.1016/j.marpolbul.2006.04.007
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97. https://doi.org/10.1007/BF02472006
Machado W, Villar LS, Monteiro FF, Viana LCA, Santelli RE (2010) Relation of acid-volatile sulfides (AVS) with metals in sediments from eutrophicated estuaries: is it limited by metal-to-AVS ratios? J Soils Seds 10:1606–1610. https://doi.org/10.1007/s11368-010-0297-0
Molisani MM, Marins RV, Machado W, Paraquetti HHM, Bidone ED, Lacerda LD (2004) Environmental changes in Sepetiba Bay, SE, Brazil. Reg Environm Chaqnge 4:17–27. https://doi.org/10.1007/s10113-003-0060-9
Mora A, Laraque A, Moreira-Turcq P, Alfonso JA (2014) Temporal variation and fluxes of dissolved and particulate organic carbon in the Apure, Caura and Orinoco rivers, Venezuela. J S Am Earth Sci 54:47–56. https://doi.org/10.1016/j.jsames.2014.04.010
Müller G (1979) Schwermetalle in sedimenten des Rheins - Veranderungen seit (1971). Umschau 79:778–783
Nasrabadi T (2015) Risky pollution index: an integrated approach towards determination of metallic pollution risk in sediments. J Eng Geol 9:2841–2868. https://doi.org/10.18869/acadpub.jeg.9.2.2841
Perin G, Craboledda L, Lucchese M, Cirillo R, Dotta L (1985) Heavy metal speciation in the sediments of Northern Adriatic Sea: a new approach for environmental toxicity determination. In: 5th International Conference on Heavy Metals in the Environment, Athens (Greece), pp 454–456
Pinto AFS, Ramalho JCM, Borghi L, Carelli TG, Plantz JB, Pereira E, Terroso D, Santos WHd, Geraldes MC, Rocha F, Rodrigues MAdC, Laut L, Martins MVA (2019) Background concentrations of chemical elements in Sepetiba Bay (SE Brazil). J Sediment Environ 4:108–123. https://doi.org/10.12957/jse.2019.40992
Ribeiro AP, Figueiredo AMG, dos Santos JO, Dantas E, Cotrim MEB, Figueira RCL, Silva EV, Wasserman JC (2013) Combined SEM/AVS and attenuation of concentration models for the assessment of bioavailability and mobility of metals in sediments of Sepetiba Bay (SE Brazil). Mar Poll Bull 68:55–63. https://doi.org/10.1016/j.marpolbul.2012.12.023
Sabadini-Santos E, Senez TM, Silva TS, Moreira MR, Mendonca JG, Santelli RE, Crapez MAC (2014) Organic matter and pyritization relationship in recent sediments from a tropical and eutrophic bay. Mar Poll Bull 89:220–228. https://doi.org/10.1016/j.marpolbul.2014.09.055
Salazar GJP, Alfaro-De la Torre MC, Aguirre RNJ, Briones-Gallardo R, Cedeño CJ, Peñuela MGA (2013) Geochemical fractionation of manganese in the Riogrande II reservoir, Antioquia, Colombia. Environ Earth Sci 69:197–208. https://doi.org/10.1007/s12665-012-1947-x
Salomons W, Förstner U (1984) Sediments and the transport of metals. In: Salomons W, Förstner U (eds) Metals in the hydrocycle. Springer Berlin Heidelberg, Berlin, pp 63–98. https://doi.org/10.1007/978-3-642-69325-0_3
Scofield V, Jacques SMS, Guimarães JRD, Farjalla VF, Scofield V, Jacques SMS, Guimarães J (2015) Potential changes in bacterial metabolism associated with increased water temperature and nutrient inputs in tropical humic lagoons. Front Microbiol 6:310. https://doi.org/10.3389/fmicb.2015.00310
Trevisan CL, Vicente MC, Rocha BCS, Wasserman JC (2020) Development of a Dredging Sensitivity Index, applied to an industrialized coastal environment in Brazil. Sci Total Environ 748:141294. https://doi.org/10.1016/j.scitotenv.2020.141294
US-EPA (2007) Method 3051A. Microwave assisted acid digestion of sediments, sludges, soils and oils. SW-846. United States Environmental Protection Agency, Washington, DC, pp 1–30. https://www.epa.gov/sites/default/files/2015-12/documents/3051a.pdf
Wang S-L, Xu X-R, Sun Y-X, Liu J-L, Li H-B (2013) Heavy metal pollution in coastal areas of South China: a review. Mar Poll Bull 76:7–15. https://doi.org/10.1016/j.marpolbul.2013.08.025
Wang HJ, Hu XP, Wetz MS, Hayes KC (2018) Oxygen consumption and organic matter remineralization in two subtropical, eutrophic coastal embayments. Environ Sci Technol 52:13004–13014. https://doi.org/10.1021/acs.est.8b02971
Wasserman JC, Barros SR, Lima GBA (2013) Planning dredging services in contaminated sediments for balanced environmental and investment costs. J Environ Manag 121:48–56. https://doi.org/10.1016/j.jenvman.2013.02.024
Wasserman JC, Wasserman MA, Barrocas PRG, Almeida AM (2016) Predicting pollutant concentrations in the water column during dredging operations: implications for sediment quality criteria. Mar Poll Bull 108:24–32. https://doi.org/10.1016/j.marpolbul.2016.05.005
Wasserman JC, Almeida AM, Perez DV, Wasserman MA, Machado W (2019) Evaluation of contaminants spreading from sludge piles, applying geochemical fractionation and attenuation of concentrations model in a tropical reservoir. Eviron Monitor Assess 191:426. https://doi.org/10.1007/s10661-019-7507-z
Windom HL, Smith RG, Rawlinson C (1989) Particulate trace metal composition and flux across the southeastern U.S. continental shelf. Mar Chem 27:283–297. https://doi.org/10.1016/0304-4203(89)90052-2
Yang HJ, Bong KM, Kang T-W, Hwang SH, Na EH (2021) Assessing heavy metals in surface sediments of the Seomjin River Basin, South Korea, by statistical and geochemical analysis. Chemosphere 284:131400. https://doi.org/10.1016/j.chemosphere.2021.131400
Yu J, Chen Q, Zhang J, Zhong J, Fan C, Hu L, Shi W, Yu W, Zhang Y (2019) In situ simulation of thin-layer dredging effects on sediment metal release across the sediment-water interface. Sci Total Environ 658:501–509. https://doi.org/10.1016/j.scitotenv.2018.12.226
Zhang J, Zhang Y, Zhang P, Li Y, Li J, Luo X, Xu J, Zhao L (2021) Seasonal phosphorus variation in coastal water affected by the land-based sources input in the eutrophic Zhanjiang Bay, China. Estuar Coast Shelf Sci 252:107277. https://doi.org/10.1016/j.ecss.2021.107277
Zheng LG, Liu GJ, Kang Y, Yang RK (2010) Some potential hazardous trace elements contamination and their ecological risk in sediments of western Chaohu Lake, China. Eviron Monitor Assess 166:379–386. https://doi.org/10.1007/s10661-009-1009-3
Funding
This work was supported by Coordenação de Pessoal de Nível Superior—CAPES PROAP (grant # 001). CER, WM and JCW are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq) for research grants # 305217/2017–8; 312849/2019-2 and 310425/2020–4, respectively. MCV received a Doctorate scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq), grant number 143523/2018–0. CER also received a support from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ, rant # E-26/200.893/2021.
Author information
Authors and Affiliations
Contributions
JCW, WM, and MCV developed the study conception and design. Material preparation and data collection were carried out by MCV, CLT, ACBC, and JCW. Analysis were performed by BCVO and CER. The first draft of the manuscript was written by MCV, and all the authors commented on the previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The present research did not involve human participants or animals.
Ethics approval
The authors accept principles of ethical and professional conduct as established in the submission guidelines.
Consent to participate
The authors consented to participate as contributors in the present article.
Consent for publication
The authors will consent to publish the present article after acceptance by Environmental Science and Pollution Research.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Carvalho Vicente, M., Trevisan, C.L., de Carvalho, A.C.B. et al. Geochemical fractionation of trace metals and ecological risk assessment of surface sediments in Sepetiba Bay, Brazil. Environ Sci Pollut Res 31, 14254–14269 (2024). https://doi.org/10.1007/s11356-024-32095-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32095-6