Abstract
The aim of this study was to determine the mobility of metals in contaminated sediments in the water reservoir Ružín No.I, which is situated on the Hornád and Hnilec Rivers (Eastern Slovakia). Bottom sediments are contaminated above all by heavy metals, namely As, Cu, Cd, Cr, Hg, Mn and Zn, which were alluvial into the reservoir from localities of former mining activities, and thus they represent ecological load mainly at the inputs into reservoir. The mobilities and solid-state distributions of As, Cd, Cr, Cu, Hg, Ni, Pb, Sb, and Zn ions in impoundment materials were investigated using a five-step sequential extraction procedure. The greatest quantity of Hg was released in the organic–sulfide fraction (F4), in the range of 75.2–87.6 % in both samples. The highest percentage of the total metal content for As, Cr, and Sb was between 82.0 and 94.6 % in the fifth step for both samples also. Cu and Pb were released in the greatest quantities in the third step in the reducible fraction, from 45 to 65.7 % in sample HN and up to 68–50 % in sample HR. High bioavailability was observed for copper, zinc, lead, and cadmium. Nickel, arsenic, antimony, and chromium were only extracted in the residue fraction and displayed low bioavailability. The bioavailability of the metals in the sediments followed the sequence: Cu > Hg > Pb > Cd > Zn > Ni > As > Cr > Sb. The sediment phytotoxicity was evaluated based on the germination of seeds and decreases in the root growth of the plant Sinapis alba. The percentage inhibition of seed germination was 8.9–41.1 % in the 2010–2011 HR samples and 9.1–45.1 % in the 2010–2011 HN samples, which was higher than the inhibition of root growth. In the majority of tested Sinapis alba seeds, the metals displayed no phytotoxic effect. This indicates that the Sinapis alba test still exhibited a strong tolerance to contaminated sediment as this plant species has developed distinct detoxification mechanisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For the reason of not leaving toxic heritage for the future generations, contaminated sediments should be carefully examined qualitatively and quantitatively. In addition for the proper hazard assessment of polluted sediments and the monitoring of cleanup processes, it is important to combine toxicity data, chemical analyses, and biological information (Plaza et al. 2010). It is necessary to understand the relationship between biological and physicochemical properties in order to interpret and characterize contaminated environmental sites (Quan et al. 2014). Ecotoxicological tests have been successfully applied to monitor the contamination and bioremediation efficiencies of sediments and are important in the ecological assessment of hazardous waste sites and in supporting management decisions for subsequent remediation. Integrated methodologies provide information not only about the quality and quantity of contaminants, but also about the effects of the sediment, its biological status, and the degradative activity on the soil microflora (Leitgib et al. 2007; Mohamed et al. 2010). Several authors (Hallare et al. 2011; Fragoso et al. 2006) have proposed that a single biomarker alone may not be sufficient to characterize the sublethal effects of pollutants. Thus, a battery of simple bioassays is recommended to provide rapid, holistic, and relative toxicity values that reflect the bioavailability, interactions, and even the mechanisms of actions of pollutants. The majority of bioassays applied to contaminated sediments are based on the toxic effects of sediment solutions or of the sediment itself on a living organism [e.g., animals, algae, plant, and bacterial bioassays (Aboudrar et al. 2013; Czerniawska-Kusza and Kusza 2011)]. Currently, alternative ecotoxicity tests (microbiotests) that are low-cost, readily available, and rapid are being increasingly applied. These tests can be successfully used to evaluate acute toxicity and to assess the environmental dangers of compounds (Dolezalova-Weissmanova et al. 2013; Pretti et al. 2009). Plant biomarkers, in particular, offer a variety of advantages, such as a large array of assessment endpoints [e.g., germination rate, biomass weigh, enzyme activity, etc. (Ferrari et al. 1999; Bagur-Gonzale et al. 2011; Clement et al. 2010)]. The pH of the nutrient solution is an extremely important property in regulating the solubility, speciation, and toxicity of trace metals. The measurement of these criteria can provide reliable data on the relationship between the depression and the concentration of a toxic metal in solution (Kopittke et al. 2010). The phytotoxicity test is based on an analysis of the phytotoxic effects (Sinapis alba) of contaminants in the germination phase of the seeds and in the development of the seedlings during the first 3 days of growth (75 h of exposure at 25 °C temperature). It is well known that sediment plays a significant role in aquatic ecosystems (Katalay et al. 2012). Sediments in aquatic ecosystems are often contaminated with metals as a result of natural background or anthropogenic activities. Large quantities of sediments can become trapped in dams and reservoirs, and their adequate management requires accurate prediction of the fates and effects of metals in aquatic environments (Roulier et al. 2010). Sediments are typically mixtures of several components, including different mineral species as well as organic debris (Bettinetti et al. 2003). Metal pollution in sediments has received extensive attention due to their toxicity, slow degradation, and facile accumulation. The metals from anthropogenic sources primarily reside in the labile fractions of sediments and may be consumed by organisms as the environmental parameters change. The metal enrichment factor is considered a useful indicator of the environmental pollution by metals (Devesa-Rey et al. 2013; Moukhchan et al. 2013). Each evaluation method possesses limitations (Mukherjee and Kumar 2012; Das and Chakrapani 2011). For example, it is problematic to use the total metal concentration in sediment as a measure of its toxicity and ability of bioaccumulate because different sediments exhibit varying degrees of bioavailability for the same total metal content (Yang et al. 2012). Bioavailability, which is determined by the chemical form, mobility, and degree of transformation of the contaminant, is an important indicator of toxicity (Vojtekova and Krakovska 2006; Urban et al. 2010; de Miguel et al. 2005). The fractionated assessment of the metal distribution provides approximate information about the liquid and solid phases of the sediment. Sequential extraction (SE) methods are suitable for the evaluation of element mobility in sediments environments and are accepted by IRMM (Institute for Reference Materials and Measurements) as standard methods for given certified reference materials (Blaskova et al. 2013; Vojtekova et al. 2010; Houben et al. 2013). The aim of this work was to determine the mobility of As, Cd, Cr, Cu, Hg, Ni, Pb, Sb, and Zn to evaluate their ecological risk by means of the enrichment factor (K EF,) and to assess the potential phytotoxic effects of the contaminated sediments from the water reservoir Ružín No.I in Slovakia.
Study area
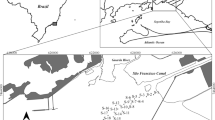
Location of historical mining, metallurgical activities, and sampling points of the water reservoir Ružín situated in the Valley of Volov´s Mountains and Hornad basin, Eastern Slovakia (Europe) is shown in Fig. 1. The long-term monitoring of the metal content in the area has confirmed that these activities influenced the quality of the bottom sediments in the water reservoir Ružín No.I. The filling of the reservoir began in 1972. Over 42 years, its volume was diminished due to the accumulation of 12 million m3 of sediment. The bottom sediments in the water reservoir are the result of erosion and sedimentation processes in their river basin, such as the weathering of rocks, and volcanic activities also play a prominent role in enriching the water of the reservoirs with metals. The Hornád River (HR) stems on the eastern rise of Kráľova Hoľa in an altitude of 500 m above sea level. Thence, it flows through melaphyres, paleogene slates, and sandstones and further provides river flow in the limestone bedrock of Slovak Paradise, where it is moving into the quartz subsoil. The River Hnilec springs on the eastern massif rise of the Kráľova Hoľa and flows through the Slovak paradise, Volov´s Mountains. In the river basin Hornád and Hnilec, there are several old, abandoned, and flooded mining works as well as mining dumps resulting as mining, treatment, and metallurgical processing of Cu, Fe, and Hg ores containing impurities of toxic elements such as As and Sb. As a result of erosive–abrasive processes, these toxic elements have been get into the water reservoir Ružín. From this point of view, the bottom sediments of reservoir Ružín are contaminated by trace and semi-trace metals, namely As, Cd, Cr, Cu, Hg, Ni, Pb, Sb, and Zn. Fe and Cu ores were exploited in the Smolník area, and Cu, Fe, Hg, and Ba ores were mined in the Rudňany area. Additionally, Cu ores from home production and import (87 %) were treated in Krompachy. These sediments accumulate various pollutants, which are also of anthropogenic origin (Sestinova 2012).
Materials and methods
Sediment and sampling methods
The study was conducted on sediments collected from the area of Eastern Slovakia (Fig. 1) in which the water reservoir Ružín No.I is located. Sample HR was collected from the Hornád branch, and sample HN was collected from the Hnilec branch. The sampling was performed in the years 2010 and 2011. The sediments were sampled in ground glass bottles using a “Multisampler” device at a depth of 50 cm. The samples were first dried at room temperature, and the sediments were thoroughly mechanically homogenized immediately prior to the experiments and then quartered. The sediments contain sand, silt, and clay fractions (Table 1). The silt and clay fractions were determined as the percentage of the sediments passing through a sieve with an opening size of 63 μm. The total extractable metal concentrations in the sediments were determined after mineralization with a mixture of acids (HCl/HNO3/HF) in a microwave pressure digestion system (MWS-3, Berghof, Germany). The metal concentrations in the sediments were determined by atomic absorption spectrometry (AAS-VARIAN, Australia). The certified river sediment reference material LGC6187 (CRM) was used as a standard and was collected from a monitoring station lagoon on the river Elbe close to the Czech–German border. This material (CRM) was systematically used to control the analytical precision and bias.
Sequential extraction procedure and enrichment factor (K EF)
Fractionation using SE methods provides comprehensive information about the mobility of metal contaminants. The SE procedure used in this study is a modified BCR procedure for the fractionated analysis of sediments (Tessier et al. 1979; Vojtekova and Krakovska 2006). The five fractions (SE) and chemical reagents are listed below:
Water soluble (F1)
0.5 g of sediment was added to 50 ml of deionized H2O, extracted for 16 h at t = 20 °C, and centrifuged at 3000 rpm for 20 min;
Acid soluble (ion exchanged and carbonate) (F2)
The sample was extracted in an acid environment by adding 40 ml of 0.11 mol l−1 CH3COOH to the remainder of the 1st step and then extracting for 16 h at t = 20 °C, centrifuging at 3000 rpm for 20 min, and washing;
Reducible (F3)
40 ml of 0.1 mol l−1 NH2OH.HCl, pH 2 was added to the remainder of the 2nd step, extracted for 16 h at t = 20 °C, centrifuged at 3000 rpm for 20 min, and washed;
Oxidizable, organic, and sulfide (F4)
10 ml of 8.8 mol l−1 H2O2, pH 2 was added to the remainder of the 3rd step, extracted for 1 h at t = 20 °C, heated to 85 °C, and dried to near completion and then 10 ml of 8.8 mol l−1 H2O2 was again added; the solution was heated to 85 °C and dried. Finally, 50 ml of 1 mol l−1 CH3COONH4, pH 2 was added, and the sample was extracted for 16 h at t = 20 °C, centrifuged at 3000 rpm for 20 min, and washed;
Residue (F5)
Total degradation was achieved by adding 10 ml of HF and 1 ml of HClO4 to the remainder of the 4th step, drying, adding 10 ml of HF and again drying, and lastly adding 5 ml of HNO3 (1:1) (Sestinova et al. 2012).
The sediment reference material LGC6187 (CRM) was used.
The evaluation of ecological risk was determined by calculating the enrichment factor (K EF), as described below:
[K EF = (Labile fraction: F1–F4 (%))/Residue fraction F5 (%))sample/(Labile fraction: F1–F4 (%)/Residue fraction F5 (%))reference] (Moukhchan et al. 2013).
Toxicity testing using Phytotoxkit tests
The toxicity assays of the fresh sediments were based on the norm ISO 11269-1:2012. Soil quality describes a method for the determination of the effects of contaminated soils or contaminated samples on the root elongation of terrestrial plants (OECD 208). The Phytotoxkit microbiotest is a flexible method for determining the impact of pollutants on higher plants and can be applied to any type of plant seed and test soil in comparison to a control soil (CS). Three replicates were performed for each sample set. Phytotoxkit is an alternative test procedure that enables determination of the biological effects of chemical compounds on plants. The sediment was covered with the filter plate, and ten seeds of Sinapis alba were placed on top of the filter in a single row. After closing the test plates with the transparent cover, they were placed vertically and incubated for 72 h at 25 °C. The test was considered to be valid if the number of germinated seeds in the control was at least 90 %. Pictures of the test plates were analyzed with the free image analysis program Image Tools. The sediment samples were used to evaluate potential phytotoxic effects using the parameters (1) percentage inhibition of seed germination (ISG) and (2) percentage inhibition of root growth (IRG) in the test sediment (S).
where NSG is the number of seeds germinated, S is the sediment, CS is the control soil, and MRL is the mean root length.
All experimental bioassay results were expressed in terms of the inhibition of the plant and/or root growth (EC50) caused by the test sediment compared with the growth observed in the control experiment cultivated in the reference medium.
Results and discussion
Physicochemical properties of the sediments
Table 1 presented the pH, oxidation–reduction potential (E h), dry weight, organic matter dry weight, and granulometric distribution in the sediments. The pH of the sediment samples was in the range 7.25–7.59, which indicate an alkaline nature of sediments. Organic matter of the sediments ranged from 8.1 to 14.2 %. The highest percentage of the organic matter of the sediments was observed in the sample 2011 HR, according to Table 1. The organic matter probably comes from soil run-off from river basins but also decomposition of the organic material in the sediment at the bottom of rivers and water reservoirs themselves (Blaskova et al. 2013). Table 2 summarizes the results of the chemical analyses of the metals in the sediments, revealing significant contamination with arsenic and mercury according to the laws of the Methodological Instruction of the Ministry of Environment of the Slovak Republic No. 549/1998-2 for Assessment of Risks from Pollution of Sediments of Streams and Water Reservoirs and National Council of Slovak Republic No. 203/2009. The remaining trace elements were under the imposed limits (LVC) by law 203/2009. In terms of the environmental risk, Cu, Ni, As, Sb, and Hg exceeded the permitted concentrations in all samples. The water reservoir Ružín No.I, branches of the HR and HN drained a former mining area, has been polluted in the long term by heavy metals (Hg, Cu, Zn, Cd, Cr, Pb, Ni, Sb, and As), which significantly contributed to environmental degradation (Takac et al. 2009; Sestinova et al. 2012). Elements could be released from the sediments or precipitated in accordance with the changes in the physicochemical properties of the environment, i.e., pH, E h, dissolved oxygen, presence of organic chelates, etc. For these reasons, the quantification of the total metal contents does not provide sufficient information about the potential interactions between biotic and non-biotic components in the environment (Vojtekova and Krakovska 2006).
Sequential extraction of metals in sediments
Figure 2 provides the results of the As, Cd, Cr, Cu, Hg, Ni, Pb, Sb, and Zn determinations after the five-step SE of the contaminated sediments, from the branches of the HN and HR. It is evident in Fig. 2 that Cu and Pb were released in the greatest quantities in the third step in the reducible fraction, from 45 to 65.7 % in sample HN and up to 68–50 % in sample HR. The SE revealed the percentage contents of both samples in the second and fourth steps to be in the range of 15.8–25.2 % of the total Cu extract content and from 1.7 to 9.8 %, for Pb. The copper and lead were bonded to Fe and Mn oxides, which are unstable (higher mobility). They are released into aquatic environments in response to changes in oxidation–reduction potential (E h). According to Fig. 2, Ni was released most predominantly in the fifth step, to a residue fraction of 32.1 % of the total nickel content in sample HN and to 68.5 % in sample HR. In the second, third and fourth steps, 6.6 –46.7 % of the total Zn and Cd content, were released in both samples. The greatest quantity of Hg was released in the organic–sulfide fraction (F4), in the range of 75.2–87.6 %, characteristic of elements bound to organic matter and sulfides, which are released into aquatic environments upon the degradation of organic matter and changes in the physico-chemical properties of sulfides. The SE revealed low proportions of metals in the water-soluble fraction in both samples. The highest percentage of the total metal content for Sb, Cr, and As was between 82.0 and 94.6 % in the fifth step for both samples. The minimal bioavailability of Sb, Cr, and As was observed in this case. Fractions 1 and 4 represent the bioavailable metal content, and fraction 5 (residue) represents the insoluble residue. In the residue fractions, the metals are distributed between silicates, phosphates, and refractory oxides (Serafimovska et al. 2013).
Evaluation of ecological risk
The enrichment factor (K EF) was developed to evaluate the metal contamination using chemical form data that is usually obtained by SE analysis. The (K EF) denotes the enrichment factor of the examined element in the labile fraction of the examined environment (Yang et al. 2012). The enrichment of trace metals can be classified into the following categories: (K EF) ≤ 1 non-enrichment, 1 < (K EF) ≤ 3 minor enrichment, 3 < (K EF) ≤ 5 moderate enrichment, 5 < (K EF) ≤ 10 moderately severe enrichment, (K EF) > 10 severe enrichment.
The enrichment factors (K EF) of the studied sediments are presented in Fig. 3. All sediments exhibited (K EF) > 10 values for Cu (K EF = 18.3–21.9) except for 2010 HR (K EF = 9.2), indicating severe enrichment of this metal. Additionally, for Hg in the sample 2010 HR (K EF = 15.1), the enrichment was most related to the discharge of old mining loads following the treatment of the sulfide ores connected with the abandoned mines (Krompachy, Rudňany). The Hg in the samples 2010 HN and 2011 HR (K EF = 5.1–6.9) exhibited 5 < (K EF) ≤ 10, indicating moderately severe enrichment. All sediments displayed high values of 5 < (K EF) ≤ 10 for Pb and Cd, indicating moderately severe enrichment, and moderate values of 3 < (K EF) ≤ 5 for Ni and Zn, indicating moderate enrichment. Not even minor enrichments of 0 < (K EF) < 3 were observed for Cr, As, and Sb. According to the enrichment factor (K EF), the metal contamination level in the studied sediments followed the sequence: 2011 HR > 2010 HR > 2010 HN > 2011 HN, with severe enrichment of Cu and Hg in all of the samples. The bioavailability of the metals in the sediments followed the sequence: Cu > Hg > Pb > Cd > Zn > Ni > As > Cr > Sb. The higher the total content of metals is, the higher percentage of the labile fraction is, especially for Hg and Cu. This indicates that the anthropogenic metals are present dominantly in the labile fraction. Metals of natural origin reach coastal areas from rivers in the form of particulate material. These metals are mainly chemically bound to aluminosilicates and therefore lowly bioavailability (Yang et al. 2012). The bioavailability of metals plays a key role in risk assessment for metal contaminated sites, such as highly industrialized areas under former and present pressure from the metal—ore mining and smelting industries (Takac et al. 2009).
Germination bioassay by Phytotoxkit
Germination is the most sensitive assay for low toxicity effects. The sediment phytotoxicity was evaluated based on decreases in seed germination and root growth of Sinapis alba seeds in comparison with CS. Over 90 % of the seeds in the control samples were germinated. The percentage ISG was 8.9–41.1 % in the 2010–2011 HR samples and 9.1–45.1 % in the 2010–2011 HN samples, which was higher than the IRG. The percentage IRG was 9.3–41.2 % in the 2010–2011 HR samples and 11.6–40.1 % in the 2010–2011 HN sediments, respectively. The inhibitions (ISG and IRG) are summarized in Fig. 4. According to the Phytotoxkit microbiotest, the experimental concentration at which growth inhibition rises above 50 % after 72 h can be considered the effective concentration 72/EC50. The inhibition of the germination rate did not differ significantly from the controls. Based on the phytotoxicity testing, no phytotoxic effects of the heavy metal contaminated sediments from the water reservoir Ružin No.I on Sinapis alba seeds were observed.
Conclusions
This study aimed to gather more detailed information about the total metal concentration, SE analysis, and potential ecotoxicity of metals in river sediments to further understand the anthropogenic load and bioavailability of metals in environments affected by industrial activities. The results of our chemical analyses indicate significant contamination with As and Hg in the sediments from the branches of the Hornád River (2010–2011 HR) and the Hnilec River (2010–2011 HN). Moderate bioavailability of Cd, Zn, and Ni and minimal bioavailability of As, Sb, and Cr were observed. We therefore conclude that As, Sb, and Cr are not present in a form that is bio-accessible to plants but remain fixed in crystal lattices and mineral matrices. SE revealed high bioavailability of Cu, Hg, and Pb in the sediments, which are primarily of anthropogenic origin. The bioavailability of the metals evaluated in the sediments followed the sequence: Cu > Hg > Pb > Cd > Zn > Ni > As > Cr > Sb. The enrichment factors (K EF) were also calculated, revealing metal enrichment in the following sequence: Cu > Hg > Pb > Cd > Ni > Zn > Cr > As > Sb. According to the KEF, the metal contamination level in the studied sediments followed the sequence: 2011 HR > 2010 HR > 2010 HN > 2011 HN, with severe enrichment of Cu and Hg observed in all of the samples. Based on the experimental results of the phytotoxicity testing, no potential phytotoxic effect of the metals on the contaminated sediments from the branches of the Hornád River (2010–2011 HR) and the Hnilec River (2010–2011 HN) on Sinapis alba seeds was observed. Based on the results of the assays, it was found that the total concentrations of As, Sb, and Hg in the sediments, which were 1.5–2 times higher than the legal standards, did not exert phytotoxic effects on Sinapis alba seeds. These results suggest that combinations of metals and organic proportion may exert either antagonistic or synergistic effects on metals uptake by plants, depending on plant species, their growth stages, concentrations and characteristics of pollutants, and sediment conditions such as pH, redox potential, and content of organic matter. This indicates that the Sinapis alba test still exhibited a strong tolerance to contaminated sediment as this plant species has developed distinct detoxification mechanisms.
References
Aboudrar W, Schwartz Ch, Morel JL, Boularbah A (2013) Effect of nickel-resistant rhizosphere bacteria on the uptake of nickel by the hyperaccumulator Noccaea caerulescens under controlled conditions. J Soils Sediments 13(501):507
Bagur-Gonzale MG, Estepa-Molina C, Martin-Peinado F, Morales-Ruano S (2011) Toxicity assessment using Lactuca sativa L. bioassay of the metal (loid) As, Cu, Mn, Pb and Zn in soluble-in-water saturated soil extracts from an abandoned mining site. J Soils Sediments 11:281–289
Bettinetti R, Giarei C, Provini A (2003) A chemical analysis and sediment toxicity bioassays to assess the contamination of the River Lambro (Northern Italy). Arch Environ Contam Toxicol 45:72–80
Blaskova J, Vojtekova V, Novakova J, Mackovych D, Bazel Y, Lapcik L, Popernikova Z, Abussenaina AMM (2013) Sono-extraction as a pretreatment approach for the screening evaluation of element mobility of sediment samples. Cent Eur J Chem 11(7):1201–1212
Clement B, Raevel V, Renard O (2010) Ecotoxicological assessment of road runoff residues for aquatic surface ecosystems in a scenario of reuse. J Soils Sediments 10:1255–1266
Czerniawska-Kusza I, Kusza G (2011) The potential of the Phytotoxkit microbiotest for hazard evaluation of sediments in eutrophic freshwater ecosystems. Environ Monit Assess 179:113–121
Das KS, Chakrapani GJ (2011) Assessment of trace metal toxicity in soils of Raniganj Coalfield, India. Environ Monit Assess 177:63–71
de Miguel E, Charlesworth S, Ordóňez A, Seijas E (2005) Geochemical fingerprints and controls in the sediments of an urban river: River Manzanares, Madrid (Spain). Sci Total Environ 340:137–148
Devesa-Rey R, Iglesias ML, Perez-Moreira R, Diaz-Fierros F, Barral MT (2013) Application of the Weng’s ratio for the identification of Zn, Cu and Pb contamination in soils and sediments. J Soils Sediments 13:932–942
Dolezalova-Weissmanova H, Stepankova I, Vavrova M, Lapcikova A (2013) Determination of toxicity of musk compounds using alternative test of ecotoxicity. Chem Lett 107:172–177
Ferrari B, Radetski CM, Veber AM, Ferard JF (1999) Ecotoxicological assessment of solid wastes: a combined liquid and solid phase testing approach using a battery of bioassays and biomarkers. Environ Toxicol Chem 18:1195–1202
Fragoso N, Hodson PV, Zambon S (2006) Evaluation of an exposure assay to measure uptake of sediment PAH by fish. Environ Monit Assess 116(481):511
Hallare AV, Seiler TB, Hollert H (2011) The versatile, changing, and advancing roles of fish in sediment toxicity assessment—a review. J Soils Sediments 11:141–173
Houben D, Coder E, Sonnet P (2013) Leachability of cadmium, lead and zinc in a long-term spontaneously revegetated slag heap: implications for phytostabilization. J Soils Sediments 13:543–554
Katalay S, Boyacioglu M, Arslan OC, Parlak H, Karaaslan MA (2012) Phytotoxicity of water and sediment from Nif Brook (Izmir, Turkey) on green algae Desmodesmus (=Scenedesmus) subspicatus. Ekoloji 21(83):25–31
Kopittke PM, Blamey FP, Asher CJ, Menzies NW (2010) Trace metal phytotoxicity in solution culture: a review. J Exp Bot 61(4):945–954
Leitgib L, Kalman J, Gruiz K (2007) Comparison of bioassays by testing whole soil and their water extract from contaminated sites. Chemosphere 66:428–434
Low No. 203/2009 about application of sewage sludge and bottom sediments into the soil and amending Low No. 188/2003
Methodological instruction ME SR No. 549/1998 Library low VI/5, supplement No. 2 on risk assessment of contaminated sediments in rivers and water reservoirs
Mohamed I, Ahamadou B, Li M, Gong Ch, Cai P, Lian W, Huang Q (2010) Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. J Soils Sediments 10:973–982
Moukhchan F, March JG, Cerda V (2013) Distribution of trace metals in marine sediments of the Bay of Palma de Mallorca (Mallorca Island, Spain). Environ Monit Assess 185:695–706
Mukherjee DP, Kumar B (2012) Evaluation of metal contamination in freshly deposited sediment of Hugli Estuary, India. Arch Appl Sci Res 4(2):1155–1168
Norm STN EN 12879 Determination of loss when burning solids
Norm STN EN 75791 Determination the total content of substances and content of water
OECD 208 Terrestrial plant test: seedling emergence and seedling growth test. Determination of the effects of pollutants on soil flora: effects of contaminated soil on the emergence and early growth of higher plants
Phytotoxkit™ (2004) Seed germination and early growth microbiotest with higher plants, Standard operational procedure, MicroBioTest, Nazareth
Plaza GA, Nalecz-Jawecki G, Pinyakong O, Illmer P, Margesin R (2010) Ecotoxicological and microbiological characterization of soils from heavy-metal-and hydrocarbon-contaminated sites. Environ Monit Assess 163:477–488
Pretti C, Chiappe C, Baldetti S, Monni G, Intorre L (2009) Acute toxicity of ionic liquids for three freshwater organisms Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Ecotox Environ Safe 72(4):1170–1176
Quan SXQ, Yan B, Lei Ch, Yang F, Li N, Xiao X-M, Fu J-M (2014) Distribution of heavy metal pollution in sediments from an acid leaching site of e-waste. Sci Total Environ 499:349–355
Roulier JL, Belaund S, Coquery M (2010) Comparison of dynamic mobilization of Co, Cd and Pb in sediments using DGT and metal mobility assessed by sequential extraction. Chemosphere 79:839–843
Serafimovska JM, Arpadjan S, Stafilov T, Tsekova K (2013) Study of the antimony species distribution in industrially contaminated soils. J Soils Sediments 13:294–303
Sestinova O (2012) The forms study occurrence of metals in the sediments from the water reservoir Ružín No.I and their phytotoxicity and genotoxicity. Dissertation, Institute of Geotechnics, Slovak Academy of Sciences, Kosice
Sestinova O, Hanculak J, Findorakova L, Spaldon T, Kurbel T (2012) Analytical methods for the determination of copper and mercury in sediments. SGEM, 2012. Ecol Environ Prot Bulg 2012:51–57
Takac P, Szabova T, Kozakova L, Benkova M (2009) Heavy metals and their bioavailability from soils in the long-term polluted Central Spiš region of SR. Plant Soil Environ 55(4):167–172
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 57:844
Urban SR, Correa AXR, Schettini CAF, Schwingel PR, Sperb RM, Radetski CM (2010) Physicochemical and ecotoxicological evaluation of estuarine water quality during a dredging operation. J Soils Sediments 10:65–76
Vojtekova V, Krakovska E (2006) Fractionation analysis of sediments-limitations in the extractant selectivity. Chem Let 100:1096–1104
Vojtekova V, Novakova J, Mackovych D (2010) Simple assessment of the reactive aluminium in sediments. Environ Chem Lett 8:45–51
Yang Y, Chen F, Zhang L, Liu J, Wu S, Kang M (2012) Comprehensive assessment of heavy metal contamination in sediment of the Pearl River Estuary and adjacent shelf. Mar Pollut Bull 64:1947–1955
Acknowledgments
The authors greatly acknowledge the fellowship support of VEGA—Slovak Grant Agency for Science (Grant Nos. 2/0194/15 and 2/0079/16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Šestinova, O., Findoráková, L., Hančuľák, J. et al. Study of metal mobility and phytotoxicity in bottom sediments that have been influenced by former mining activities in Eastern Slovakia. Environ Earth Sci 74, 6017–6025 (2015). https://doi.org/10.1007/s12665-015-4625-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4625-y